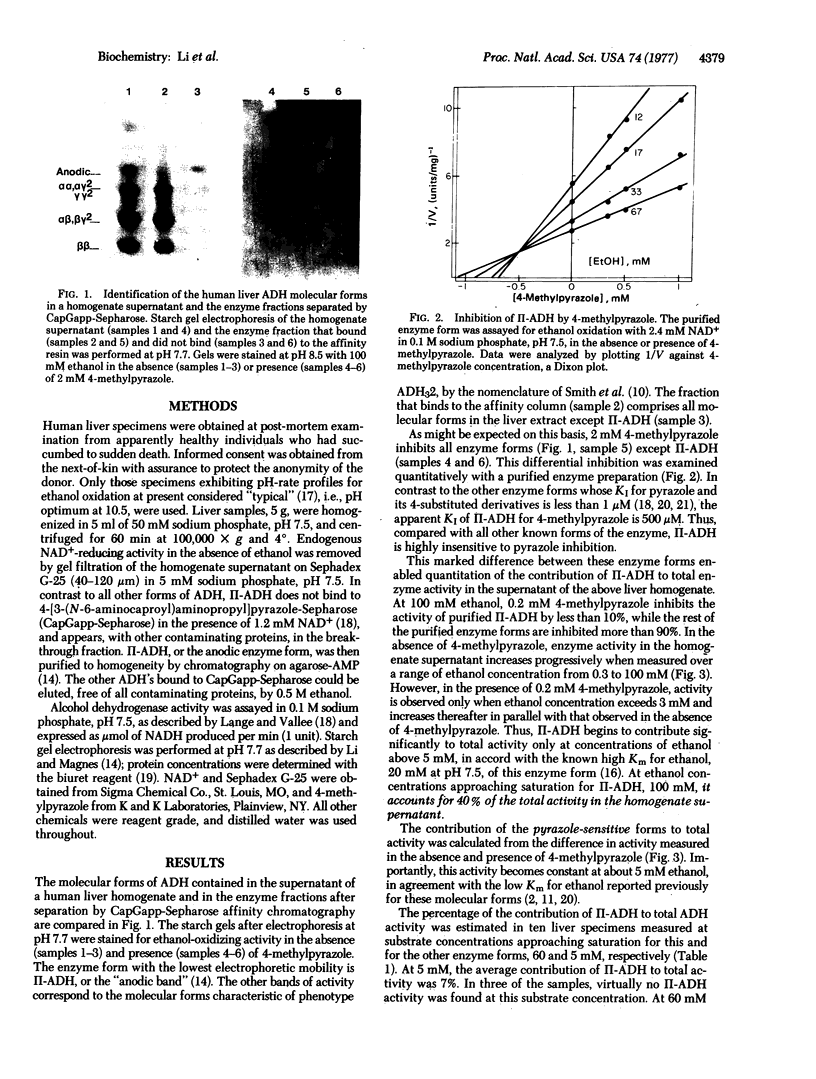
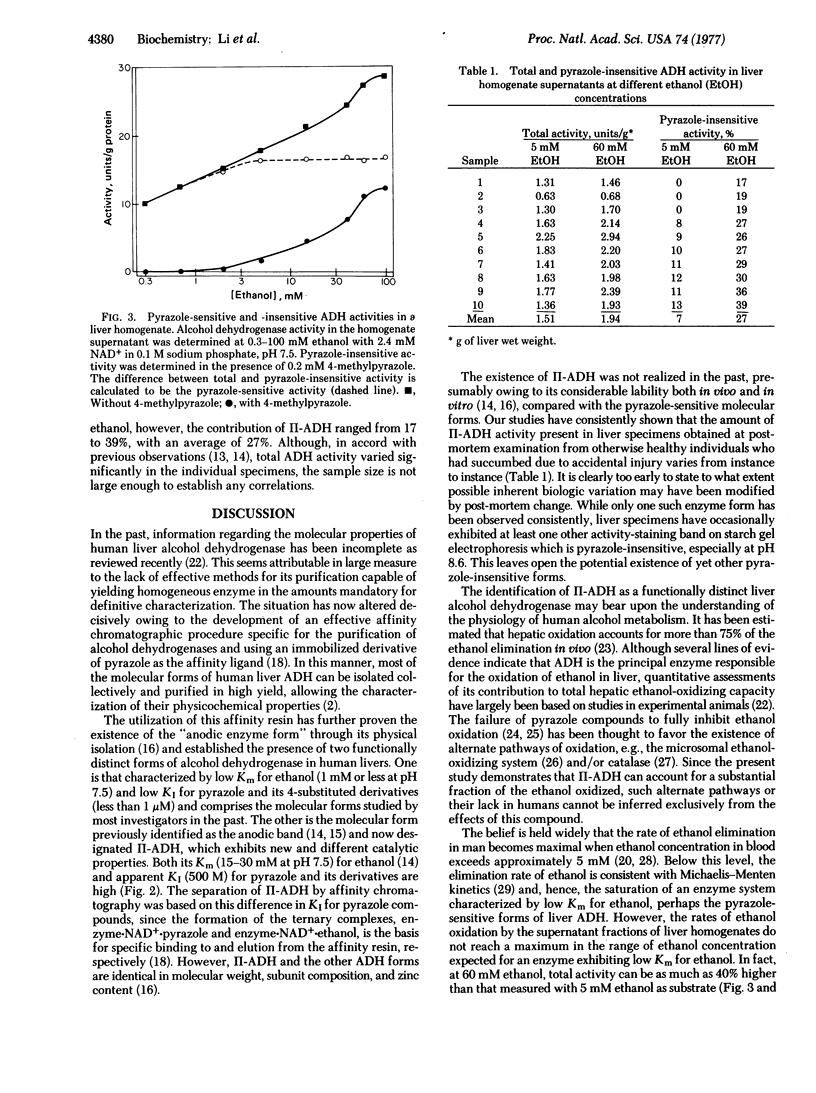
Abstract
Human liver alcohol dehydrogenase (alcohol: NAD+ oxidoreductase, EC 1.1.1.1), homogeneous by physicochemical criteria, has been available in quantity only recently [Lange, L. G. & Vallee, B. L. (1976) Biochemistry 15, 4681-4686]. Until now, the biochemical basis of human alcohol metabolism had to be extrapolated from the properties and behavior of enzymes from other species, primarily horses and yeast. The biological determinants of human alcoholism have remained obscure, although recent evidence indicates a genetic predisposition, requiring delineation. A functionally distinct form of human liver alcohol dehydrogenase (ADH), which we have designated II-ADH, is provocative since, thus far, it seems to be unique to human beings. It has a high Km for ethanol and is remarkably insensitive (apparent KI, 500 μM) to pyrazole and its derivatives, which are usually potent ADH inhibitors (KI, 1 μM), a property that is the basis for the isolation of II-ADH. The affinity resin 4-[3-(N-6-aminocaproyl)aminopropyl]pyrazole-Sepharose binds all other known forms of ADH but not II-ADH, thereby separating it selectively by affinity chromatography. In turn, this has led to the establishment of its identity with that enzyme form which was previously known as the anodic band and characterized by a high Km for ethanol (20 mM at pH 7.5). The remarkable insensitivity of II-ADH to pyrazole inhibition has also permitted quantitation of its role in hepatic ethanol oxidation. At 5 mM ethanol, a saturating concentration for virtually all other forms of ADH, II-ADH contributes less than 15% to total ethanol oxidation. However, at intoxicating concentrations, e.g., 60 mM, it can account for as much as 40% of the total ethanol oxidation rate of liver, indicating a seemingly unique role for this enzyme form in ethanol elimination. Thus far, we have found the amount of II-ADH varies from liver to liver of individuals and is considerably more labile than the other molecular forms, phenomena whose inter- or independence requires further study. The isolation of human II-ADH advances efforts to recognize and understand biochemical mechanisms that may be biological determinants of alcoholism and alcohol-related disease states, now generally approached and managed largely as psychosocial disorders.
Keywords: ethanol metabolism, multiple molecular forms, affinity chromatography
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azevêdo E., Smith M., Hopkinson D. A., Harris H. A study of possible factors influencing the variation in liver alcohol dehydrogenase activity in individuals of the 'usual' ADH phenotype. Ann Hum Genet. 1974 Jul;38(1):31–37. doi: 10.1111/j.1469-1809.1974.tb01990.x. [DOI] [PubMed] [Google Scholar]
- Bennion L. J., Li T. K. Alcohol metabolism in American Indians and whites. Lack of racial differences in metabolic rate and liver alcohol dehydrogenase. N Engl J Med. 1976 Jan 1;294(1):9–13. doi: 10.1056/NEJM197601012940103. [DOI] [PubMed] [Google Scholar]
- Blair A. H., Vallee B. L. Some catalytic properties of human liver alcohol dehydrogenase. Biochemistry. 1966 Jun;5(6):2026–2034. doi: 10.1021/bi00870a034. [DOI] [PubMed] [Google Scholar]
- Blomstrand R., Theorell H. Inhibitory effect on ethanol oxidation in man after administration of 4-methylpyrazole. Life Sci II. 1970 Jun 8;9(11):631–640. doi: 10.1016/0024-3205(70)90214-6. [DOI] [PubMed] [Google Scholar]
- Borson W. F., Li T. K. Isolation and characterization of an anodic form of human liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1977 Jan 10;74(1):85–91. doi: 10.1016/0006-291x(77)91378-x. [DOI] [PubMed] [Google Scholar]
- Goldberg L., Rydberg U. Inhibition of ethanol metabolism in vivo by administration of pyrazole. Biochem Pharmacol. 1969 Jul;18(7):1749–1762. doi: 10.1016/0006-2952(69)90164-6. [DOI] [PubMed] [Google Scholar]
- Goodwin D. W., Schulsinger F., Moller N., Hermansen L., Winokur G., Guze S. B. Drinking problems in adopted and nonadopted sons of alcoholics. Arch Gen Psychiatry. 1974 Aug;31(2):164–169. doi: 10.1001/archpsyc.1974.01760140022003. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Kalant H. The metabolism of ethanol and its metabolic effects. Pharmacol Rev. 1972 Mar;24(1):67–157. [PubMed] [Google Scholar]
- ISBELL H., FRASER H. F., WIKLER A., BELLEVILLE R. E., EISENMAN A. J. An experimental study of the etiology of rum fits and delirium tremens. Q J Stud Alcohol. 1955 Mar;16(1):1–33. [PubMed] [Google Scholar]
- LUNDQUIST F., WOLTHERS H. The kinetics of alcohol elimination in man. Acta Pharmacol Toxicol (Copenh) 1958;14(3):265–289. doi: 10.1111/j.1600-0773.1958.tb01164.x. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Sytkowski A. J., Vallee B. L. Human liver alcohol dehydrogenase: purification, composition, and catalytic features. Biochemistry. 1976 Oct 19;15(21):4687–4693. doi: 10.1021/bi00666a023. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Vallee B. L. Double-ternary complex affinity chromatography: preparation of alcohol dehydrogenases. Biochemistry. 1976 Oct 19;15(21):4681–4686. doi: 10.1021/bi00666a022. [DOI] [PubMed] [Google Scholar]
- Li T. K., Magnes L. J. Identification of a distinctive molecular form of alcohol dehydrogenase in human livers with high activity. Biochem Biophys Res Commun. 1975 Mar 3;63(1):202–208. doi: 10.1016/s0006-291x(75)80030-1. [DOI] [PubMed] [Google Scholar]
- Li T. K., Theorell H. Human liver alcohol dehydrogenase: inhibition by pyrazole and pyrazole analogs. Acta Chem Scand. 1969;23(3):892–902. doi: 10.3891/acta.chem.scand.23-0892. [DOI] [PubMed] [Google Scholar]
- Lieber C. S., DeCarli L. M. The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J Pharmacol Exp Ther. 1972 May;181(2):279–287. [PubMed] [Google Scholar]
- Mezey E., Tobon F. Rates of ethanol clearance and activities of the ethanol-oxidizing enzymes in chronic alcoholic patients. Gastroenterology. 1971 Nov;61(5):707–715. [PubMed] [Google Scholar]
- Misra P. S., Lefévre A., Ishii H., Rubin E., Lieber C. S. Increase of ethanol, meprobamate and pentobarbital metabolism after chronic ethanol administration in man and in rats. Am J Med. 1971 Sep;51(3):346–351. doi: 10.1016/0002-9343(71)90270-1. [DOI] [PubMed] [Google Scholar]
- Newman H. W. Maximal Consumption of Ethyl Alcohol. Science. 1949 Jun 10;109(2841):594–595. doi: 10.1126/science.109.2841.594. [DOI] [PubMed] [Google Scholar]
- Pietruszko R. Human liver alcohol dehydrogenase--inhibition of methanol activity by pyrazole, 4-methylpyrazole, 4-hydroxymethylpyrazole and 4-carboxypyrazole. Biochem Pharmacol. 1975 Sep 1;24(17):1603–1607. doi: 10.1016/0006-2952(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Pietruszko R., Theorell H., De Zalenski C. Heterogeneity of alcohol dehydrogenase from human liver. Arch Biochem Biophys. 1972 Nov;153(1):279–293. doi: 10.1016/0003-9861(72)90446-8. [DOI] [PubMed] [Google Scholar]
- Schenker T. M., Teeple L. J., Von Wartburg J. P. Heterogeneity and polymorphism of human-liver alcohol dehydrogenase. Eur J Biochem. 1971 Dec;24(2):271–279. doi: 10.1111/j.1432-1033.1971.tb19681.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Alcohol dehydrogenase isozymes in adult human stomach and liver: evidence for activity of the ADH 3 locus. Ann Hum Genet. 1972 Mar;35(3):243–253. doi: 10.1111/j.1469-1809.1957.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Smith M., Hopkinson D. A., Harris H. Developmental changes and polymorphism in human alcohol dehydrogenase. Ann Hum Genet. 1971 Feb;34(3):251–271. doi: 10.1111/j.1469-1809.1971.tb00238.x. [DOI] [PubMed] [Google Scholar]
- TYGSTRUP N., WINKLER K., LUNDQUIST F. THE MECHANISM OF THE FRUCTOSE EFFECT ON THE ETHANOL METABOLISM OF THE HUMAN LIVER. J Clin Invest. 1965 May;44:817–830. doi: 10.1172/JCI105194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- VONWARTBURG J. P., BETHUNE J. L., VALLEE B. L. HUMAN LIVER--ALCOHOL DEHYDROGENASE. KINETIC AND PHYSICOCHEMICAL PROPERTIES. Biochemistry. 1964 Nov;3:1775–1782. doi: 10.1021/bi00899a033. [DOI] [PubMed] [Google Scholar]
- Vesell E. S., Page J. G., Passananti G. T. Genetic and environmental factors affecting ethanol metabolism in man. Clin Pharmacol Ther. 1971 Mar-Apr;12(2):192–201. doi: 10.1002/cpt1971122part1192. [DOI] [PubMed] [Google Scholar]
- li T. K. Enzymology of human alcohol metabolism. Adv Enzymol Relat Areas Mol Biol. 1977;45:427–483. doi: 10.1002/9780470122907.ch6. [DOI] [PubMed] [Google Scholar]
- von Wartburg J. P., Papenberg J., Aebi H. An atypical human alcohol dehydrogenase. Can J Biochem. 1965 Jul;43(7):889–898. doi: 10.1139/o65-102. [DOI] [PubMed] [Google Scholar]