Abstract
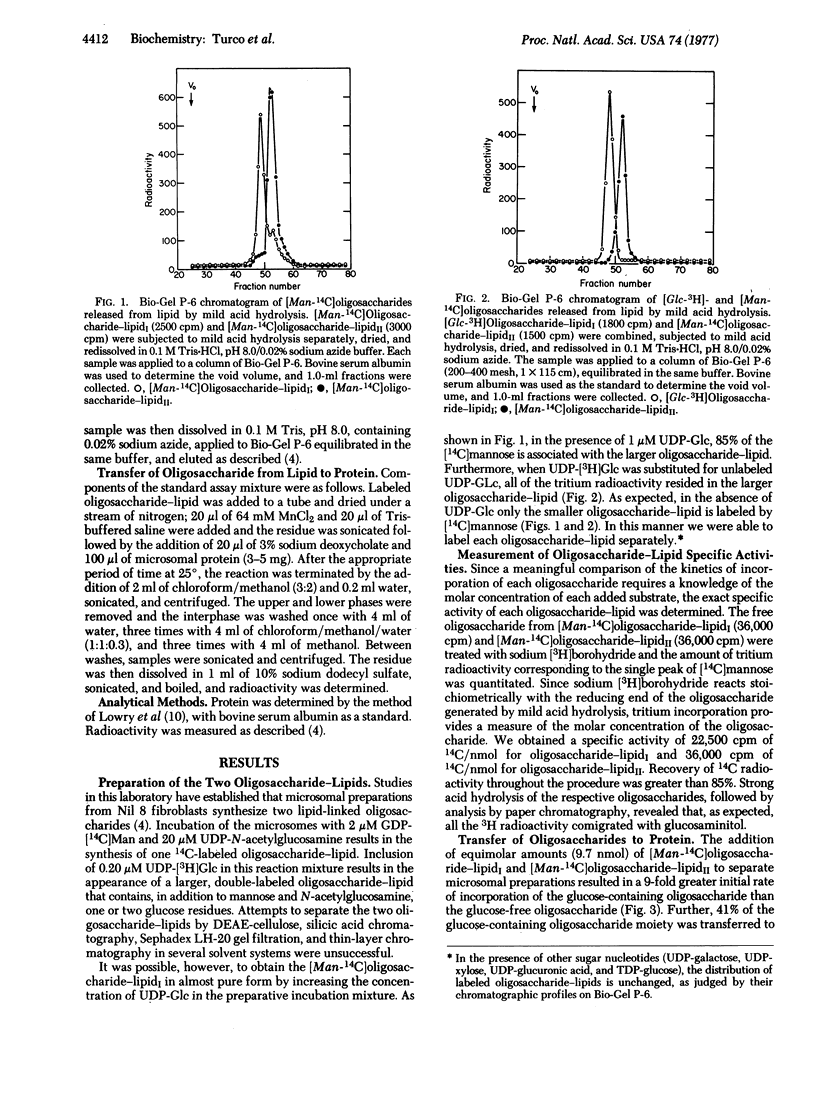
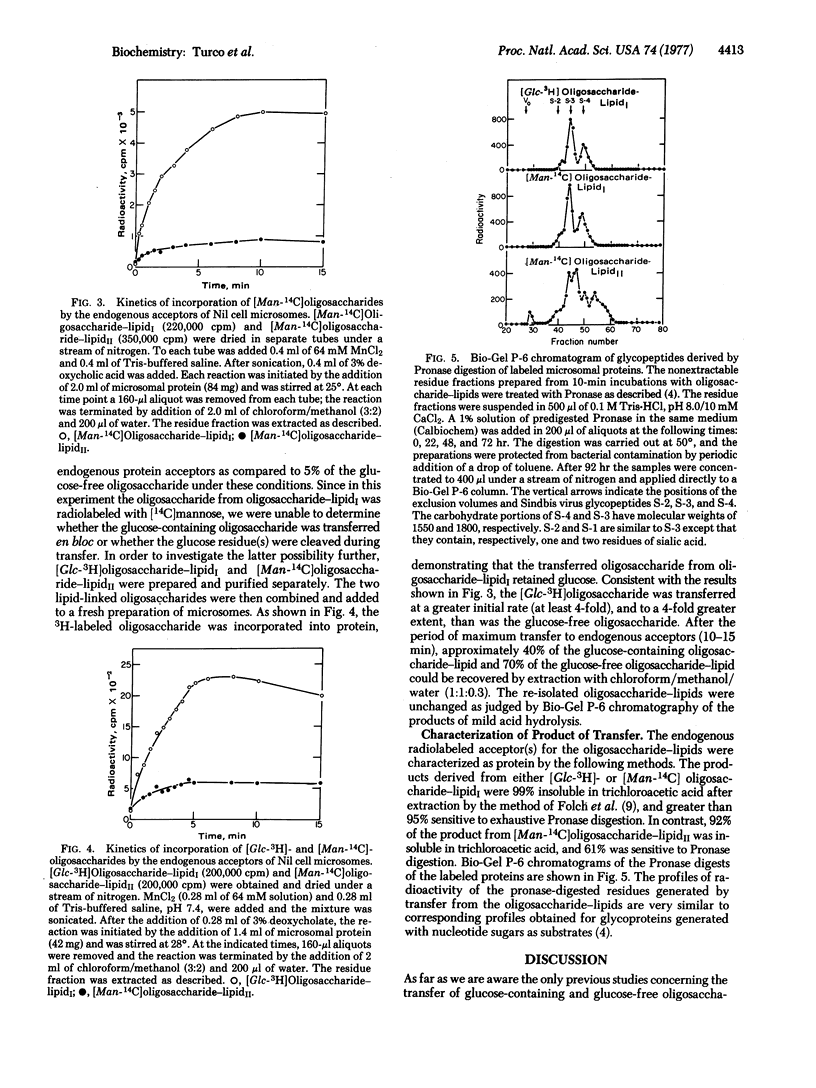
We have shown previously that particulate preparations of Nil 8 fibroblasts catalyze the synthesis of two oligosaccharide-lipids: one composed of N-acetylglucosamine and mannose residues and the other containing, in addition to mannose and N-acetylglucosamine, one or two glucose residues. These two oligosaccharide-lipids were purified and added to fresh microsomal preparations. In comparative studies, we find that the glucose-containing lipid-linked oligosaccharide is transferred much more rapidly to endogenous protein acceptors than the glucose-free compound. With materials of comparable specific activities, as much as 41% of the glucose-containing oligosaccharide was transferred to protein as compared to 5% for the glucose-free compound. These results suggest that the attachment of glucose to mannosyl lipid-linked oligosaccharide serves an important role in the transfer of these compounds from lipid carrier to protein acceptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens N. H., Parodi A. J., Leloir L. F. Glucose transfer from dolichol monophosphate glucose: the product formed with endogenous microsomal acceptor. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2857–2860. doi: 10.1073/pnas.68.11.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- GINSBURG V. SUGAR NUCLEOTIDES AND THE SYNTHESIS OF CARBOHYDRATES. Adv Enzymol Relat Areas Mol Biol. 1964;26:35–88. doi: 10.1002/9780470122716.ch2. [DOI] [PubMed] [Google Scholar]
- Herscovics A., Bugge B., Jeanloz R. W. Glucosyltransferase activity in calf pancreas microsomes. Formation of dolichyl D[14C]glucosyl phosphate and 14C-labeled lipid-linked oligosaccharides from UDP-D-[14C]glucose. J Biol Chem. 1977 Apr 10;252(7):2271–2277. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Opheim D. J., Touster O. The purification and characterization of rat liver lysosomal alpha-L-fucosidase. J Biol Chem. 1977 Jan 25;252(2):739–743. [PubMed] [Google Scholar]
- Parodi A. J., Behrens N. H., Leloir L. F., Carminatti H. The role of polyprenol-bound saccharides as intermediates in glycoprotein synthesis in liver. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3268–3272. doi: 10.1073/pnas.69.11.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Krag S. S., Liu T. Effects of UDP-glucose addition on the synthesis of mannosyl lipid-linked oligosaccharides by cell-free fibroblast preparations. J Biol Chem. 1977 Mar 10;252(5):1780–1785. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. I. Formation of an oligosaccharide-lipid by thyroid slices and evaluation of its role in protein glycosylation. J Biol Chem. 1976 Oct 25;251(20):6400–6408. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. III. Comparison of oligosaccharide-lipids formed by slices from several tissues. J Biol Chem. 1976 Oct 25;251(20):6420–6425. [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J., Bhoyroo V. D. Lipid-saccharide intermediates in glycoprotein biosynthesis. II. Studies on the structure of an oligosaccharide-lipid from thyroid. J Biol Chem. 1976 Oct 25;251(20):6409–6419. [PubMed] [Google Scholar]
- Tomino S., Paigen K., Tulsiani D. R., Touster O. Purification and chemical properities of mouse liver lysosomal (L form) beta-glucuronidase. J Biol Chem. 1975 Nov 10;250(21):8503–8509. [PubMed] [Google Scholar]
- Tulsiani D. R., Keller R. K., Touster O. The preparation and chemical composition of the multiple forms of beta-glucuronidase from the female rat preputial gland. J Biol Chem. 1975 Jun 25;250(12):4770–4776. [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]



