The changing spectrum of HIV-related thoracic diseases and their clinical and radiologic manifestations are discussed to promote familiarity with and expedite diagnosis and treatment of these diseases in the era of antiretroviral therapy.
Abstract
The human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) pandemic has entered its 4th decade. Since the introduction of combination antiretroviral therapy (ART) in 1996, the number of AIDS-related deaths has plateaued worldwide. Today, owing to the effectiveness of ART, the HIV-infected population is aging and HIV infection has become a chronic illness. Non-AIDS comorbidities are increasing, and the spectrum of HIV-related thoracic diseases is evolving. In developed countries, bacterial pneumonia has become more common than Pneumocystis pneumonia. Its imaging appearance depends on the responsible organism, most commonly Streptococcus pneumoniae. Mycobacterium tuberculosis continues to be a major threat. Its imaging patterns vary depending on CD4 count. Primary lung cancer and Hodgkin lymphoma are two important non–AIDS-defining malignancies that are increasingly encountered at chest imaging. Human herpesvirus 8, also known as Kaposi sarcoma–associated herpesvirus (KSHV), is strongly linked to HIV-related diseases, including Kaposi sarcoma, multicentric Castleman disease, KSHV inflammatory cytokine syndrome, and primary effusion lymphoma. Immune reconstitution inflammatory syndrome is a direct complication of ART whose manifestations vary with the underlying disease. Given the high rate of smoking among HIV-infected patients, chronic obstructive pulmonary disease is another important cause of morbidity and mortality. A high degree of suspicion is required for the early diagnosis of pulmonary arterial hypertension and lymphocytic interstitial pneumonia, given their nonspecific manifestations. Finally, multilocular thymic cyst manifests as a cystic anterior mediastinal mass. Recognition of the clinical and radiologic manifestations of these less traditional HIV-related diseases can expedite diagnosis and treatment in the ART era.
© RSNA, 2014
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Discuss the widening spectrum of HIV-related thoracic diseases in an aging population in the ART era.
■ List the important clinical findings of these HIV-related diseases.
■ Describe the imaging appearances of the less traditional HIV-related thoracic diseases.
Introduction
Considered the modern-day plague at the turn of the 21st century, the human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) pandemic has entered its 4th decade. AIDS-related illnesses had claimed approximately 25 million lives worldwide by 2006 (1). At the end of 2009, an estimated 33.3 million people were living with HIV (2).
HIV was present in central Africa as early as 1959 and likely originated with cross-species transmission of the simian immunodeficiency virus from chimpanzees (3). In 1981, the Centers for Disease Control reported five cases of Pneumocystis jirovecii pneumonia in Los Angeles, announcing the pandemic (4). In 1982, the first imaging report described the radiographic findings of Pneumocystis pneumonia in a previously healthy man with concurrent Kaposi sarcoma and Cryptococcus infection, making radiologists aware of the new immunodeficiency syndrome (5). In 1981, the hallmark of AIDS, a low CD4 count, was recognized (6), and in 1983, the retrovirus responsible for AIDS, initially termed human T-cell lymphotropic (leukemia) virus–III (HTLV-III) and later named HIV, was isolated and identified (7). In 1987, the U.S. Food and Drug Administration approved azidothymidine as the first medication for the treatment of AIDS, followed by many other antiviral drugs in subsequent years. From this time up until 1995, AIDS was the leading cause of death among Americans 25–44 years of age (8). Finally, in 1996, combination antiretroviral therapy (ART) delivered a breakthrough in treatment.
Once regarded as a death sentence, HIV infection can now be effectively managed with ART. Today, more people are “living with” than are “dying from” HIV. The incidence of new HIV infections has declined, and the number of AIDS-related deaths in adults and children worldwide has plateaued (2). Although more people were living with HIV in 2009 than in 2001 (33.3 million versus 28.6 million), the number of AIDS-related deaths (1.8 million annually) was the same. AIDS-related deaths declined 19% between 2004 and 2009 (2). Globally, an estimated 14.4 million life-years have been gained since 1996 with the availability of ART (2). The dramatic reduction in HIV-related deaths is clearly associated with ART (9).
With the effectiveness of ART, the HIV-infected population is now aging. By 2015, over one-half of the 1.5 million people living with HIV in the United States will be over 50 years of age (10). At the same time, the incidences of the classic AIDS-defining conditions are declining (11). However, even with ART, the health and life expectancy of HIV-infected patients are not normal (11). HIV-infected patients have a chronic inflammatory state that appears to accelerate the aging process (12), and they can suffer non-AIDS comorbidities associated with HIV, ART, and aging, which contribute to mortality. Non–AIDS-defining diseases account for nearly two-thirds of intensive care unit admissions of HIV-infected patients, with respiratory failure and pulmonary diseases being the most common (13). The respiratory system is one of the most frequently affected organ systems in HIV-infected patients (14).
ART has turned HIV infection into a complex chronic illness and has changed the spectrum of HIV-related thoracic diseases. Traditional diseases, such as Pneumocystis pneumonia, can still be seen in chronic HIV infection, even in the ART era, due to noncompliance, ineffectiveness of medication, or lack of access to medication.
In this article, we describe the clinical and radiologic findings of a wide variety of HIV-related thoracic diseases, including bacterial pneumonia; malignancies such as lung cancer, Hodgkin lymphoma, and virus-induced neoplasms; immune reconstitution inflammatory syndrome (IRIS); chronic obstructive pulmonary disease (COPD); pulmonary hypertension; interstitial pneumonia; and multilocular thymic cyst (MTC). We also revisit the importance and describe the clinical and radiologic findings of coinfection with Mycobacterium tuberculosis, which remains a serious—perhaps worse—threat to people living with HIV compared with the pre-ART era.
Bacterial Pneumonia
Infections remain the predominant cause of respiratory disease in HIV (14). The rate of bacterial pneumonia in HIV-infected patients has dropped considerably since the introduction of ART (15). However, this decline was not as dramatic as the overall decline in opportunistic pneumonia in the ART era (16). ART has thus transformed the epidemiology of pulmonary infections, particularly in developed countries, and bacterial pneumonia has become more common than Pneumocystis pneumonia (14,16).
Bacterial pneumonia is 10–25 times more common in HIV-infected patients than in the general population (14,17). Recurrent bacterial pneumonia is an AIDS-defining disease (14). A variety of HIV-induced immune abnormalities increase the risk of infection, particularly by encapsulated bacteria (18). Cigarette smoking and use of injected drugs increase the risk. Bacterial pneumonia can occur at any CD4 count, but the risk increases as the count decreases (14). The median CD4 count in patients with bacterial pneumonia is 200 cells/μL (14).
Clinical features of bacterial pneumonia in HIV-infected patients are similar to those in non–HIV-infected patients (cough, fever, chills, pleuritic chest pain, and dyspnea), but the infection may progress more rapidly, and bacteremia can occur more frequently (14,17,18). Bacterial pneumonia is rarely asymptomatic if there are radiographic abnormalities (18).
Streptococcus pneumoniae is the most common cause of community-acquired pneumonia in both HIV-infected patients and the general population (Fig 1) (14), but pneumococcal septicemia is 100 times more common in HIV-infected patients (18). Haemophilus influenzae is the second most common cause, followed by Staphylococcus aureus (14). S aureus infection is associated with intravenous drug use and can cause tricuspid endocarditis with septic pulmonary emboli. Methicillin-resistant S aureus (MRSA) pneumonia should be considered, particularly given its growing prevalence in both community and healthcare settings.
Figure 1a.
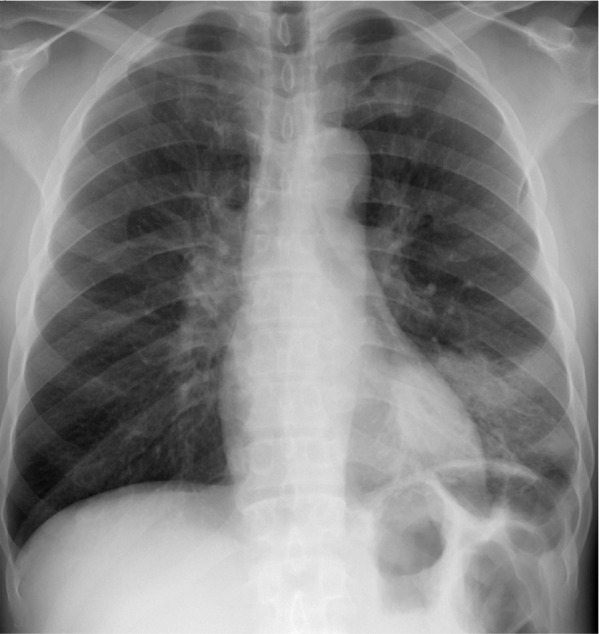
Streptococcus pneumonia in a 42-year-old man with HIV infection who had been receiving ART for 6 months. He had a CD4 count of 400 cells/μL and a 5-day history of fever, chills, and cough productive of purulent sputum. Frontal chest radiograph (a) and axial computed tomographic (CT) image (b) show consolidation in the left lower lobe. Sputum culture grew S pneumoniae.
Figure 1b.
Streptococcus pneumonia in a 42-year-old man with HIV infection who had been receiving ART for 6 months. He had a CD4 count of 400 cells/μL and a 5-day history of fever, chills, and cough productive of purulent sputum. Frontal chest radiograph (a) and axial computed tomographic (CT) image (b) show consolidation in the left lower lobe. Sputum culture grew S pneumoniae.
S aureus and Pseudomonas aeruginosa are the main pathogens causing nosocomial bacterial pneumonia in HIV-infected patients (19), although infection by P aeruginosa has declined in the ART era (14). Legionella infection, although uncommon, has been reported to have a 40-fold higher incidence in AIDS patients (14). Rhodococcus equi, a much less common pathogen, is an acid-fast coccobacillus that can manifest in patients with advanced HIV disease as an indolent course of fever, cough, and dyspnea accompanied by consolidation or cavitary masses, typically in the upper lobes and sometimes resembling tuberculosis (Fig 2) (14,18). Nocardia asteroides, which is partially acid fast, is also uncommon, particularly because of trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis for Pneumocystis pneumonia, but the incidence is still 140 times greater in HIV-infected patients than in the general population (14,18). Radiographic findings include single or multiple nodules that cavitate more often in patients with AIDS; lobar or multilobar consolidation, often with upper lobe involvement; and, less commonly, pleural involvement from direct extension, pleural effusion, or pleural thickening (14,18,20). Pulmonary nocardiosis can also extend into the chest wall (20). Mycoplasma pneumoniae and Chlamydia pneumoniae are other rarely encountered pathogens (14).
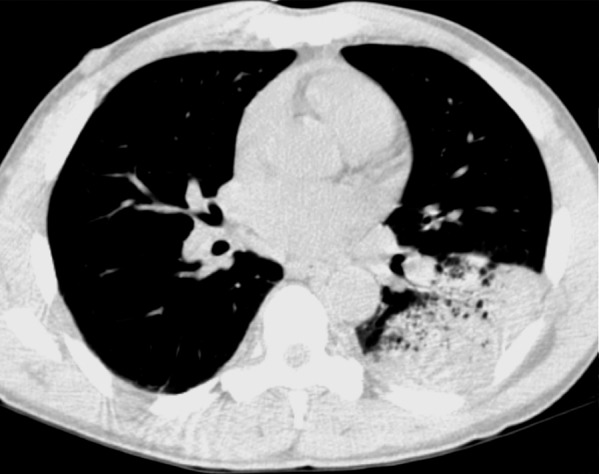
Figure 2.
Rhodococcus pneumonia in a 37-year-old man with HIV infection who was not receiving ART (CD4 count = 0). The patient presented with a 10-day history of fever, chills, and hemoptysis. Similar symptoms had been occurring intermittently for a year, and tuberculosis was suspected. Chest radiograph shows left apical lung consolidation with possible cavitation. Sputum culture grew R equi.
In general, the imaging features of bacterial pneumonia in HIV-infected patients are similar to those in non–HIV-infected patients, typically consisting of unilateral segmental or lobar consolidation (Fig 1) (14). However, almost one-half of bacterial pneumonias have a pattern other than focal consolidation (18). In HIV-infected patients, there is a greater likelihood of bilateral and multifocal reticulonodular patterns. Bacterial pneumonia is the most likely cause of pulmonary nodules in HIV-infected patients (18). More than one-half of H influenzae infections manifest with bilateral interstitial opacities, mimicking Pneumocystis pneumonia (14). S aureus and P aeruginosa infection may cause cavitary nodules or consolidation that resembles mycobacterial or fungal infection (14,18). S aureus infection predisposes to empyema (18). CT helps diagnose the complications of empyema or abscess.
Mycobacterium tuberculosis
Despite the availability of ART and the plateauing of mortality rates for HIV infection, M tuberculosis remains a major threat to HIV-infected patients. It accounted for approximately 380,000 deaths among HIV-infected patients in 2009 and 22% of HIV-related deaths in 2010 (2). The risk of developing tuberculosis is 50 times higher for an HIV-infected patient than for the average person (21). Moreover, drug-resistant tuberculosis is more common among HIV-infected patients than non–HIV-infected patients (2).
The imaging patterns of tuberculosis in HIV-infected patients vary depending on CD4 count. Above 200 cells/μL, a reactivation tuberculosis pattern predominates, with classic findings of upper lung consolidation and multiple nodules, which may cavitate (Fig 3) (21,22). Endobronchial spread of tuberculosis manifests as centrilobular nodules in a “tree-in-bud” configuration (Fig 4) (21,22). At a CD4 count of 50–200 cells/μL, reactivation tuberculosis resembles primary tuberculosis at imaging and can manifest as mediastinal lymphadenopathy with rim enhancement and low-attenuation central necrosis (Fig 5a, 5b) (21,22). A miliary pattern of tuberculosis can also be seen at this stage of HIV infection (Fig 5c) (21,22). Below 50 cells/μL, findings are not specific and include diffuse consolidation, ground-glass opacities, and pleural effusion.
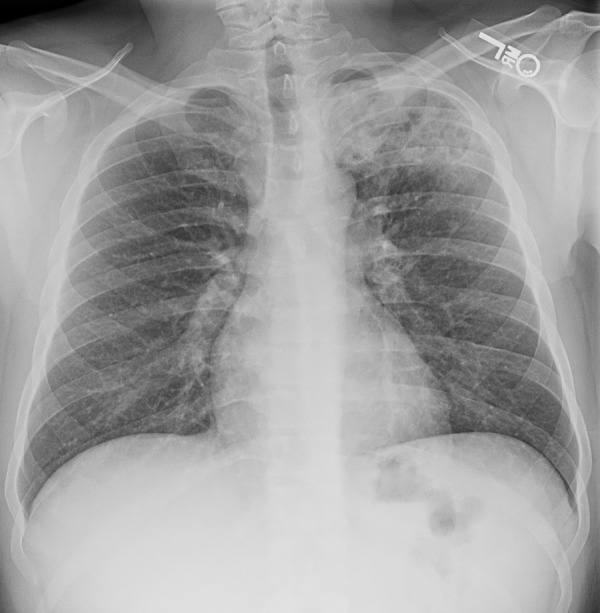
Figure 3.
Scarring from reactivation tuberculosis in a patient with HIV infection. Chest radiograph shows scarring and volume loss caused by reactivation tuberculosis in the right upper lobe.
Figure 4.
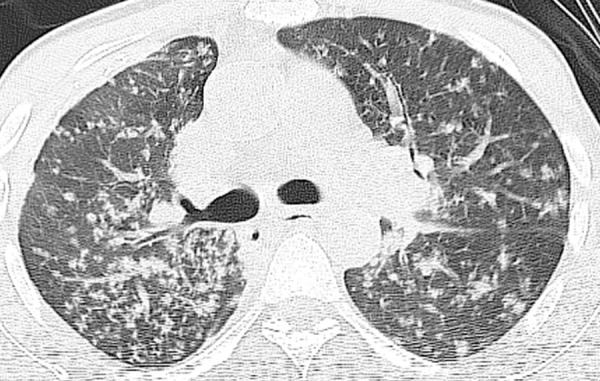
Reactivation tuberculosis in a 43-year-old man with a 2-month history of weakness, recurrent fever, night sweats, decreased appetite, weight loss, and nonproductive cough. He had a CD4 count of 34 cells/μL and was diagnosed with HIV infection. Chest CT image shows extensive centrilobular nodules in a tree-in-bud configuration, reflecting the endobronchial spread of tuberculosis. Additional findings included mediastinal lymphadenopathy (not shown). Sputum culture grew M tuberculosis, with reactivation of latent tuberculosis that was attributed to the low CD4 count.
Figure 5a.
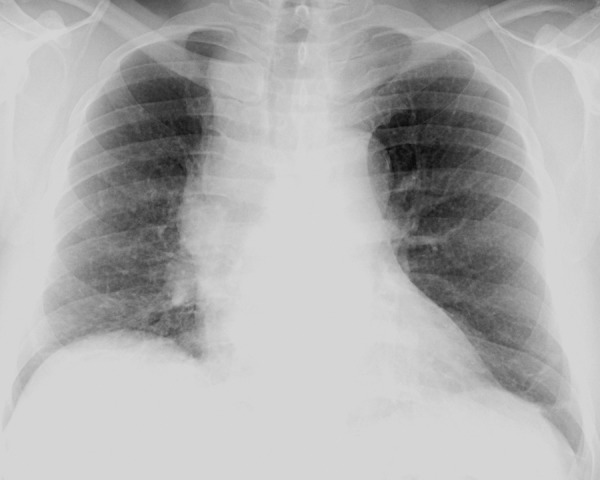
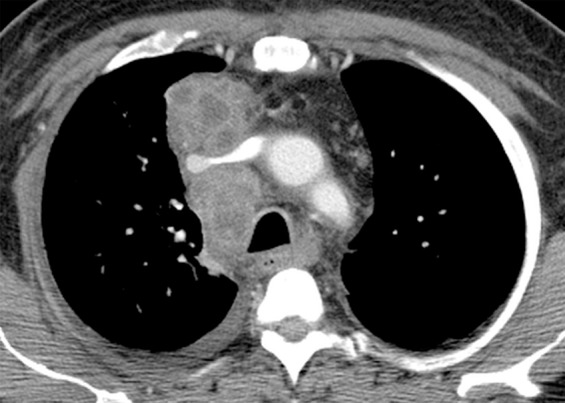
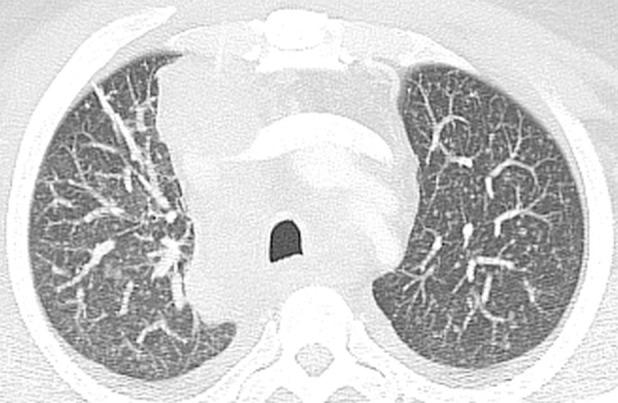
Primary-pattern tuberculosis in a 46-year-old man with a recent diagnosis of HIV infection and a CD4 count of 166 cells/μL. The patient presented with a history of several months duration of generalized weakness, night sweats, and weight loss. (a) Chest radiograph shows right paratracheal and hilar lymphadenopathy. (b) Chest CT image helps confirm lymphadenopathy with central necrosis and shows compression of the superior vena cava. (c) Maximum intensity projection CT image more clearly depicts miliary lung nodules. Pulmonary tuberculosis was diagnosed with a sputum M tuberculosis DNA probe.
Figure 5b.
Primary-pattern tuberculosis in a 46-year-old man with a recent diagnosis of HIV infection and a CD4 count of 166 cells/μL. The patient presented with a history of several months duration of generalized weakness, night sweats, and weight loss. (a) Chest radiograph shows right paratracheal and hilar lymphadenopathy. (b) Chest CT image helps confirm lymphadenopathy with central necrosis and shows compression of the superior vena cava. (c) Maximum intensity projection CT image more clearly depicts miliary lung nodules. Pulmonary tuberculosis was diagnosed with a sputum M tuberculosis DNA probe.
Figure 5c.
Primary-pattern tuberculosis in a 46-year-old man with a recent diagnosis of HIV infection and a CD4 count of 166 cells/μL. The patient presented with a history of several months duration of generalized weakness, night sweats, and weight loss. (a) Chest radiograph shows right paratracheal and hilar lymphadenopathy. (b) Chest CT image helps confirm lymphadenopathy with central necrosis and shows compression of the superior vena cava. (c) Maximum intensity projection CT image more clearly depicts miliary lung nodules. Pulmonary tuberculosis was diagnosed with a sputum M tuberculosis DNA probe.
Malignancies
HIV and AIDS predispose to certain malignancies notably, Kaposi sarcoma and non-Hodgkin lymphoma, whose incidences have declined in the ART era. Conversely, the number of cases of non–AIDS-defining malignancies has increased, with a threefold increase in 2001–2005 compared with 1991–1995 (23–25). This rise reflects more than the longer survival of HIV-infected patients, and HIV infection appears to be an independent risk factor. Primary lung cancer and Hodgkin lymphoma are two important non–AIDS-defining malignancies that have become more common in HIV-infected patients. An additional group of intrathoracic neoplasms related to specific concurrent viral superinfections in the HIV-infected population are discussed separately under “Human Herpesvirus 8–related Diseases.”
Primary Lung Cancer
Among the non–AIDS-defining malignancies, lung cancer is the most prevalent and a chief cause of cancer mortality in HIV-infected patients (24,25). Cigarette smoking is the principal risk factor for lung cancer, and the 60%–80% smoking rate in HIV-infected patients in the United States is two to three times higher than in the general U.S. population (26,27). However, even after statistical adjustment for smoking and other demographic factors, lung cancer incidence remains higher in HIV-infected patients than in the general population (27–30). Thus, HIV infection is an independent risk factor for lung cancer (28–30), but the mechanism is unclear. Proposed causes include (a) chronic inflammation, such as from recurrent lung infection, and (b) immunosuppression, given that immunosuppressed organ transplant recipients also have a high rate of lung cancer (27). No clear relationship has been established between lung cancer rates and CD4 count, viral load, or use of ART (29).
HIV-infected patients are younger than non–HIV-infected patients at the time of diagnosis of lung cancer (27,31), and their cancers are most often stage III or IV (30), with a poor prognosis. The distribution of histologic subtypes is similar to that for non–HIV-infected patients, with adenocarcinoma being the most common (Fig 6), followed by squamous cell carcinoma (Fig 7) and small cell carcinoma (30). Imaging findings are the same in both HIV-infected and non–HIV-infected patient groups, although advanced-stage disease may be more common and the lesions tend to be more peripheral in the former group (32). Peripheral upper lobe lesions are more commonly found in patients who have had tuberculosis or Pneumocystis pneumonia, whereas central masses are more common in patients without prior lung infections (32).
Figure 6.
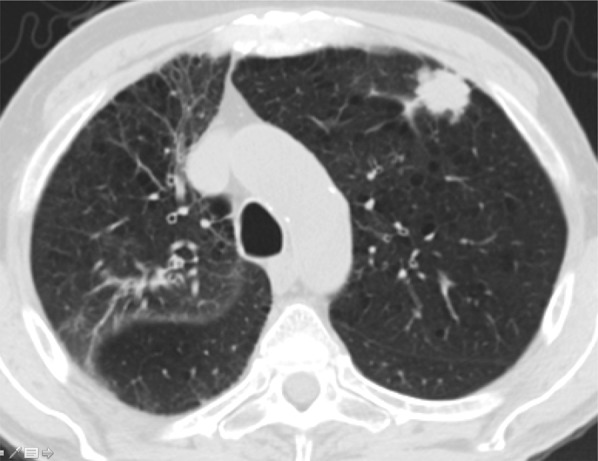
Adenocarcinoma in a 52-year-old man with HIV infection who was receiving ART. The patient presented with dyspnea and had a CD4 count of 287 cells/μL. CT image shows mild emphysema and an irregular peripheral nodule in the left upper lobe. Biopsy demonstrated lung adenocarcinoma.
Figure 7.
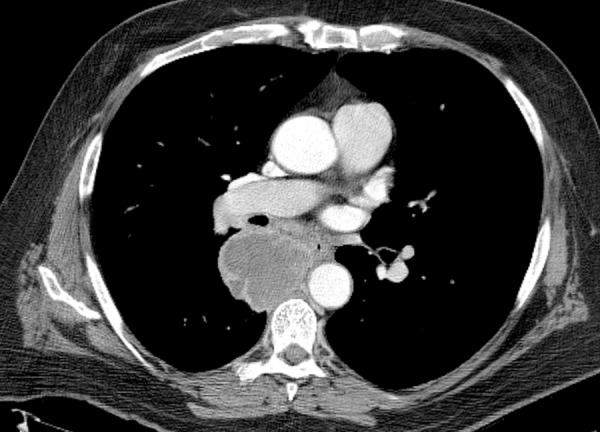
Squamous cell carcinoma in a 58-year-old man with HIV infection (CD4 count = 319 cells/μL) who was receiving ART. The patient had a 40-pack-year smoking history and presented with fatigue and dyspnea on exertion. CT image shows a central mass with peripheral enhancement and central necrosis. Biopsy demonstrated squamous cell carcinoma of the lung.
Hodgkin Lymphoma
The incidence of Hodgkin lymphoma, which is strongly linked to Epstein-Barr virus (EBV) infection, has increased substantially in the ART era (23–25,33,34). Its incidence is up to 14 times higher in HIV-infected patients than in the general population (23). EBV is found in one-half of cases of Hodgkin lymphoma in the general population, but in virtually all cases of Hodgkin lymphoma in HIV-infected patients (34). The risk for Hodgkin lymphoma is highest in HIV-infected patients with moderate immunosuppression, although the risk decreases with decreasing CD4 count (33,34). In one study, the risk for Hodgkin lymphoma was highest at CD4 counts between 225 and 249 cells/μL and lower at counts less than 200 cells/μL (33). ART-induced improvement in CD4 counts from severe to moderate immunosuppression correlates with an increased incidence of Hodgkin lymphoma (33). It has been postulated that stimulation of B lymphocytes after the initiation of ART in patients with latent EBV infection promotes Hodgkin lymphoma (23,34). Although the incidence of Hodgkin lymphoma has increased in the ART era, treatment outcomes and survival rates have improved (34,35).
Hodgkin lymphoma in HIV-infected patients manifests with typical systemic symptoms of fever, night sweats, and weight loss and is accompanied by lymphadenopathy, typically in the mediastinum (Fig 8a, 8b) (36,37). Lung involvement is unusual, especially when it is unaccompanied by mediastinal or hilar lymphadenopathy; when it does occur, masses or masslike consolidation are the most common finding, and one-half of lesions have air bronchograms (36). Other findings include lung nodules, pleural masses, and pleural effusions (36). Most patients with AIDS have extranodal lesions (Fig 8c) (37).
Figure 8a.
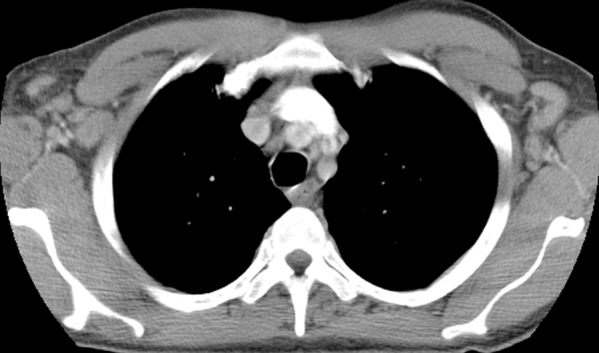
Hodgkin lymphoma in a 46-year-old man with HIV infection who presented with recurrent fever, chills, generalized body aches, cough, loose stools, axillary lymphadenopathy, and pancytopenia. He had been receiving ART for 4 months, during which time his CD4 count increased from 43 cells/μL (nadir) to 144 cells/μL. (a, b) CT images show axillary (a) and mediastinal (b) lymphadenopathy. There was also hilar, mesenteric, and retroperitoneal lymphadenopathy. (c) CT image shows extranodal findings including hepatosplenomegaly and small hypoattenuating areas in the spleen. Axillary lymph node biopsy demonstrated nodular sclerosing Hodgkin lymphoma.
Figure 8b.
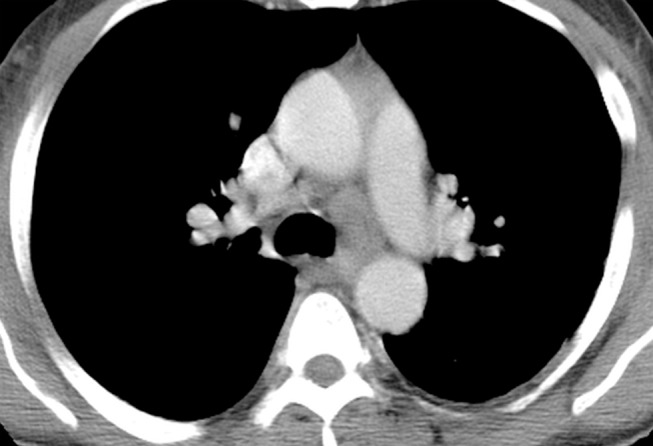
Hodgkin lymphoma in a 46-year-old man with HIV infection who presented with recurrent fever, chills, generalized body aches, cough, loose stools, axillary lymphadenopathy, and pancytopenia. He had been receiving ART for 4 months, during which time his CD4 count increased from 43 cells/μL (nadir) to 144 cells/μL. (a, b) CT images show axillary (a) and mediastinal (b) lymphadenopathy. There was also hilar, mesenteric, and retroperitoneal lymphadenopathy. (c) CT image shows extranodal findings including hepatosplenomegaly and small hypoattenuating areas in the spleen. Axillary lymph node biopsy demonstrated nodular sclerosing Hodgkin lymphoma.
Figure 8c.
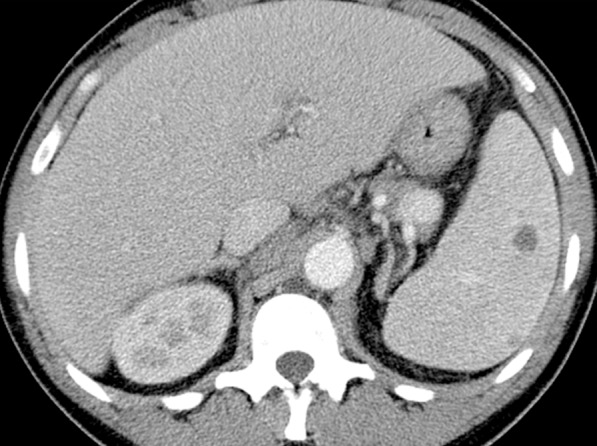
Hodgkin lymphoma in a 46-year-old man with HIV infection who presented with recurrent fever, chills, generalized body aches, cough, loose stools, axillary lymphadenopathy, and pancytopenia. He had been receiving ART for 4 months, during which time his CD4 count increased from 43 cells/μL (nadir) to 144 cells/μL. (a, b) CT images show axillary (a) and mediastinal (b) lymphadenopathy. There was also hilar, mesenteric, and retroperitoneal lymphadenopathy. (c) CT image shows extranodal findings including hepatosplenomegaly and small hypoattenuating areas in the spleen. Axillary lymph node biopsy demonstrated nodular sclerosing Hodgkin lymphoma.
Human Herpes-virus 8–related Diseases
Kaposi Sarcoma
Kaposi sarcoma is the most common AIDS-defining malignancy worldwide (2). It is briefly discussed in this article because of its association with human herpesvirus 8 (HHV-8), also known as Kaposi sarcoma–associated herpesvirus (KSHV). Risk factors for Kaposi sarcoma include a CD4 count of less than 200 cells/μL and an elevated HHV-8 antibody titer (38). ART has drastically reduced the incidence of Kaposi sarcoma by restoring CD4 count, but its morbidity and mortality rates remain high (38). Many patients with established Kaposi sarcoma respond to ART and enter remission, but some progress or relapse (38). The classic imaging feature of pulmonary Kaposi sarcoma is peribronchovascular consolidation with flame-shaped hilar radiation (Fig 9), and poorly defined lung nodules are common (39). Other CT findings include interlobular septal thickening, patchy ground-glass opacities, fissural nodularity with distortion, and pleural effusion, which is often bilateral and small (39).
Figure 9.
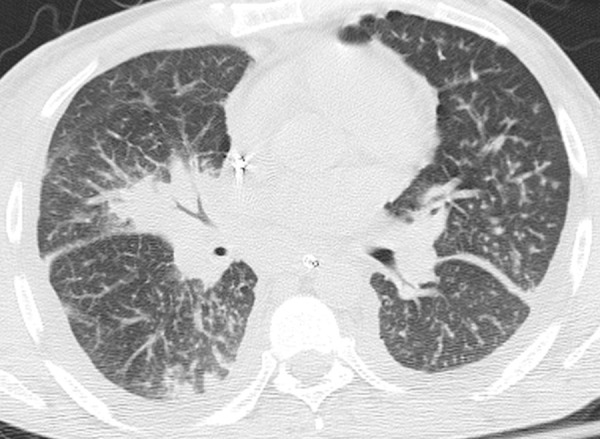
Kaposi sarcoma in a 48-year-old man with a history of over a decade of HIV infection. The patient, who had a CD4 count of 50 cells/μL, was receiving ART and presented with acute respiratory distress after 3 weeks of cough and dyspnea. CT image demonstrates flame-shaped peribronchovascular consolidation caused by Kaposi sarcoma. A high level of plasma HHV-8 DNA was detected.
Multicentric Castleman Disease
In the setting of HIV infection, multicentric Castleman disease (MCD) is a less well-known HHV-8–related disease. Unlike Kaposi sarcoma, HIV-related MCD has actually become more prevalent in the ART era (40). Castleman disease is a lymphoproliferative disorder with two main histologic types (hyaline-vascular and plasma cell), a unicentric or multicentric distribution, and involvement of multiple nodal stations and organs (41). In MCD, the plasma cell type predominates (41). In HIV-infected patients, nearly 100% of MCD is associated with HHV-8, which can be detected at node biopsies (41,42). Most HIV-infected patients with MCD have a CD4 count of over 200 cells/μL (40).
HIV-infected patients with MCD experience relapsing constitutional symptoms of fever, malaise, and weight loss that can fluctuate over days (43,44). Respiratory symptoms, pleural effusion, ascites, peripheral edema, hepatosplenomegaly, and diffuse lymphadenopathy can occur (40,41,43). Laboratory abnormalities include hypoalbuminemia, hyponatremia, high interleukin-6 and C-reactive protein levels, and cytopenia with coagulopathy due to hemophagocytic syndrome (41,43). High levels of HHV-8 DNA in the peripheral blood correlate with symptoms and may drop to undetectable levels between “attacks” (42), suggesting that active HHV-8 viral replication is central in MCD pathogenesis (42,43). Concurrent malignancies, particularly Kaposi sarcoma and lymphoma, may accompany MCD (41). Overall, the prognosis is poor and the mortality rate is high.
Imaging features of MCD during acute flare-ups include ill-defined centrilobular nodules, interlobular septal thickening, peribronchovascular thickening, mediastinal widening, and small pleural effusions (44). Hilar, mediastinal, and axillary lymphadenopathy are common, and lymphadenopathy may enhance with intravenous injection of contrast material (Fig 10) (45). Ground-glass opacities, consolidation, and bronchiectasis are less common CT findings (45). Hepatosplenomegaly, ascites, diffuse lymphadenopathy, and thickening of the retroperitoneal fascia may also occur (46).
Figure 10.
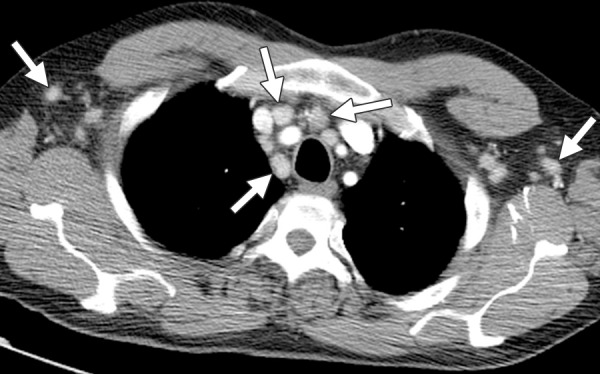
MCD in the same patient as in Figure 9, who presented with recurrent fever, chills, and bulky cervical, axillary, and inguinal lymphadenopathy. CT image shows diffuse, moderately enhancing axillary and mediastinal lymphadenopathy (arrows). A diagnosis of MCD was made with biopsy of an inguinal lymph node, with the patient having a CD4 count of 438 cells/μL at the time of diagnosis.
KSHV Inflammatory Cytokine Syndrome
A novel inflammatory syndrome associated with concurrent HIV and HHV-8 (ie, KSHV) infections was described in 2010 and given the name KSHV inflammatory cytokine syndrome (KICS) (47). This syndrome shares many of the features of MCD but is different pathologically. As with MCD, blood levels of interleukin-6 and HHV-8 are elevated, and hypoalbuminemia and hyponatremia are common. Symptoms are similar and include fever, night sweats, fatigue, and cachexia, reflecting heightened systemic inflammation. Lymphadenopathy, pleural effusion, and hepatosplenomegaly may also occur (Fig 11) (47). Ultimately, tissue or lymph node biopsy fails to demonstrate MCD. The differential diagnosis includes MCD and sepsis, and therefore tissue or lymph node biopsy is required (47).
Figure 11a.
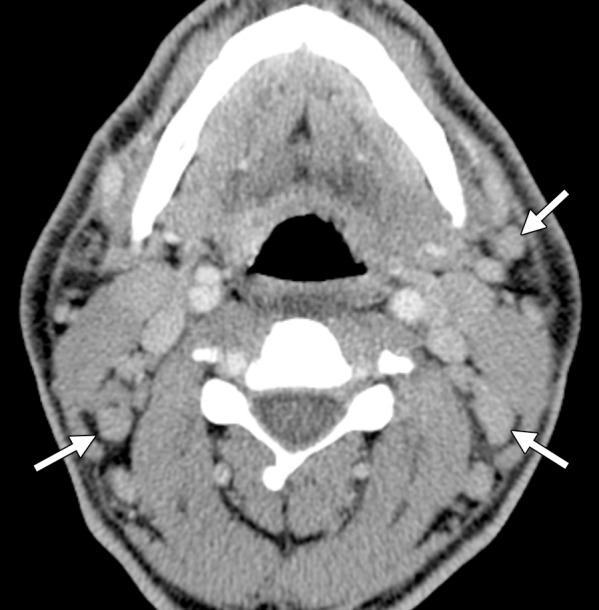
KICS in a 38-year-old man with HIV infection and a CD4 count of 506 cells/μL. The patient, who was not receiving ART, presented with a 2-day history of high fever, tachycardia, and altered mentation, as well as thrombocytopenia, hyponatremia, and hemophagocytosis. (a) CT image shows lymphadenopathy in the neck (arrows). (b) CT image shows lymphadenopathy in the axillae (arrows), along with bilateral pleural effusion. Enlargement of the mesenteric lymph nodes (not shown) was also noted. Plasma contained 15 million copies per milliliter of HHV-8 (ie, KSHV) and low amounts of reactivated EBV. Biopsy of a cervical node showed atypical reactive hyperplasia with scattered HHV-8–positive cells, leading to the diagnosis of KICS. The patient’s condition improved with dexamethasone and cyclosporine treatment.
Figure 11b.
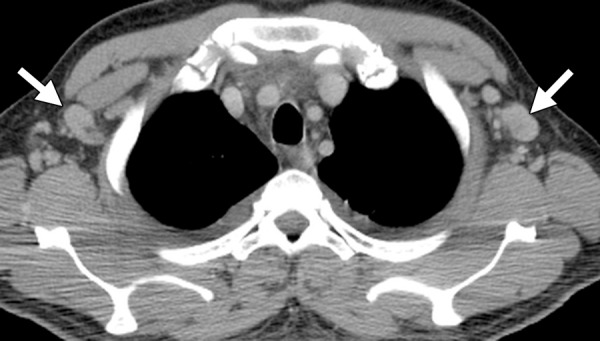
KICS in a 38-year-old man with HIV infection and a CD4 count of 506 cells/μL. The patient, who was not receiving ART, presented with a 2-day history of high fever, tachycardia, and altered mentation, as well as thrombocytopenia, hyponatremia, and hemophagocytosis. (a) CT image shows lymphadenopathy in the neck (arrows). (b) CT image shows lymphadenopathy in the axillae (arrows), along with bilateral pleural effusion. Enlargement of the mesenteric lymph nodes (not shown) was also noted. Plasma contained 15 million copies per milliliter of HHV-8 (ie, KSHV) and low amounts of reactivated EBV. Biopsy of a cervical node showed atypical reactive hyperplasia with scattered HHV-8–positive cells, leading to the diagnosis of KICS. The patient’s condition improved with dexamethasone and cyclosporine treatment.
Primary Effusion Lymphoma
Primary effusion lymphoma is an even rarer condition associated with HHV-8, which is present in all cases (48). Primary effusion lymphoma is a subgroup of B-cell lymphoma that occurs in advanced HIV disease and AIDS. It manifests as malignant pleural, peritoneal, or pericardial effusion in the absence of nodal disease or solid tumor nodules (49). Symptoms are caused by the effusion, which is the only imaging finding.
Immune Reconstitution Inflammatory Syndrome
Pathophysiologic Features
Up to 30% of patients receiving ART can experience worse symptoms as their CD4 count increases, despite having no progression of HIV disease and no new secondary infection (50). This paradoxic exacerbation of symptoms reflects the clinical unveiling or exacerbation of opportunistic infection or some other inflammatory disorder despite ongoing treatment (51) and is known as IRIS. IRIS can also occur when non-AIDS immunodeficiencies are treated (50).
The mechanism of IRIS depends on the responsible pathogen or antigenic stimulus (52). With therapy, the HIV load decreases and the CD4 count increases, which can initiate a delayed hypersensitivity reaction (eg, to tuberculosis) (52). On the other hand, the development of IRIS associated with herpesvirus infection may largely reflect dysregulation of CD8 T cells (52).
Risk Factors
Risk factors for IRIS include advanced HIV infection and very low CD4 count. Other factors include the presence of concurrent disseminated opportunistic infection, inflammatory disorder, or antigenic load; a greater degree of immune recovery as indicated by a very rapid initial decrease in HIV titer; possible genetic factors; and a short interval between (a) the start of treatment for concurrent infection or disease besides HIV, and (b) the initiation of ART, particularly in patients who have never previously received ART (53).
Clinical and Radiologic Manifestations
Many underlying infections or systemic diseases can lead to IRIS (Table) (52). Clinical and radiologic manifestations vary with these underlying diseases. Tuberculosis-IRIS is the most common form of IRIS worldwide (52,54), consisting of either paradoxic exacerbation or unmasking of latent tuberculosis, causing fever, recurrent tuberculosis symptoms, and lymphadenopathy within the first 2 months of ART. Imaging findings include lymphadenopathy (sometimes suppurative) and miliary nodules (Fig 12) (55). Cavitation, pleural effusion, and worsening consolidation may also occur (Fig 13) (55).
Infections or Systemic Diseases in HIV-infected Patients That May Lead to IRIS
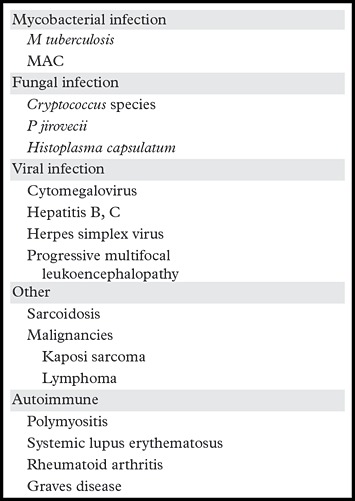
Note.—Reprinted, with permission, from reference 17. MAC = M avium complex.
Figure 12a.
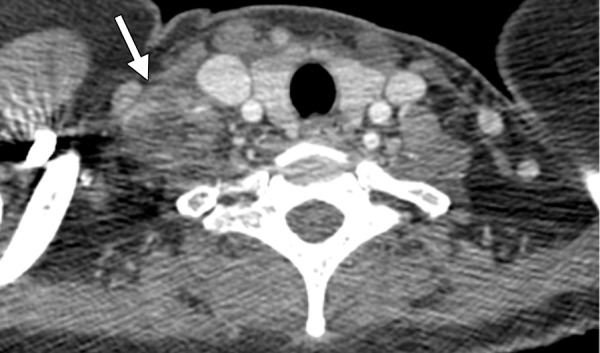
Tuberculosis-IRIS in a 45-year-old woman with HIV infection. The patient had a CD4 count of 26 cells/μL (nadir), a peak viral load of 3.8 million/μL, and known pyrazinamide-resistant tuberculosis. (a) CT image obtained prior to the initiation of ART shows right supraclavicular lymphadenopathy (arrow). After the initiation of ART and concurrent antituberculosis therapy, the lymphadenopathy decreased. As a result of ART, the patient’s CD4 count rose to 107 cells/μL, with an undetectable viral load. (b) CT image obtained after 2–3 months of ART and antituberculosis therapy shows worsened right supraclavicular lymphadenitis with drainage and necrosis (arrow). A diagnosis of tuberculosis-IRIS was made. The patient’s symptoms were managed with ibuprofen, and ART and antituberculosis therapy were continued. The lymphadenopathy resolved completely after 3 months.
Figure 13a.
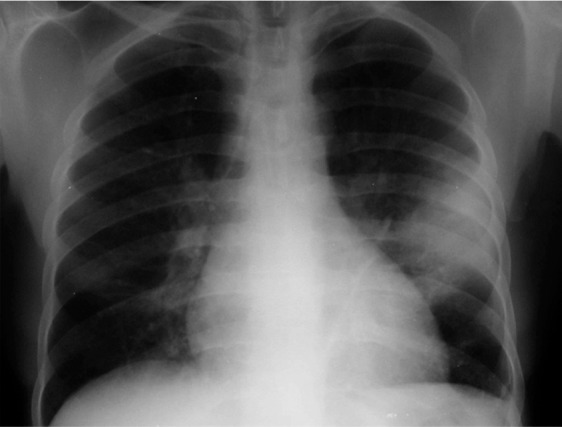
Tuberculosis-IRIS in a patient with HIV infection. (a) Initial chest radiograph shows consolidation caused by tuberculosis. (b) Radiograph obtained after the initiation of ART while the patient was receiving antituberculosis therapy shows worsening of the consolidation, a finding that reflects tuberculosis-IRIS. (Fig 13 courtesy of Laurence Huang, MD, University of California–San Francisco.)
Figure 12b.
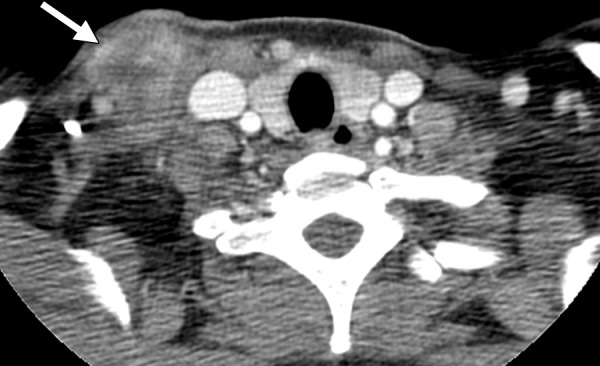
Tuberculosis-IRIS in a 45-year-old woman with HIV infection. The patient had a CD4 count of 26 cells/μL (nadir), a peak viral load of 3.8 million/μL, and known pyrazinamide-resistant tuberculosis. (a) CT image obtained prior to the initiation of ART shows right supraclavicular lymphadenopathy (arrow). After the initiation of ART and concurrent antituberculosis therapy, the lymphadenopathy decreased. As a result of ART, the patient’s CD4 count rose to 107 cells/μL, with an undetectable viral load. (b) CT image obtained after 2–3 months of ART and antituberculosis therapy shows worsened right supraclavicular lymphadenitis with drainage and necrosis (arrow). A diagnosis of tuberculosis-IRIS was made. The patient’s symptoms were managed with ibuprofen, and ART and antituberculosis therapy were continued. The lymphadenopathy resolved completely after 3 months.
Figure 13b.
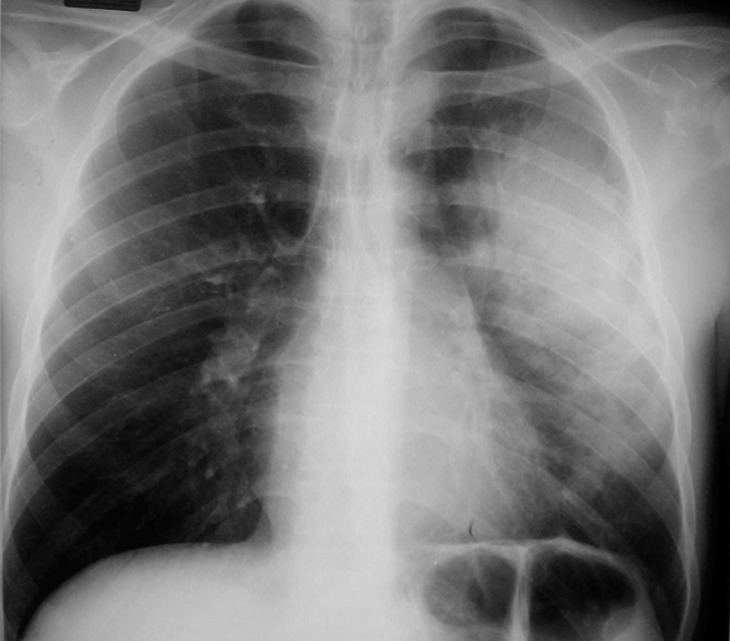
Tuberculosis-IRIS in a patient with HIV infection. (a) Initial chest radiograph shows consolidation caused by tuberculosis. (b) Radiograph obtained after the initiation of ART while the patient was receiving antituberculosis therapy shows worsening of the consolidation, a finding that reflects tuberculosis-IRIS. (Fig 13 courtesy of Laurence Huang, MD, University of California–San Francisco.)
IRIS associated with MAC is common, particularly in regions with a lower prevalence of tuberculosis (52,54). MAC-IRIS typically unmasks the initially asymptomatic and undiagnosed underlying MAC infection, causing fever, cough, night sweats, dyspnea, hemoptysis, occasional weight loss, and painful suppurative lymphadenitis in the neck, chest, and abdomen within the first 3 months of ART (52,56). Imaging shows regional or disseminated lymphadenopathy, often with central necrosis (Fig 14) (56). Pulmonary findings include consolidation and centrilobular nodules that are at times masslike (56,57). Extrapulmonary findings include ascites, splenomegaly, and low-attenuation splenic lesions (57).
Figure 14a.
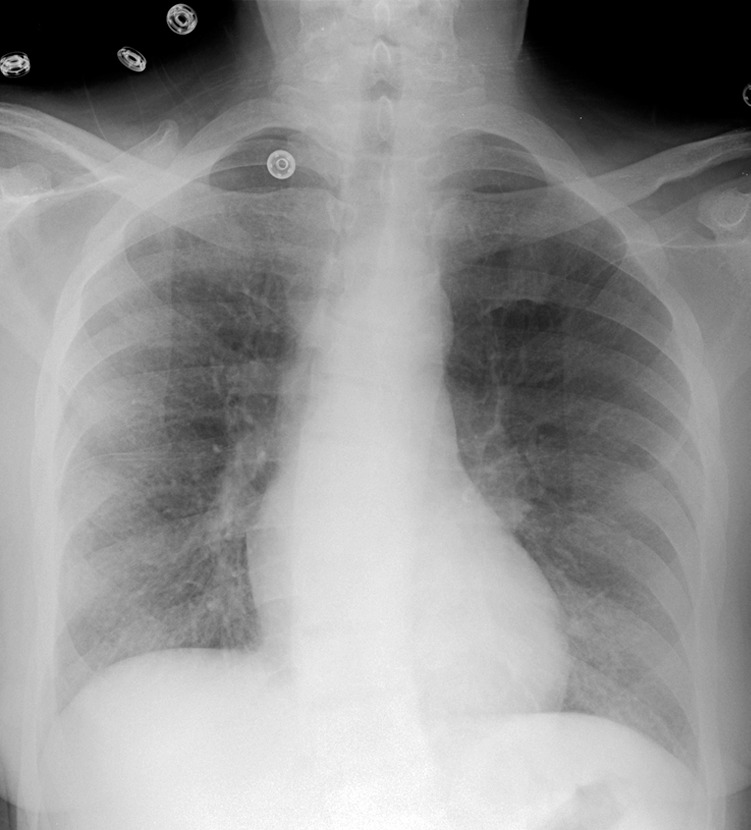
MAC-IRIS in a 42-year-old woman with HIV infection who had received treatment for disseminated MAC for 4 months. (a) Chest radiograph obtained 2 months prior to the initiation of ART shows mild diffuse lung disease caused by edema. The patient’s CD4 count was undetectable, with a viral load of 11,600 copies/mL. After less than 1 month of ART, the patient presented with fever and nonproductive cough, at which time her CD4 count was 34 cells/μL, with a viral load of less than 30 copies/mL. (b) Chest radiograph shows new hilar and mediastinal lymphadenopathy and increased diffuse lung disease. (c, d) CT images obtained while the patient was receiving ART help confirm mediastinal lymphadenopathy with central necrosis (c) and hilar lymphadenopathy (d). Once infection was excluded, the diagnosis of IRIS was made, ART and treatment for MAC were continued, and the patient was given ibuprofen to control the symptoms of IRIS.
Figure 14b.
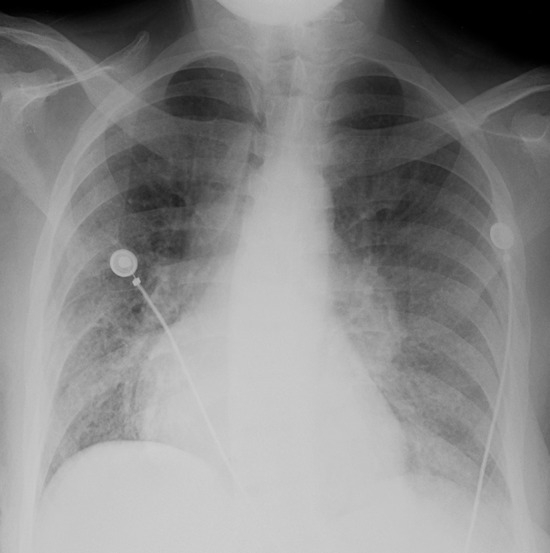
MAC-IRIS in a 42-year-old woman with HIV infection who had received treatment for disseminated MAC for 4 months. (a) Chest radiograph obtained 2 months prior to the initiation of ART shows mild diffuse lung disease caused by edema. The patient’s CD4 count was undetectable, with a viral load of 11,600 copies/mL. After less than 1 month of ART, the patient presented with fever and nonproductive cough, at which time her CD4 count was 34 cells/μL, with a viral load of less than 30 copies/mL. (b) Chest radiograph shows new hilar and mediastinal lymphadenopathy and increased diffuse lung disease. (c, d) CT images obtained while the patient was receiving ART help confirm mediastinal lymphadenopathy with central necrosis (c) and hilar lymphadenopathy (d). Once infection was excluded, the diagnosis of IRIS was made, ART and treatment for MAC were continued, and the patient was given ibuprofen to control the symptoms of IRIS.
Figure 14c.
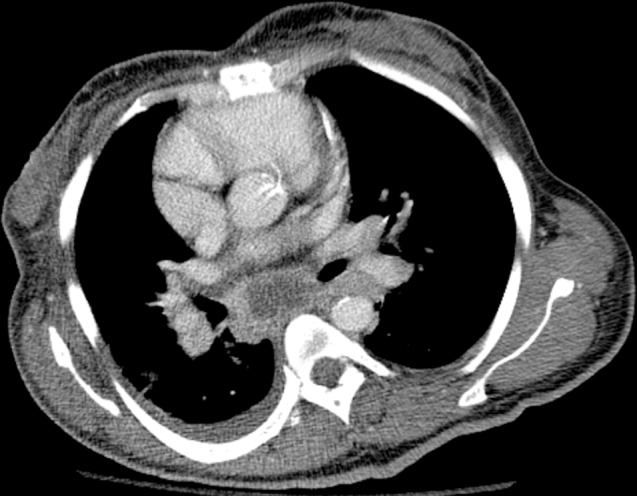
MAC-IRIS in a 42-year-old woman with HIV infection who had received treatment for disseminated MAC for 4 months. (a) Chest radiograph obtained 2 months prior to the initiation of ART shows mild diffuse lung disease caused by edema. The patient’s CD4 count was undetectable, with a viral load of 11,600 copies/mL. After less than 1 month of ART, the patient presented with fever and nonproductive cough, at which time her CD4 count was 34 cells/μL, with a viral load of less than 30 copies/mL. (b) Chest radiograph shows new hilar and mediastinal lymphadenopathy and increased diffuse lung disease. (c, d) CT images obtained while the patient was receiving ART help confirm mediastinal lymphadenopathy with central necrosis (c) and hilar lymphadenopathy (d). Once infection was excluded, the diagnosis of IRIS was made, ART and treatment for MAC were continued, and the patient was given ibuprofen to control the symptoms of IRIS.
Figure 14d.
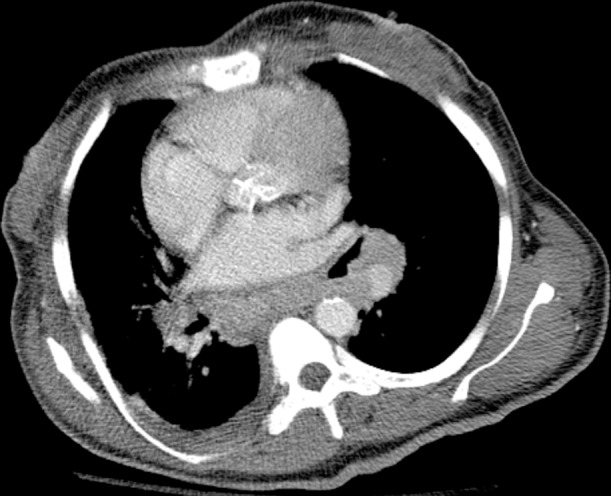
MAC-IRIS in a 42-year-old woman with HIV infection who had received treatment for disseminated MAC for 4 months. (a) Chest radiograph obtained 2 months prior to the initiation of ART shows mild diffuse lung disease caused by edema. The patient’s CD4 count was undetectable, with a viral load of 11,600 copies/mL. After less than 1 month of ART, the patient presented with fever and nonproductive cough, at which time her CD4 count was 34 cells/μL, with a viral load of less than 30 copies/mL. (b) Chest radiograph shows new hilar and mediastinal lymphadenopathy and increased diffuse lung disease. (c, d) CT images obtained while the patient was receiving ART help confirm mediastinal lymphadenopathy with central necrosis (c) and hilar lymphadenopathy (d). Once infection was excluded, the diagnosis of IRIS was made, ART and treatment for MAC were continued, and the patient was given ibuprofen to control the symptoms of IRIS.
Pneumocystis pneumonia is a well-known opportunistic infection. Part of its pathophysiologic makeup is the result of the strong inflammatory response it elicits (54). Pneumocystis IRIS occurs within days to weeks of the initiation of ART as the exaggerated CD4 inflammatory response causes severe lung injury, even as the infection resolves (54). Pneumocystis IRIS manifests in the same way as Pneumocystis pneumonia, with dyspnea, dry cough, and profound hypoxia (54). Patchy or diffuse ground-glass opacities are typically seen at radiography (Fig 15) (54). Cavitating and noncavitating granulomatous nodules and organizing pneumonia rarely develop, manifesting as bilateral peripheral or peribronchial consolidation and nodules (58,59).
Figure 15a.
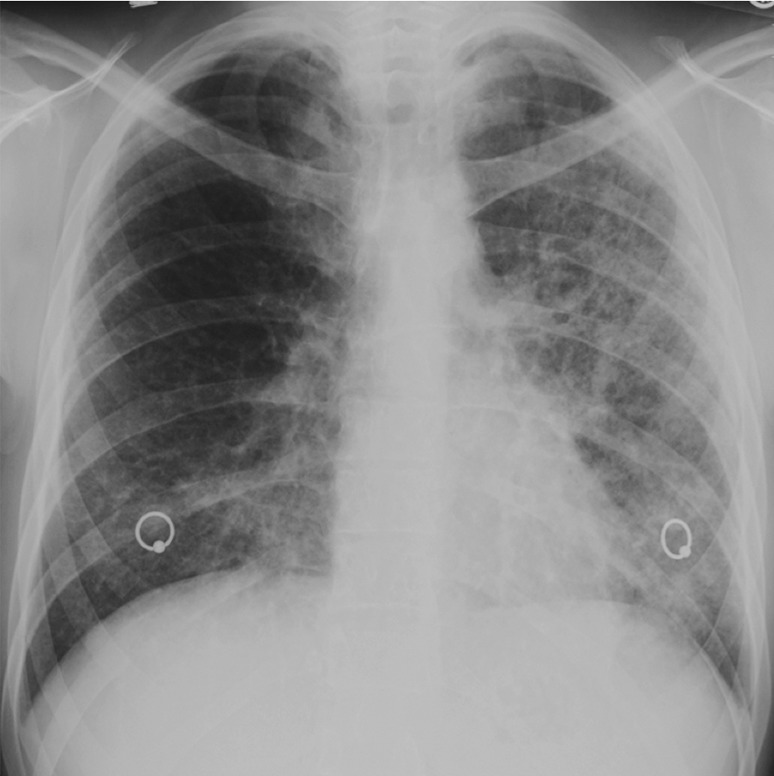
Pneumocystis pneumonia–IRIS in an HIV-infected 30-year-old man with a CD4 count of 100 cells/μL. (a) Chest radiograph shows Pneumocystis pneumonia, mostly on the left side. Clinical improvement was seen within 2 weeks of the initiation of treatment for this condition, and ART was initiated; within 5 days, however, dyspnea, cough, and hypoxia increased. (b) Follow-up chest radiograph shows worsening of lung consolidation, a finding that was attributed to Pneumocystis pneumonia–IRIS.
Figure 15b.
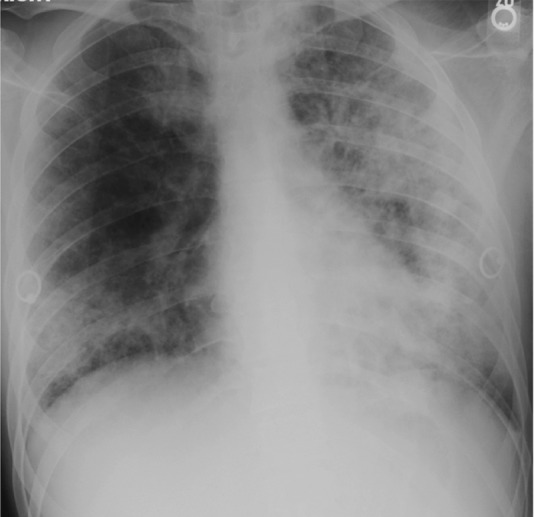
Pneumocystis pneumonia–IRIS in an HIV-infected 30-year-old man with a CD4 count of 100 cells/μL. (a) Chest radiograph shows Pneumocystis pneumonia, mostly on the left side. Clinical improvement was seen within 2 weeks of the initiation of treatment for this condition, and ART was initiated; within 5 days, however, dyspnea, cough, and hypoxia increased. (b) Follow-up chest radiograph shows worsening of lung consolidation, a finding that was attributed to Pneumocystis pneumonia–IRIS.
Sarcoidosis is a CD4 cell–mediated granulomatous inflammatory disorder (54). Although rare, its incidence appears to be increased in HIV-infected patients who receive ART (52). The development or recurrence of sarcoidosis can occur months to years after the initiation of ART, usually with a substantial drop in HIV viral load and a rise in CD4 count (52,60). In fact, most patients have a CD4 count of greater than 200 cells/μL (52). Thus, sarcoidosis is considered a possible form of IRIS. About one-half of patients with sarcoidosis-IRIS experience nonspecific constitutional and respiratory symptoms (52). Imaging findings are the same as those in non–HIV-infected patients and typically consist of perilymphatic nodules with or without hilar or mediastinal lymphadenopathy (Fig 16) (52,54,60).
Figure 16.
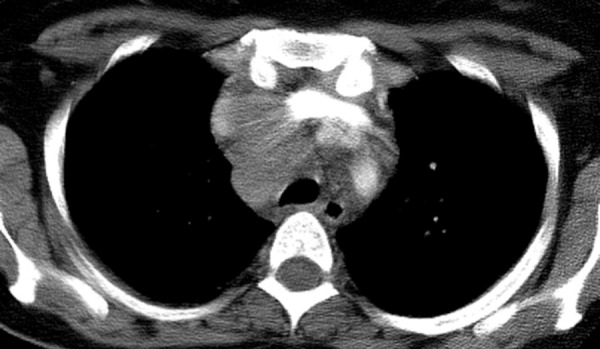
Sarcoidosis-IRIS in a 43-year-old patient with HIV infection who presented with cough and fatigue. The patient had a remote history of sarcoidosis and had been receiving ART for nearly a decade. CT image shows worsening mediastinal lymphadenopathy.
Diagnosis
IRIS is a diagnosis of exclusion, so that a high degree of suspicion is required (52). Worsening of an underlying known infection must always be considered, due to factors such as malabsorption of medications, noncompliance with treatment, drug resistance (as in drug-resistant tuberculosis), secondary infection, or treatment complications (52). For the diagnosis of IRIS to be made, the patient’s condition must improve when ART is initiated and then become worse, and viral load should decrease as the CD4 count increases.
Management and Prognosis
IRIS can cause substantial morbidity, but the mortality rate is low (54). Patients require close monitoring. Unless IRIS is life threatening, as with involvement of the central nervous system, ART is usually continued and underlying infection or other disease is treated (52). In addition, early initiation of ART in patients with opportunistic infections improves outcome despite the increased risk of IRIS (54). The role of corticosteroids and other anti-inflammatory drugs is unclear, and corticosteroids are usually reserved for the most serious cases (52).
Chronic Obstructive Pulmonary Disease
Given the increased rate of smoking among HIV-infected patients, COPD and other smoking-related diseases have become important causes of morbidity and mortality in the aging HIV-infected population (26). Other risk behaviors, such as use of injected and inhalational drugs, also damage the lungs (26). Pulmonary infections such as bacterial pneumonia and pulmonary colonization by P jirovecii contribute to the pathogenesis of COPD (61,62). However, although the prevalence of opportunistic infections such as Pneumocystis pneumonia has decreased markedly in the ART era, the prevalence of HIV-related COPD appears to be increasing.
HIV may be an independent risk factor for COPD, particularly for emphysema (63). HIV-infected patients have a higher prevalence of emphysema than do non–HIV-infected patients matched for age, pulmonary infections, and smoking history (64). Nonsmoking HIV-infected patients have higher rates of emphysema than do nonsmoking non–HIV-infected patients (64). The pathogenesis of COPD in HIV infection remains unclear and likely involves multiple pathways, including immunologic, apoptotic, proteolytic, and oxidative stress mechanisms (65–67). In addition, HIV infection is associated with lymphocytic alveolitis, in which CD8 lymphocytes in the lungs produce inflammatory cytokines that lead to tissue destruction and emphysema (62,64).
The role of ART in COPD is unclear. Some studies suggest that ART increases the risk of COPD, whereas others suggest that it lowers the risk (62–64).
COPD symptoms and pulmonary function testing are the same as for non–HIV-infected patients. CT findings are also typical and include hyperinflation, air trapping, parenchymal destruction, bullae, cysts, and, occasionally, pneumothorax (Fig 17) (68).
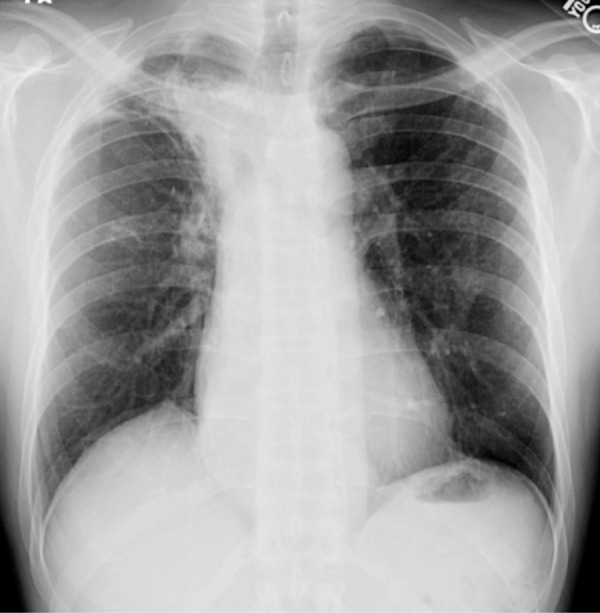
Figure 17a.
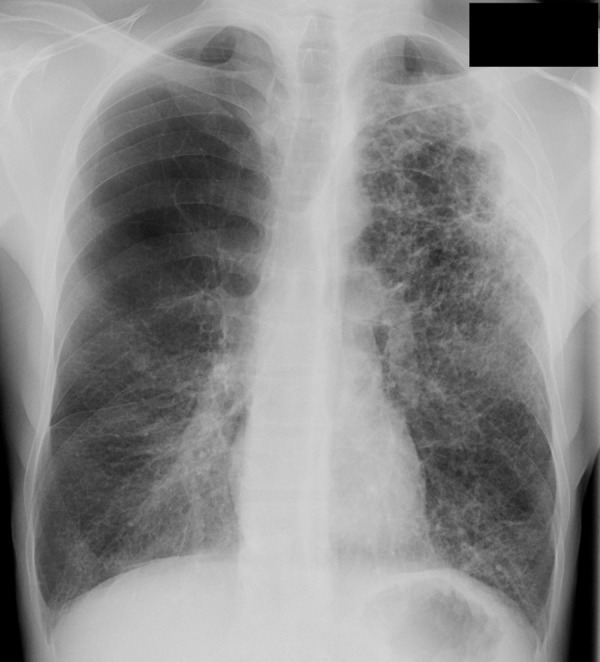
COPD and Streptococcus pneumonia in a 53-year-old man with HIV infection. The patient had been receiving ART for 7 months and was on home oxygen for COPD. He presented with a 2-week history of left chest pain, cough, and malaise, and had a CD4 count of 424 cells/μL. (a) Chest radiograph demonstrates emphysema and left upper lobe consolidation. (b) CT image helps confirm pneumonia superimposed on severe centrilobular emphysema. Bronchoalveolar lavage revealed α-hemolytic streptococci.
Figure 17b.
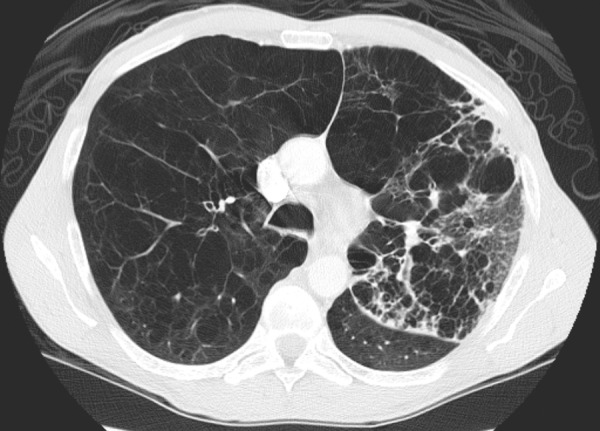
COPD and Streptococcus pneumonia in a 53-year-old man with HIV infection. The patient had been receiving ART for 7 months and was on home oxygen for COPD. He presented with a 2-week history of left chest pain, cough, and malaise, and had a CD4 count of 424 cells/μL. (a) Chest radiograph demonstrates emphysema and left upper lobe consolidation. (b) CT image helps confirm pneumonia superimposed on severe centrilobular emphysema. Bronchoalveolar lavage revealed α-hemolytic streptococci.
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is defined as a mean pulmonary arterial pressure greater than 25 mm Hg at rest, with a normal pulmonary wedge pressure of 15 mm Hg or less at right heart catheterization (69,70). PAH is diagnosed at histopathologic analysis or right heart catheterization in one of every 200 HIV-infected patients (0.5%), an approximately 25-fold increase compared with the general population, and its prevalence has not decreased in the ART era (70,71). About 35%–66% of HIV-infected patients have systolic pulmonary arterial pressures of over 30 mm Hg at echocardiography, and an elevated systolic pulmonary arterial pressure is associated with impaired pulmonary function (72).
HIV-related PAH is more common in men than in women, with an average patient age of 35–41 years (70,71). No definite relationship between PAH and CD4 count has been established (71,72). PAH can develop at any stage of HIV infection but may increase at a CD4 count of less than 200 cells/μL (70,72). There is no clear correlation between PAH and ART (71,72).
The laboratory and histopathologic features of HIV-related PAH are similar to those of primary PAH (71). The pathogenesis of PAH may involve the chronic inflammatory state accompanying HIV infection. In addition, the HIV envelope protein, glycoprotein gp-120, stimulates production of endothelin-1, which promotes vasoconstriction and proliferation of smooth muscle and endothelial cells in pulmonary vessels (69,73). Genetic predisposition and stimulation of other inflammatory markers, cytokines, and growth factors have also been implicated (69,71–73).
Patients are often asymptomatic in the early course of PAH (73,74). Exertional dyspnea is typically the first and principal symptom (73,74). Other symptoms include fatigue, weakness, and exercise intolerance (74). Angina and syncope reflect impaired cardiac output (73,74). Eventually, dyspnea at rest and right heart failure develop (74).
A high degree of suspicion is required to diagnose early PAH. Chest radiographs may be normal in asymptomatic patients. On radiographs and CT images, findings of PAH include enlarged central pulmonary arteries with rapid or abrupt tapering of peripheral branches (“pruning”) (Fig 18a) (74). Associated findings include cardiomegaly with dilatation of the right heart chambers in particular (71,74). Additional findings of PAH at CT include a main pulmonary artery diameter of 29 mm or more at the level of its bifurcation and a pulmonary artery–aorta ratio of over 1 (Fig 18b) (73,74). CT is also useful in detecting other causes of dyspnea or PAH. Doppler echocardiography is the usual noninvasive test for PAH, but right heart catheterization is more accurate and remains the standard of reference (74).
Figure 18a.
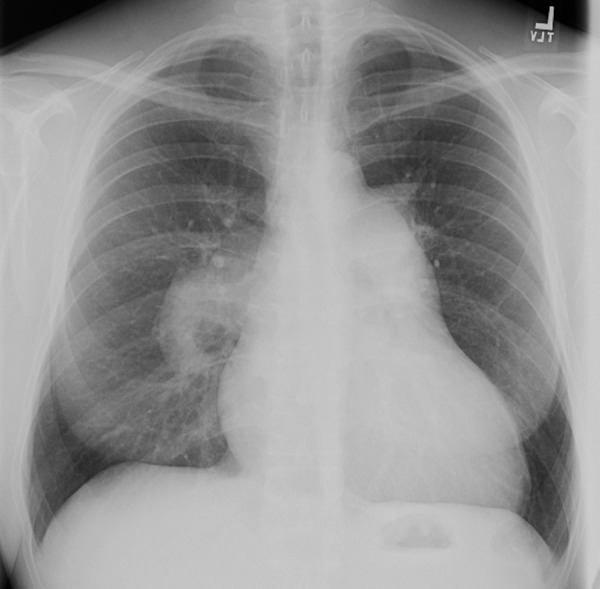
PAH in a 41-year-old man with long-standing HIV infection who had been receiving ART for 7 years. The patient presented with a 4-day history of dyspnea, nonproductive cough, pedal edema, and fatigue, and had a CD4 count of 452 cells/μL. (a) Chest radiograph shows enlarged central pulmonary arteries. (b) CT image shows the main pulmonary artery with a diameter of 36 mm and a pulmonary artery–aorta ratio of 1.3, findings that are consistent with PAH.
Figure 18b.
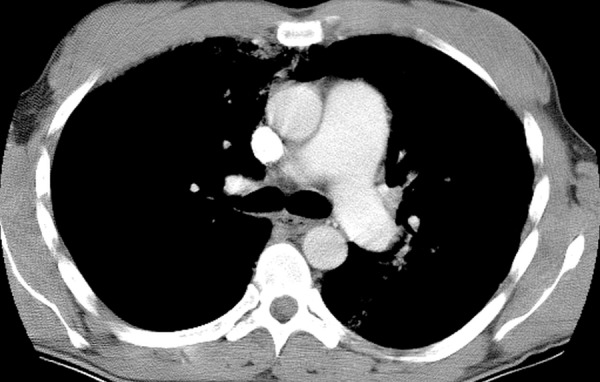
PAH in a 41-year-old man with long-standing HIV infection who had been receiving ART for 7 years. The patient presented with a 4-day history of dyspnea, nonproductive cough, pedal edema, and fatigue, and had a CD4 count of 452 cells/μL. (a) Chest radiograph shows enlarged central pulmonary arteries. (b) CT image shows the main pulmonary artery with a diameter of 36 mm and a pulmonary artery–aorta ratio of 1.3, findings that are consistent with PAH.
Lymphocytic Interstitial Pneumonia
Lymphocytic interstitial pneumonia (LIP) is an AIDS-defining illness in children under 13 years of age (75) but is less common in HIV-infected adults. It is associated with EBV (36), and HIV-infected adults with LIP have higher levels of EBV antibodies than do those without LIP (75). LIP can occur at any stage of HIV infection but usually occurs when the CD4 count is normal (76). Patients typically have nonspecific respiratory or constitutional symptoms (eg, exertional dyspnea, nonproductive cough, and fever) but can be asymptomatic. The clinical course is variable, ranging from spontaneous resolution to respiratory failure. The diagnosis of LIP is usually made at transbronchial or open lung biopsy (76). Histopathologic analysis demonstrates interstitial inflammatory infiltration by T lymphocytes, plasma cells, and histiocytes (36,76). LIP may respond to the administration of ART alone (75,76).
Imaging findings include basal reticulation, ground-glass opacities, ill-defined centrilobular and subpleural nodules, thin-walled cysts, peribronchovascular interstitial thickening, and interlobular septal thickening (Fig 19a) (36,75). Cysts may result from bronchiolar obstruction by lymphocytic infiltrates with postobstructive ectasia (Fig 19b) (75). Although its imaging features are nonspecific, LIP can be suspected when the relevant findings persist despite antibiotic treatment and wax and wane with a chronic, indolent course (75).
Figure 19a.
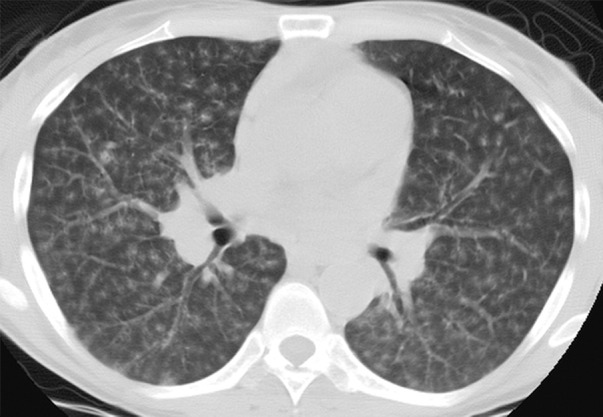
LIP in a 35-year-old woman who presented with fever, dyspnea, and cough. The patient’s CD4 count was 328 cells/μL, and a diagnosis of HIV infection was made. (a) CT image shows diffuse, ill-defined centrilobular nodules, which did not respond to antibiotic treatment. ART was initiated, but similar symptoms and radiographic abnormalities persisted for over 10 years. Bronchoalveolar lavage revealed 78% lymphocytes with a decreased CD4-CD8 ratio. Transbronchial biopsy was nondiagnostic. (b) CT image obtained 10 years after the initial examination (cf a) shows lung cysts. A presumptive diagnosis of LIP was made based on the combination of clinical, laboratory, and radiologic findings.
Figure 19b.
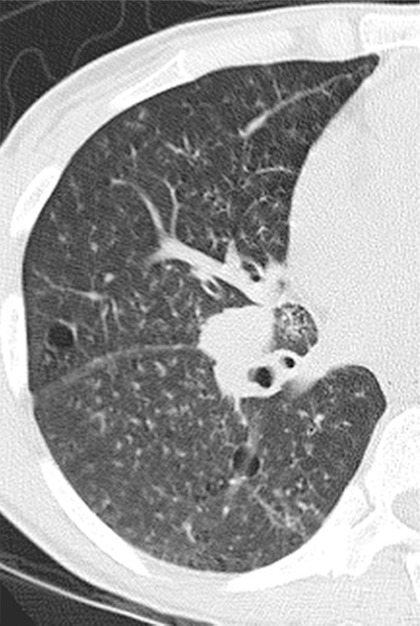
LIP in a 35-year-old woman who presented with fever, dyspnea, and cough. The patient’s CD4 count was 328 cells/μL, and a diagnosis of HIV infection was made. (a) CT image shows diffuse, ill-defined centrilobular nodules, which did not respond to antibiotic treatment. ART was initiated, but similar symptoms and radiographic abnormalities persisted for over 10 years. Bronchoalveolar lavage revealed 78% lymphocytes with a decreased CD4-CD8 ratio. Transbronchial biopsy was nondiagnostic. (b) CT image obtained 10 years after the initial examination (cf a) shows lung cysts. A presumptive diagnosis of LIP was made based on the combination of clinical, laboratory, and radiologic findings.
Multilocular Thymic Cyst
HIV stimulates the proliferation of CD8 T lymphocytes, causing a set of diseases known as diffuse infiltrative lymphocytosis syndrome (75). These disease entities include LIP in the lungs, lymphoepithelial cysts in the salivary glands, and MTCs in the thymus (75). These entities are mostly benign, with malignant transformation occurring only rarely (75,77).
Most HIV-infected patients with MTCs are children (77). Histologically, MTCs resemble lymphoepithelial cysts in the parotid glands (77). HIV-related MTCs have also been documented in patients with LIP and parotid gland enlargement (78). MTCs may manifest as anterior mediastinal masses with cystic or soft-tissue attenuation at CT and high signal intensity at T2-weighted MR imaging, with septations and a multilocular configuration (Fig 20) (77,78).
Figure 20a.
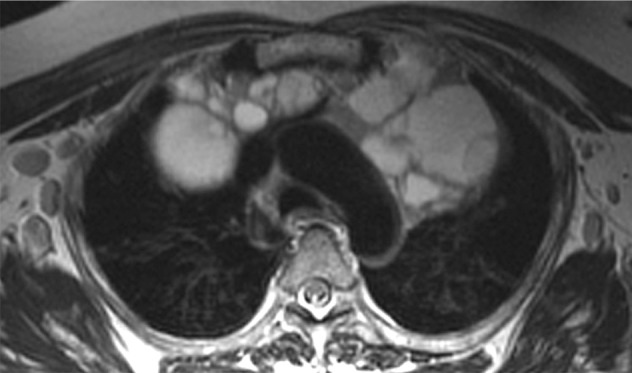
MTC in a patient with HIV infection. Axial (a) and coronal (b) T2-weighted MR images demonstrate a hyperintense MTC with septations in the superior mediastinum. (Courtesy of Daniel Ocazionez, MD, University of Washington, Seattle, and Carlos Restrepo, MD, University of Texas Health Science Center at San Antonio.)
Figure 20b.
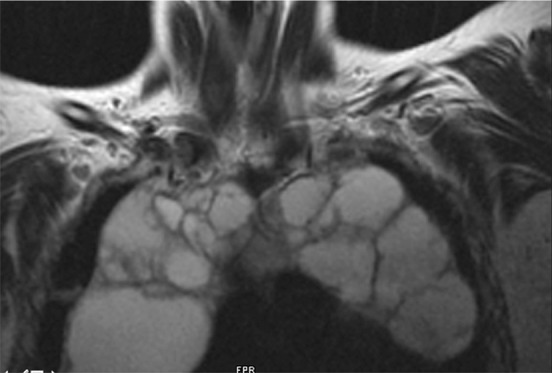
MTC in a patient with HIV infection. Axial (a) and coronal (b) T2-weighted MR images demonstrate a hyperintense MTC with septations in the superior mediastinum. (Courtesy of Daniel Ocazionez, MD, University of Washington, Seattle, and Carlos Restrepo, MD, University of Texas Health Science Center at San Antonio.)
Conclusion
Since HIV and AIDS emerged 30 years ago, the introduction of ART has transformed the demographics of HIV-infected patients and the spectrum of thoracic diseases. HIV infection is now a chronic illness. Complications from aging and chronic inflammation have emerged and have increased in frequency. These comorbidities include a multitude of thoracic diseases, of which the most important are bacterial pneumonia; malignancies such as lung cancer, Hodgkin lymphoma, and HHV-8–related neoplasms; IRIS; COPD; pulmonary hypertension; interstitial pneumonia; and MTC. Familiarity with the manifestations of these less traditional HIV-related diseases can expedite diagnosis and treatment.
Recipient of a Cum Laude award for an education exhibit at the 2012 RSNA Annual Meeting.
For this journal-based SA-CME activity, the author S.N.P. has reported financial relationships (see “Disclosures of Conflicts of Interest”); the other authors, editor, and reviewers have no relevant relationships to disclose.
Funding: The work was supported by the National Institutes of Health [grant numbers NIH/NCI R01CA173754 and NIH/NHLBI R01HL090342].
Disclosures of Conflicts of Interest.—S.N.P.: Related financial activities: grant from GE Healthcare. Other financial activities: royalties from Amirsys for authorship of EXPERTddx: Chest.
Abbreviations:
- AIDS
- acquired immunodeficiency syndrome
- ART
- antiretroviral therapy
- COPD
- chronic obstructive pulmonary disease
- EBV
- Epstein-Barr virus
- HHV-8
- human herpesvirus 8
- HIV
- human immunodeficiency virus
- IRIS
- immune reconstitution inflammatory syndrome
- KICS
- KSHV inflammatory cytokine syndrome
- KSHV
- Kaposi sarcoma–associated herpesvirus
- LIP
- lymphocytic interstitial pneumonia
- MAC
- Mycobacterium avium complex
- MCD
- multicentric Castleman disease
- MTC
- multilocular thymic cyst
- PAH
- pulmonary arterial hypertension
References
- 1.UN Joint Programme on HIV/AIDS. 2006 Report on the global AIDS epidemic: Executive summary. http://data.unaids.org/pub/GlobalReport/2006/2006_gr-executivesummary_en.pdf. Updated May 2006. Accessed April 7, 2013.
- 2.UN Joint Programme on HIV/AIDS . Global Report: UNAIDS Report on the Global AIDS Epidemic: 2010. http://www.unaids.org/globalreport/global_report.htm. Updated November 23, 2010. Accessed April 7, 2013.
- 3.Zhu T, Korber BT, Nahmias AJ, Hooper E, Sharp PM, Ho DD. An African HIV-1 sequence from 1959 and implications for the origin of the epidemic. Nature 1998;391(6667):594–597. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control (CDC) . Pneumocystis pneumonia: Los Angeles. MMWR Morb Mortal Wkly Rep 1981;30(21):250–252. [PubMed] [Google Scholar]
- 5.Godwin JD, Ravin CE, Roggli VL. Fatal pneumocystis pneumonia, cryptococcosis, and Kaposi sarcoma in a homosexual man. AJR Am J Roentgenol 1982;138(3):580–581. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb MS, Schroff R, Schanker HM, et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 1981;305(24):1425–1431. [DOI] [PubMed] [Google Scholar]
- 7.Gallo RC, Sarin PS, Gelmann EP, et al. Isolation of human T-cell leukemia virus in acquired immune deficiency syndrome (AIDS). Science 1983;220(4599):865–867. [DOI] [PubMed] [Google Scholar]
- 8.Merson MH. The HIV-AIDS pandemic at 25: the global response. N Engl J Med 2006;354(23): 2414–2417. [DOI] [PubMed] [Google Scholar]
- 9.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection: HIV Outpatient Study Investigators. N Engl J Med 1998;338(13):853–860. [DOI] [PubMed] [Google Scholar]
- 10.Work Group for HIV and Aging Consensus Project . Summary report from the Human Immunodeficiency Virus and Aging Consensus Project: treatment strategies for clinicians managing older individuals with the human immunodeficiency virus. J Am Geriatr Soc 2012;60(5):974–979. [DOI] [PubMed] [Google Scholar]
- 11.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 2009;338:a3172. [DOI] [PubMed] [Google Scholar]
- 12.Deeks SG. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2009;17(4):118–123. [PubMed] [Google Scholar]
- 13.Akgün KM, Pisani M, Crothers K. The changing epidemiology of HIV-infected patients in the intensive care unit. J Intensive Care Med 2011;26(3):151–164. [DOI] [PubMed] [Google Scholar]
- 14.Benito N, Moreno A, Miro JM, Torres A. Pulmonary infections in HIV-infected patients: an update in the 21st century. Eur Respir J 2012;39(3): 730–745. [DOI] [PubMed] [Google Scholar]
- 15.Segal LN, Methé BA, Nolan A, et al. HIV-1 and bacterial pneumonia in the era of antiretroviral therapy. Proc Am Thorac Soc 2011;8(3):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crothers K, Thompson BW, Burkhardt K, et al. HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc 2011;8(3):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohli R, Lo Y, Homel P, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis 2006;43(1): 90–98. [DOI] [PubMed] [Google Scholar]
- 18.Brecher CW, Aviram G, Boiselle PM. CT and radiography of bacterial respiratory infections in AIDS patients. AJR Am J Roentgenol 2003;180(5):1203–1209. [DOI] [PubMed] [Google Scholar]
- 19.Petrosillo N, Nicastri E, Viale P. Nosocomial pulmonary infections in HIV-positive patients. Curr Opin Pulm Med 2005;11(3):231–235. [DOI] [PubMed] [Google Scholar]
- 20.Kanne JP, Yandow DR, Mohammed TL, Meyer CA. CT findings of pulmonary nocardiosis. AJR Am J Roentgenol 2011;197(2):W266–W272. [DOI] [PubMed] [Google Scholar]
- 21.Saurborn DP, Fishman JE, Boiselle PM. The imaging spectrum of pulmonary tuberculosis in AIDS. J Thorac Imaging 2002;17(1):28–33. [DOI] [PubMed] [Google Scholar]
- 22.Marchiori E, Müller NL, Soares Souza A, Jr, Escuissato DL, Gasparetto EL, Franquet T. Pulmonary disease in patients with AIDS: high-resolution CT and pathologic findings. AJR Am J Roentgenol 2005; 184(3):757–764. [DOI] [PubMed] [Google Scholar]
- 23.Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 2008;148(10):728–736. [DOI] [PubMed] [Google Scholar]
- 24.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008;123(1):187–194. [DOI] [PubMed] [Google Scholar]
- 25.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 2009;52(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc 2011;8(3):313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winstone TA, Man SF, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest 2013;143(2):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol 2006;24(9):1383–1388. [DOI] [PubMed] [Google Scholar]
- 29.Kirk GD, Merlo CA; Lung HIV Study. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc 2011;8(3):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sigel K, Wisnivesky J, Gordon K, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012;26(8):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med 2010;153(7):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fishman JE, Schwartz DS, Sais GJ, Flores MR, Sridhar KS. Bronchogenic carcinoma in HIV-positive patients: findings on chest radiographs and CT scans. AJR Am J Roentgenol 1995;164(1):57–61. [DOI] [PubMed] [Google Scholar]
- 33.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood 2006;108(12):3786–3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goedert JJ, Bower M. Impact of highly effective antiretroviral therapy on the risk for Hodgkin lymphoma among people with human immunodeficiency virus infection. Curr Opin Oncol 2012;24(5):531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan LD. Management of HIV-associated Hodgkin lymphoma: how far we have come. J Clin Oncol 2012;30(33):4056–4058. [DOI] [PubMed] [Google Scholar]
- 36.Hare SS, Souza CA, Bain G, et al. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol 2012;85(1015):848–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns J, Shaknovich R, Lau J, Haramati LB. Oncogenic viruses in AIDS: mechanisms of disease and intrathoracic manifestations. AJR Am J Roentgenol 2007;189(5):1082–1087. [DOI] [PubMed] [Google Scholar]
- 38.El Amari EB, Toutous-Trellu L, Gayet-Ageron A, et al. Predicting the evolution of Kaposi sarcoma, in the highly active antiretroviral therapy era. AIDS 2008;22(9):1019–1028. [DOI] [PubMed] [Google Scholar]
- 39.Traill ZC, Miller RF, Shaw PJ. CT appearances of intrathoracic Kaposi’s sarcoma in patients with AIDS. Br J Radiol 1996;69(828):1104–1107. [DOI] [PubMed] [Google Scholar]
- 40.Chadburn A. Immunodeficiency-associated lymphoid proliferations (ALPS, HIV, and KSHV/HHV8). Semin Diagn Pathol 2013;30(2):113–129. [DOI] [PubMed] [Google Scholar]
- 41.Cesarman E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett 2011;305(2):163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gantt S, Casper C. Human herpesvirus 8-associated neoplasms: the roles of viral replication and antiviral treatment. Curr Opin Infect Dis 2011;24(4):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oksenhendler E. HIV-associated multicentric Castleman disease. Curr Opin HIV AIDS 2009;4(1):16–21. [DOI] [PubMed] [Google Scholar]
- 44.Guihot A, Couderc LJ, Rivaud E, et al. Thoracic radiographic and CT findings of multicentric Castleman disease in HIV-infected patients. J Thorac Imaging 2007;22(2):207–211. [DOI] [PubMed] [Google Scholar]
- 45.Johkoh T, Müller NL, Ichikado K, et al. Intrathoracic multicentric Castleman disease: CT findings in 12 patients. Radiology 1998;209(2):477–481. [DOI] [PubMed] [Google Scholar]
- 46.Ko SF, Hsieh MJ, Ng SH, et al. Imaging spectrum of Castleman’s disease. AJR Am J Roentgenol 2004;182(3):769–775. [DOI] [PubMed] [Google Scholar]
- 47.Polizzotto MN, Uldrick TS, Hu D, Yarchoan R. Clinical manifestations of Kaposi sarcoma herpesvirus lytic activation: multicentric Castleman disease (KSHV-MCD) and the KSHV inflammatory cytokine syndrome. Front Microbiol 2012;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simonelli C, Spina M, Cinelli R, et al. Clinical features and outcome of primary effusion lymphoma in HIV-infected patients: a single-institution study. J Clin Oncol 2003;21(21):3948–3954. [DOI] [PubMed] [Google Scholar]
- 49.Carbone A, Cesarman E, Gloghini A, Drexler HG. Understanding pathogenetic aspects and clinical presentation of primary effusion lymphoma through its derived cell lines. AIDS 2010;24(4): 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barber DL, Andrade BB, Sereti I, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol 2012;10(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS 2004;18(12):1615–1627. [DOI] [PubMed] [Google Scholar]
- 52.Crothers K, Huang L. Pulmonary complications of immune reconstitution inflammatory syndromes in HIV-infected patients. Respirology 2009;14(4): 486–494. [DOI] [PubMed] [Google Scholar]
- 53.Shelburne SA, Visnegarwala F, Darcourt J, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS 2005;19(4):399–406. [DOI] [PubMed] [Google Scholar]
- 54.Calligaro G, Meintjes G, Mendelson M. Pulmonary manifestations of the immune reconstitution inflammatory syndrome. Curr Opin Pulm Med 2011;17(3):180–188. [DOI] [PubMed] [Google Scholar]
- 55.Rajeswaran G, Becker JL, Michailidis C, Pozniak AL, Padley SP. The radiology of IRIS (immune reconstitution inflammatory syndrome) in patients with mycobacterial tuberculosis and HIV co-infection: appearances in 11 patients. Clin Radiol 2006; 61(10):833–843. [DOI] [PubMed] [Google Scholar]
- 56.Berman EJ, Iyer RS, Addrizzo-Harris D, Ko JP. Immune-reconstitution syndrome related to atypical mycobacterial infection in AIDS. J Thorac Imaging 2008;23(3):182–187. [DOI] [PubMed] [Google Scholar]
- 57.Nunweiler CG, Brown JA, Phillips P, Ellis J, Müller NL. The imaging features of nontuberculous mycobacterial immune reconstitution syndrome. J Comput Assist Tomogr 2009;33(2):242–246. [DOI] [PubMed] [Google Scholar]
- 58.Godoy MC, Silva CI, Ellis J, Phillips P, Müller NL. Organizing pneumonia as a manifestation of Pneumocystis jirovecii immune reconstitution syndrome in HIV-positive patients: report of 2 cases. J Thorac Imaging 2008;23(1):39–43. [DOI] [PubMed] [Google Scholar]
- 59.Klein JS, Warnock M, Webb WR, Gamsu G. Cavitating and noncavitating granulomas in AIDS patients with Pneumocystis pneumonitis. AJR Am J Roentgenol 1989;152(4):753–754. [DOI] [PubMed] [Google Scholar]
- 60.Naccache JM, Antoine M, Wislez M, et al. Sarcoid-like pulmonary disorder in human immunodeficiency virus-infected patients receiving antiretroviral therapy. Am J Respir Crit Care Med 1999;159(6):2009–2013. [DOI] [PubMed] [Google Scholar]
- 61.Norris KA, Morris A. Pneumocystis infection and the pathogenesis of chronic obstructive pulmonary disease. Immunol Res 2011;50(2-3):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raynaud C, Roche N, Chouaid C. Interactions between HIV infection and chronic obstructive pulmonary disease: clinical and epidemiological aspects. Respir Res 2011;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crothers K, Huang L, Goulet JL, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011;183(3):388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morris A, George MP, Crothers K, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc 2011;8(3):320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crothers K. Chronic obstructive pulmonary disease in patients who have HIV infection. Clin Chest Med 2007;28(3):575–587, vi. [DOI] [PubMed] [Google Scholar]
- 66.Petrache I, Diab K, Knox KS, et al. HIV associated pulmonary emphysema: a review of the literature and inquiry into its mechanism. Thorax 2008;63(5):463–469. [DOI] [PubMed] [Google Scholar]
- 67.Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1(+) smokers with early emphysema. J Leukoc Biol 2009;86(4):913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuhlman JE, Knowles MC, Fishman EK, Siegelman SS. Premature bullous pulmonary damage in AIDS: CT diagnosis. Radiology 1989;173(1):23–26. [DOI] [PubMed] [Google Scholar]
- 69.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc, and the Pulmonary Hypertension Association. Circulation 2009;119(16): 2250–2294. [DOI] [PubMed] [Google Scholar]
- 70.Sitbon O, Lascoux-Combe C, Delfraissy JF, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med 2008;177(1):108–113. [DOI] [PubMed] [Google Scholar]
- 71.Janda S, Quon BS, Swiston J. HIV and pulmonary arterial hypertension: a systematic review. HIV Med 2010;11(10):620–634. [DOI] [PubMed] [Google Scholar]
- 72.Morris A, Gingo MR, George MP, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS 2012; 26(6):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bugnone AN, Viamonte M, Jr, Garcia H. Imaging findings in human immunodeficiency virus-related pulmonary hypertension: report of five cases and review of the literature. Radiology 2002; 223(3):820–827. [DOI] [PubMed] [Google Scholar]
- 74.McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest 2004;126(1 suppl):14S–34S. [DOI] [PubMed] [Google Scholar]
- 75.Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest 2002;122(6):2150–2164. [DOI] [PubMed] [Google Scholar]
- 76.Das S, Miller RF. Lymphocytic interstitial pneumonitis in HIV infected adults. Sex Transm Infect 2003;79(2):88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi X, Nasseri F, Berger DM, Nachiappan AC. Large multilocular thymic cyst: a rare finding in an HIV positive adult female. J Clin Imaging Sci 2012;2:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leonidas JC, Berdon WE, Valderrama E, et al. Human immunodeficiency virus infection and multilocular thymic cysts. Radiology 1996;198(2):377–379. [DOI] [PubMed] [Google Scholar]


