Abstract
Support for the contribution of reactive oxygen species (ROS) to antimicrobial lethality has been refined and strengthened. Killing by diverse antimicrobials is enhanced by defects in genes that protect against ROS, inhibited by compounds that block hydroxyl radical accumulation, and is associated with surges in intracellular ROS. Moreover, support has emerged for a genetic pathway that controls the level of ROS. Since some antimicrobials kill in the absence of ROS, ROS must add to, rather than replace, known killing mechanisms. New work has addressed many of the questions concerning the specificity of dyes used to detect intracellular ROS and the specificity of perturbations that influence ROS surges. However, complexities associated with killing under anaerobic conditions remain to be resolved. Distinctions among primary lesion formation, resistance, direct lesion-mediated killing and a self-destructive stress response are discussed to facilitate efforts to potentiate ROS-mediated bacterial killing and improve antimicrobial efficacy.
Keywords: ROS, antibiotics, killing, post-damage cellular response
Reactive oxygen species (ROS) involvement in antimicrobial action
In 2007 Kohanski et al.1 proposed that ROS (superoxide, peroxide and hydroxyl radicals) contribute to lethality for fluoroquinolones, β-lactams and aminoglycosides. The work explained why antioxidants suppress quinolone-mediated mutagenesis2 and why oxidative stress is detected in bacteria treated with antimicrobials.3,4 Follow-up work showed that the deletion of genes encoding catalase/peroxidase increases lethality for the three antibiotic classes,5 and upstream genes appeared to be part of a death pathway.6–10 Moreover, the contribution of DNA repair genes to killing by ampicillin and kanamycin was explained as a consequence of ROS-mediated DNA damage.11 Stress-induced, ROS-mediated bacterial self-destruction opened new avenues for antimicrobial enhancement with the clear understanding that the contribution of ROS to antimicrobial killing is complex—ROS adds to, but does not replace, previously established killing mechanisms specific to each compound class.12 The level of ROS contribution to antimicrobial killing depends on compound type and drug exposure. Indeed, some quinolones rely fully on ROS for rapid killing, while others do not.13,14
Contrary opinions on the ROS hypothesis
In 2013 four reports addressed aspects of the ROS hypothesis. One contrary view actually confirmed that some antimicrobials kill in the absence of ROS15 using norfloxacin, a quinolone known to kill Escherichia coli by an ROS-dependent mechanism at low concentrations and an ROS-independent mechanism at high concentrations.13 As expected, thiourea and anaerobic conditions interfered with norfloxacin-mediated killing at low but not high concentrations.15 Ofloxacin, a more potent fluoroquinolone, was affected little by anoxia, as expected for a compound that kills largely by the ROS-independent pathway. This behaviour of quinolones is described in Figure 1: killing can derive directly from the primary lesion or from an ROS-mediated stress response. For ampicillin and kanamycin, the absence of AhpCF peroxidase was reported to enhance activity,5 but activity was still seen with ampicillin when experiments were conducted in an anaerobic chamber.15 These observations suggest that ampicillin has a mode of killing that does not involve ROS.
Figure 1.
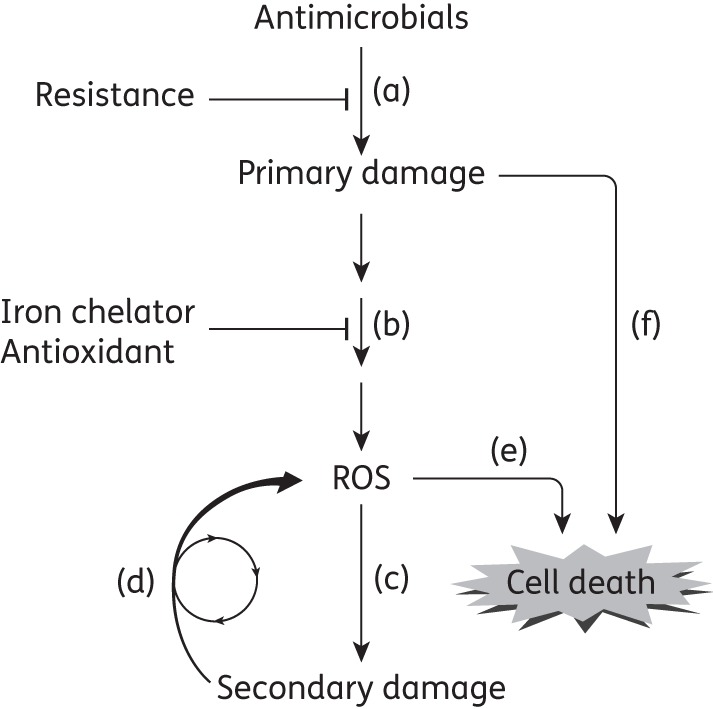
Major steps in lethal action of antimicrobials. (a) Treatment with a lethal antimicrobial leads to primary damage that is characteristic of the agent. Growth inhibition, a surrogate for primary damage formation, is usually measured as MIC or efficiency of plating, parameters that reflect drug uptake, efflux and drug-target affinity; high MIC values are associated with resistance. These parameters are not designed to measure killing. (b) Primary damage stimulates a pathway that leads to ROS accumulation. This pathway can be blocked by treating cells with iron chelators and antioxidants; it is stimulated by deficiencies in catalase/peroxidases. (c) ROS cause secondary damage to nucleic acids, proteins and lipids. (d) Secondary damage stimulates additional ROS production. When secondary damage exceeds a critical threshold, it becomes self-amplifying. (e) Self-amplification of ROS assures cell death. (f) If primary damage is severe enough, it can result in death directly, i.e. without the need for ROS even though ROS accumulate.14 Killing due to primary damage can be measured by blocking the accumulation of ROS. To study factors specifically involved in death rather than primary lesion formation, factors influencing step (a) (e.g. drug uptake, efflux and target affinity) need to be eliminated from consideration. This elimination can be achieved by normalizing drug concentration to MIC when survival is measured. Rapid killing may require drug concentrations above MIC, as seen for quinolones.14,41,42 Not shown are ROS-mediated effects on drug uptake and efflux18,35 and on feedback regulatory loops controlling ROS.
A second report16 raised issues concerning chemical probes of ROS effects. For example, off-target effects are difficult to rule out for antioxidants and iron chelators, compounds widely used to correlate ROS with antimicrobial-mediated killing. Questions were also raised about the specificity of dyes used to assess ROS surges associated with killing. As mentioned above,15 some agents (ampicillin and norfloxacin) were lethal when E. coli was treated in an anaerobic chamber. These data are consistent with some killing being ROS-independent. In these experiments ambiguity was introduced by plating in air following anoxic antimicrobial treatment, a procedure that may affect bacterial survival.17
Two other reports emphasize the utility of Figure 1. One defines features that affect gentamicin uptake and therefore primary lesion formation and direct killing.18 Such experiments cannot distinguish effects on growth inhibition (MIC) from killing, nor can they separate antimicrobial-specific mechanisms of killing from a secondary, lethal stress response common to multiple antimicrobials. The other report used an assay, efficiency of plating, that measures primary lesion formation and resistance, not killing.19 Thus, neither report specifically addressed ROS-mediated killing.
In summary, the follow-up work confirmed that ROS does not replace known lethal mechanisms and emphasized that killing under anaerobic conditions is far from understood. Apparent weaknesses in the support for ROS being an additional killing mechanism do not allow the hypothesis to be rejected. Nevertheless, a set of commentaries has emerged,20–24 revealing a need for clarification and another round of experimentation.
Solidifying the ROS–antimicrobial lethality hypothesis
Many of the objections to the ROS–antimicrobial lethality hypothesis were subsequently addressed experimentally17 and in reviews.25,26 For example, dye specificity for intracellular ROS detection was addressed by examining seven different dyes acting through a variety of chemistries. Norfloxacin, ampicillin and gentamicin did indeed differ in the level of response elicited, but lethal antimicrobials generally elevated ROS levels. In another example, an intracellular assay for peroxide revealed an increase in ROS associated with antibiotic treatment, a result not seen with an extracellular assay.16 Moreover, an Hpx catalase/peroxidase triple mutant (katG, katE, ahpCF) was found to constitutively express factors expected to protect from oxidative stress. This observation explained a previous failure to observe elevated antimicrobial lethality with this mutant.16 As additional evidence, a variety of superoxide- and peroxide-sensitive promoters were activated by norfloxacin and ampicillin. Finally, overexpression of katG reduced antibiotic-mediated lethality, thereby complementing earlier work in which the deletion of catalase/peroxidase genes increased lethality.5
While the role of ROS in antimicrobial-mediated killing is imperfectly understood, the recent follow-up work17 forces us to consider whether antioxidant consumption is advisable during antibiotic therapy, since it appears to affect antibiotic action.27 These observations also encourage work to find enhancers of ROS-mediated antimicrobial lethality. To facilitate that effort, we briefly consider measurements of lethal stress responses.
Assays for factors involved in lethal stress responses
Distinguishing between bacteriostatic and bactericidal activity is a key issue (Figure 1). Bacteriostatic action, which is associated with primary lesion formation (Figure 1a), is affected by processes such as drug uptake, efflux and target affinity. Bactericidal activity may derive from both primary lesions and the cellular response to primary damage. Focus on the lethal response, rather than on the initial lesion, can be achieved by expressing lethal drug concentrations as a multiple of MIC.5 That normalizes treatments with respect to growth inhibition, a surrogate for primary lesion formation. However, even after normalization to MIC, the extent of antimicrobial exposure can be important. For example, high drug concentration and long treatment time can eliminate most of the major, growing bacterial population. Then tests for the contribution of ROS on antimicrobial lethality reflect the response of persister cells. Such cells may be metabolically inactive28,29 and show little contribution of ROS; thus, the absence of an effect on subpopulations of persister cells by agents expected to perturb ROS15 has no bearing on ROS involvement in antimicrobial action with the major, growing bacterial population. Duration of the experiment is also important: ROS can accelerate killing without increasing the extent of killing.30 Consequently, overnight killing assays, such as those represented by MBC, are uninformative for detecting a transient stress response. Using a broad range of both drug concentrations and treatment times is important to avoid missing the exposure window in which the contribution of ROS to killing can be observed.
Concluding remarks
We conclude that ROS contribute to the lethal action of many antimicrobials. Exceptions deepen our understanding of antimicrobials, and apparent paradoxes promise new insights. For example, the MazF toxin is proposed to be part of the pathway that communicates information about antimicrobial-mediated lesions to the respiratory chain for ROS production.7,8 Why do some laboratories conclude that the MazF toxin is protective while others conclude the opposite?31–33 Why do subinhibitory doses of a superoxide generator protect E. coli from some types of antimicrobial-mediated killing,5,34–36 while high doses of the generator enhance lethality?37 It appears that bacteria contain a set of bifunctional factors that allow cells to make a live-or-die decision based on whether the stress-mediated damage is repairable.8,38,39 Since the cost of double-strand DNA break repair can be high,40 bacterial populations may use ROS-mediated cell death to maximize resource utilization. Perhaps we can exploit this feature to help control infections.
Funding
The authors' work was supported by NIH grants 1DP2OD007423, 2R01AI073491 and 1R21AI103781.
Transparency declarations
None to declare.
Acknowledgements
We thank Arnold Bendich and Marila Gennaro for critical comments on the manuscript prior to sunmission.
References
- 1.Kohanski M, Dwyer D, Hayete B, et al. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Arriaga-Alba M, Rivera-Sanchez R, Parra-Cervantes G, et al. Antimutagenesis of β-carotene to mutations induced by quinolone on Salmonella typhimurium. Arch Med Res. 2000;31:156–61. doi: 10.1016/s0188-4409(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 3.Becerra M, Albesa I. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochem Biophys Res Commun. 2002;297:1003–7. doi: 10.1016/s0006-291x(02)02331-8. [DOI] [PubMed] [Google Scholar]
- 4.Albesa I, Becerra M, Battan P, et al. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem Biophys Res Commun. 2004;317:605–9. doi: 10.1016/j.bbrc.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Zhao X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother. 2009;53:1395–402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohanski M, Dwyer D, Wierzbowski J, et al. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–90. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies B, Kohanski M, Simmons L, et al. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol Cell. 2009;36:845–60. doi: 10.1016/j.molcel.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorsey-Oresto A, Lu T, Mosel M, et al. YihE kinase is a novel regulator of programmed cell death in bacteria. Cell Rep. 2013;3:528–37. doi: 10.1016/j.celrep.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gusarov I, Shatalin K, Starodubtseva M, et al. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science. 2009;325:1380–4. doi: 10.1126/science.1175439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shatalin K, Shatalina E, Mironov A, et al. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334:986–90. doi: 10.1126/science.1209855. [DOI] [PubMed] [Google Scholar]
- 11.Foti J, Devadoss B, Winkler J, et al. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–9. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohanski M, Dwyer D, Collins J. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8:423–35. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik M, Hussain S, Drlica K. Effect of anaerobic growth on quinolone lethality with Escherichia coli. Antimicrob Agents Chemother. 2007;51:28–34. doi: 10.1128/AAC.00739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Zhao X, Malik M, et al. Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death. J Antimicrob Chemother. 2010;65:520–4. doi: 10.1093/jac/dkp486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keren I, Wu Y, Inocencio J, et al. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–6. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Imlay J. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–3. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwyer D, Belenky P, Yang J, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc Natl Acad Sci USA. 2014;111:E2100–9. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezraty B, Vergnes A, Banzhaf M, et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science. 2013;340:1583–7. doi: 10.1126/science.1238328. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney TF, Silhavy TJ. The Cpx stress response confers resistance to some, but not all, bactericidal antibiotics. J Bacteriol. 2013;195:1869–74. doi: 10.1128/JB.02197-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang F. Antibiotic and ROS linkage questioned. Nat Biotech. 2013;31:415–6. doi: 10.1038/nbt.2574. [DOI] [PubMed] [Google Scholar]
- 21.Wright G, Hung D, Helmann J. How antibiotics kill bacteria: new models needed? Nat Med. 2013;19:544–5. doi: 10.1038/nm.3198. [DOI] [PubMed] [Google Scholar]
- 22.Hadlington S. Battleground develops over antibiotic killing mechanism. ChemistryWorld. 2013. 8 March. [Google Scholar]
- 23.Balaban N, Gerdes K, Lewis K, et al. A problem of persistence: still more questions than answers? Nat Rev Microbiol. 2013;11:587–91. doi: 10.1038/nrmicro3076. [DOI] [PubMed] [Google Scholar]
- 24.Kuhnert N. One size does not fit all--bacterial cell death by antibiotics cannot be explained by the action of reactive oxygen species. Angew Chem Int Ed. 2013;52:10946–8. doi: 10.1002/anie.201304548. [DOI] [PubMed] [Google Scholar]
- 25.Dwyer DJ, Collins JJ, Walker GC. Unraveling the physiological complexities of antibiotic lethality. Annu Rev Pharmacol Toxicol. 2015;55:9.1–9.20. doi: 10.1146/annurev-pharmtox-010814-124712. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, Drlica K. Reactive oxygen species and the bacterial response to lethal stress. Current Opin Microbiol. 2014;21:1–6. doi: 10.1016/j.mib.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marathe S, Kumar R, Ajitkumar P, et al. Curcumin reduces the antimicrobial activity of ciprofloxacin against Salmonella typhimurium and Salmonella typhi. J Antimicrob Chemother. 2013;68:139–52. doi: 10.1093/jac/dks375. [DOI] [PubMed] [Google Scholar]
- 28.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–72. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 29.Gerdes K, Maisonneuve E. Bacterial persistence and toxin-antitoxin loci. Annu Rev Microbiol. 2012;66:103–23. doi: 10.1146/annurev-micro-092611-150159. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Liu X, Qu Y, et al. Inhibitors of reactive oxygen species accumulation delay and/or reduce the lethality of several antistaphylococcal agents. Antimicrob Agents Chemother. 2012;56:6048–50. doi: 10.1128/AAC.00754-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, et al. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2006;2:e135. doi: 10.1371/journal.pgen.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerdes K, Christensen SK, Lobner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol. 2005;3:371–82. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhang J, Hoeflich K, et al. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell. 2003;12:913–23. doi: 10.1016/s1097-2765(03)00402-7. [DOI] [PubMed] [Google Scholar]
- 34.Burger RM, Drlica K. Superoxide protects Escherichia coli from bleomycin mediated lethality. J Inorg Biochem. 2009;103:1273–7. doi: 10.1016/j.jinorgbio.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosel M, Li L, Drlica K, et al. Superoxide-mediated protection of Escherichia coli from antimicrobials. Antimicrob Agents Chemother. 2013;57:5755–9. doi: 10.1128/AAC.00754-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Vulic M, Keren I, et al. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother. 2012;56:4922–6. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farr SB, Natvig DO, Kogoma T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J Bacteriol. 1985;164:1309–16. doi: 10.1128/jb.164.3.1309-1316.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Wang X, Drlica K, et al. A toxin-antitoxin module in Bacillus subtilis can both mitigate and amplify effects of lethal stress. PLoS One. 2011;6:e23909. doi: 10.1371/journal.pone.0023909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dwyer D, Camacho D, Kohanski M, et al. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol Cell. 2012;46:561–72. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoeijmakers JH. DNA damage, aging, and cancer. New Engl J Med. 2009;361:1475–85. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 41.Malik M, Zhao X, Drlica K. Lethal fragmentation of bacterial chromosomes mediated by DNA gyrase and quinolones. Mol Microbiol. 2006;61:810–25. doi: 10.1111/j.1365-2958.2006.05275.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen CR, Malik M, Snyder M, et al. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258:627–37. doi: 10.1006/jmbi.1996.0274. [DOI] [PubMed] [Google Scholar]


