Abstract
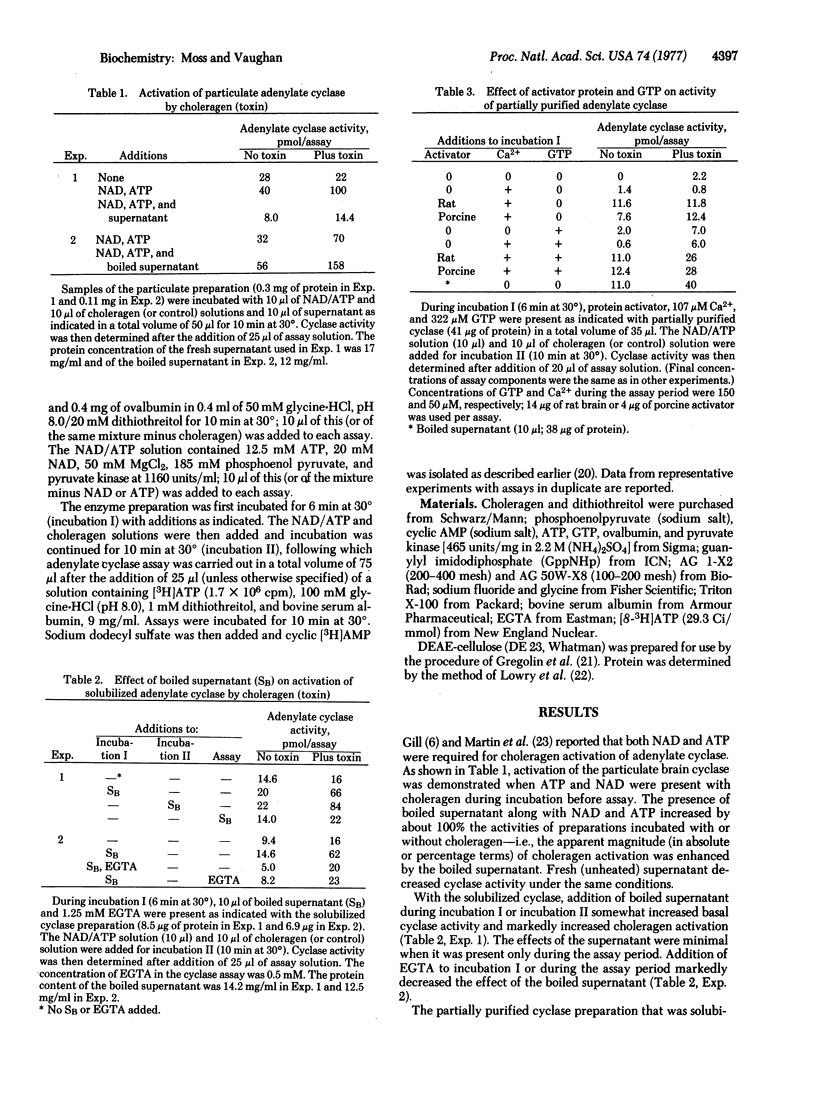
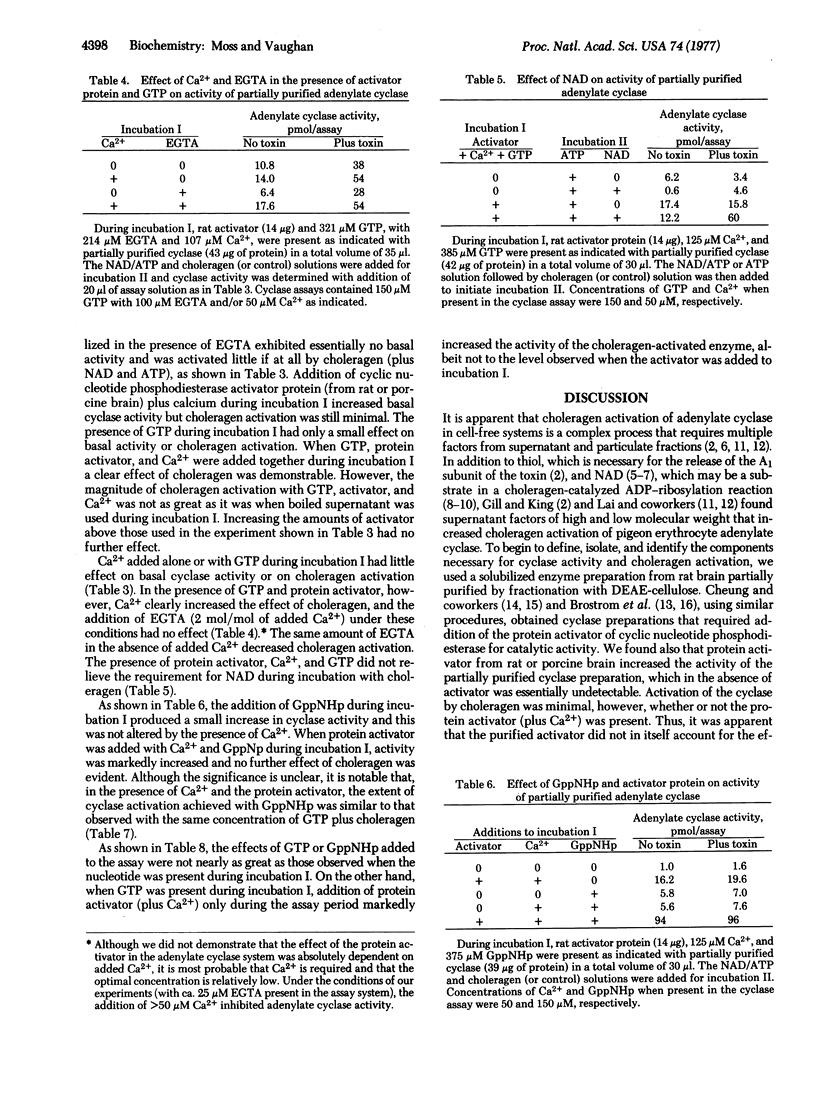
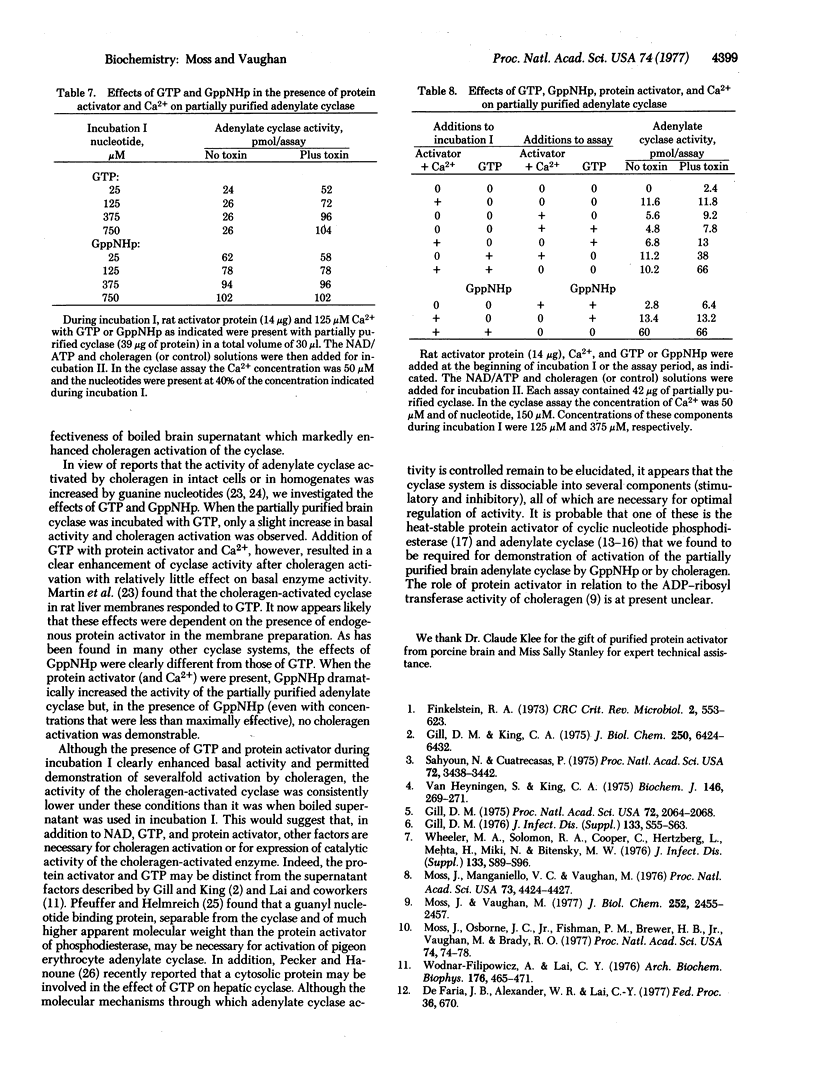
The requirements for choleragen activation of adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] were investigated by using an enzyme preparation solubilized with Triton X-100 from an extensively washed brain particulate fraction and partially purified with DEAE-cellulose. Unlike the particulate enzyme, this preparation was not activated after incubation with choleragen plus dithiothreitol, ATP, and NAD. Addition of the purified protein activator of cyclic nucleotide phosphodiesterase and calcium to the partially purified enzyme increased basal activity somewhat, but choleragen activation was minimal. When cyclase was incubated with GTP plus the protein activator (and calcium), choleragen markedly increased the activity 3- to 6-fold. When GppNHp and protein activator were incubated with the cyclase prior to assay, activity was elevated but no effect of choleragen was observed. GTP and GppNHp had relatively small effects on cyclase activity in the absence of protein activator or if they were added directly to the assay. Boiled brain supernatant was consistently more effective than protein activator (plus calcium) and GTP, suggesting that other factors are required for maximal cyclase activity after choleragen treatment.
It appears that the cyclase system is dissociable into several components, all of which may be necessary for optimal regulation of activity. It is probable that one of these is the heat-stable calcium-dependent protein activator of cyclic nucleotide phosphodiesterase and adenylate cyclase that we have found is required along with GTP for demonstration of choleragen activation of partially purified brain adenylate cyclase.
Keywords: phosphodiesterase activator, guanylyl imidodiphosphate, cholera toxin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Mong L., Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. Relations to the mode of activation by hormones. J Membr Biol. 1975 Nov 7;24(2):107–129. doi: 10.1007/BF01868618. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Huang Y. C., Breckenridge B. M., Wolff D. J. Identification of a calcium-binding protein as a calcium-dependent regulator of brain adenylate cyclase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):64–68. doi: 10.1073/pnas.72.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostrom M. A., Brostrom C. O., Breckenridge B. M., Wolff D. J. Regulation of adenylate cyclase from glial tumor cells by calcium and a calcium-binding protein. J Biol Chem. 1976 Aug 10;251(15):4744–4750. [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gregolin C., Ryder E., Lane M. D. Liver acetyl coenzyme A carboxylase. I. Isolation and cat- alytic properties. J Biol Chem. 1968 Aug 25;243(16):4227–4235. [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin Y. M., Liu Y. P., Cheung W. Y. Cyclic 3':5'-nucleotide phosphodiesterase. Purification, characterization, and active form of the protein activator from bovine brain. J Biol Chem. 1974 Aug 10;249(15):4943–4954. [PubMed] [Google Scholar]
- Lynch T. J., Tallant E. A., Cheung W. Y. Ca++-dependent formation of brain adenylate cyclase-protein activator complex. Biochem Biophys Res Commun. 1976 Jan 26;68(2):616–625. doi: 10.1016/0006-291x(76)91190-6. [DOI] [PubMed] [Google Scholar]
- Manganiello V. C., Vaughan M. Activation and inhibition of fat cell adenylate cyclase by fluoride. J Biol Chem. 1976 Oct 25;251(20):6205–6209. [PubMed] [Google Scholar]
- Martin B. R., Houslay M. D., Kennedy E. L. Cholera toxin requires oxidized nicotinamide-adenine dinucleotide to activate adenylate cyclase in purified rat liver plasma membranes. Biochem J. 1977 Mar 1;161(3):639–642. doi: 10.1042/bj1610639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Manganiello V. C., Vaughan M. Hydrolysis of nicotinamide adenine dinucleotide by choleragen and its A protomer: possible role in the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4424–4427. doi: 10.1073/pnas.73.12.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Osborne J. C., Jr, Fishman P. H., Brewer H. B., Jr, Vaughan M., Brady R. O. Effect of gangliosides and substrate analogues on the hydrolysis of nicotinamide adenine dinucleotide by choleragen. Proc Natl Acad Sci U S A. 1977 Jan;74(1):74–78. doi: 10.1073/pnas.74.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Mechanism of action of choleragen. Evidence for ADP-ribosyltransferase activity with arginine as an acceptor. J Biol Chem. 1977 Apr 10;252(7):2455–2457. [PubMed] [Google Scholar]
- Pecker F., Hanoune J. Activation of epinephrine-sensitive adenylate cyclase in rat liver by cytosolic protein-nucleotide complex. J Biol Chem. 1977 Apr 25;252(8):2784–2786. [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Sahyoun N., Cuatrecasas P. Mechanism of activation of adenylate cyclase by cholera toxin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3438–3442. doi: 10.1073/pnas.72.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen S., King C. A. Short communications. Subunit A from cholera toxin is an activator of adenylate cyclase in pigeon erythrocytes. Biochem J. 1975 Jan;146(1):269–271. doi: 10.1042/bj1460269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Lai C. Y. Stimulation of adenylate cyclase in washed pigeon erythrocyte membrane with cholera toxin and its subunits. Arch Biochem Biophys. 1976 Oct;176(2):465–471. doi: 10.1016/0003-9861(76)90189-2. [DOI] [PubMed] [Google Scholar]


