Image-guided tumor ablation is a rapidly evolving technique for treatment of benign and malignant tumors, but optimization of performance and patient outcomes requires using the most appropriate modality for each patient; this article will help provide a rationale for choosing an appropriate modality for a given clinical scenario.
Abstract
Image-guided thermal ablation is an evolving and growing treatment option for patients with malignant disease of multiple organ systems. Treatment indications have been expanding to include benign tumors as well. Specifically, the most prevalent indications to date have been in the liver (primary and metastatic disease, as well as benign tumors such as hemangiomas and adenomas), kidney (primarily renal cell carcinoma, but also benign tumors such as angiomyolipomas and oncocytomas), lung (primary and metastatic disease), and soft tissue and/or bone (primarily metastatic disease and osteoid osteomas). Each organ system has different underlying tissue characteristics, which can have profound effects on the resulting thermal changes and ablation zone. Understanding these issues is important for optimizing clinical results. In addition, thermal ablation technology has evolved rapidly during the past several decades, with substantial technical and procedural improvements that can help improve clinical outcomes and safety profiles. Staying up to date on these developments is challenging but critical because the physical properties underlying the different ablation modalities and the appropriate use of adjuncts will have a tremendous effect on treatment results. Ultimately, combining an understanding of the physical properties of the ablation modalities with an understanding of the thermal kinetics in tissue and using the most appropriate ablation modality for each patient are key to optimizing clinical outcomes. Suggested algorithms are described that will help physicians choose among the various ablation modalities for individual patients.
©RSNA, 2014
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Describe the physical properties of the most commonly used thermal ablation modalities.
■ Discuss the potential advantages and disadvantages of each thermal ablation modality.
■ Apply decision-making algorithms to select an appropriate thermal ablation modality for common clinical indications.
Introduction
Image-guided thermal ablation is becoming increasingly accepted for the treatment of certain benign and malignant tumors of the lungs, liver, kidneys, bone, and soft tissues (1–6). Numerous thermal and nonthermal ablation modalities are available, including radiofrequency (RF) ablation, microwave ablation, cryoablation, high-intensity focused ultrasonography (US), laser ablation, irreversible electroporation, chemical ablation (with ethanol and acetic acid), and brachytherapy. This article focuses on the thermal ablation devices used most frequently in the United States: RF ablation, microwave ablation, and cryoablation devices. The number of available ablation modalities, the rapid changes in the associated technology (especially recently), the relative lack of randomized controlled or comparative trials, the wide discrepancies in published results (with associated difficulties in interpreting the results), and the variability of practice in different centers have led to confusion about best practice in patients. In this article, some of the issues are elucidated, and the present and future of image-guided tumor ablation are clarified.
The mechanisms of action of various ablation modalities are at the center of many of the relative advantages, disadvantages, and limitations encountered in clinical practice. A basic understanding of the underlying physical processes is critical to determining the most advantageous modality. Therefore, we start by discussing the physical properties of the various modalities and then address the reasons why particular technologies may succeed or fail when applied clinically.
Physical Properties of Thermal Ablation
Heat-based Ablation
Heat has long been recognized as a mechanism for inducing cellular damage. The relationship between cellular temperatures and cellular death is a complex one affected by temperature, time of exposure, and tissue-level effects related to such factors as perfusion, vascular coagulation, adjunctive treatments, and cell fragility (7). For tumor ablation, however, the typical goal is to reach temperatures higher than 55°C in the target tissue to create coagulation necrosis. Temperatures would ideally decrease rapidly outside the target volume to avoid unwanted collateral damage.
RF Ablation.—The heating mechanism of RF ablation has been described in detail elsewhere (8). Briefly, an alternating electric current is applied to the target tissue through use of an interstitial electrode; in monopolar RF ablation, the current returns through grounding pads on the skin surface. The electric current rapidly oscillates tissue ions, creating frictional heating in areas of high current density adjacent to the electrode. Additional growth of the ablation zone can be attributed primarily to thermal diffusion (9).
Two primary issues associated with this heating mechanism have limited the ability of RF ablation to effectively treat the growing number and diversity of tumors being considered for minimally invasive therapies. First, heating tissue with electric current is a self-limiting process. Water vapor, desiccation, and charring created during RF ablation progressively increase tissue impedance. An increase in impedance limits the application of additional current and, hence, limits temperatures that can be achieved in the ablation zone. Second, conductive heat transfer is relatively slow in most tissues and in the in vivo setting has limited ability to overcome relatively minor competing processes, such as perfusion and ventilation. As a result, RF ablation zones can vary widely according to the local tissue environment (10,11). For example, aerated lung is characterized by high impedance to electric current flow and poor heat transfer compared with most solid organs. The high impedance of normal lung tissue surrounding a tumor can reduce power delivery, whereas poor thermal diffusion limits growth of the ablation zone (12,13). Some of these technical limitations can be overcome by increases in power and time, but, overall, RF ablation is a suboptimal choice for ablation of lung tumor (14).
Numerous attempts have been made to overcome the physical limitations of RF ablation, with varying degrees of success. Developments that have improved the size of the ablation zone include the following: (a) internal electrode cooling to limit charring in the tissues immediately adjacent to the electrode (15); (b) expandable, multi-tined, or clustered electrodes to increase the electrode surface area and facilitate greater power delivery (16); and (c) rapidly switching multiple-electrode approaches that allow the simultaneous production of separate or overlapping ablation zones (17–19). Other innovations include the use of saline infusion and high-power generators (20). Unfortunately, although several of these techniques have improved the overall performance of RF ablation, many are associated with an increased risk for complications related to an increase in applicator size or number, increased current with the potential for skin burns, and escalation in procedural complexity and the probability of collateral damage.
Microwave Ablation.—Cell death in microwave ablation is nearly identical to that observed in RF ablation (21). However, the mechanism of microwave heating may have advantages in certain clinical applications. Polar molecules (primarily water) continuously realign with the oscillating microwave field, effectively increasing kinetic energy and tissue temperature. In contrast to electric currents, microwaves radiate through all biological tissues, including those with high impedance to electric current, such as bone, lung, and charred or desiccated tissues (22). This allows microwaves to continuously generate heat in a much larger volume of tissue surrounding the applicator (23). As a result, microwave energy can produce faster, hotter, and larger ablation zones in multiple tissue types compared with RF current (Fig 1). The clinical result is that, on average, fewer applicators are needed, and ablative margins are easier to obtain.
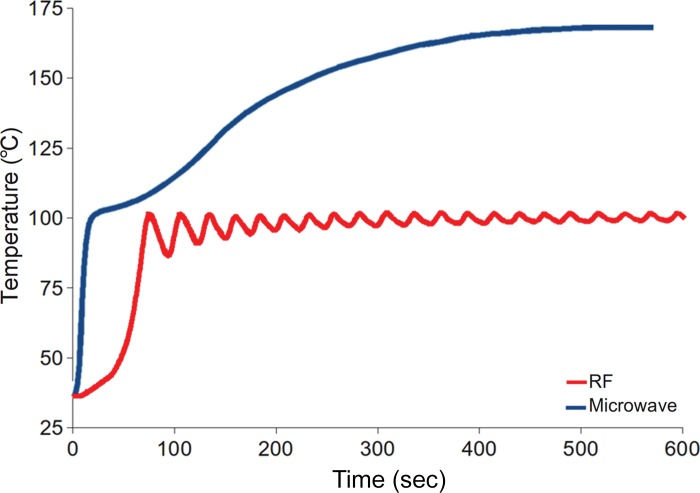
Figure 1.
Graph shows the comparison of temperatures obtained with a third-generation microwave ablation system versus a similar RF ablation system. Microwave energy produces faster and hotter ablation zones than does RF current.
Microwave systems can be divided into three types: (a) First-generation systems lack active antenna cooling and are therefore limited to low power and short durations; (b) second-generation systems have antenna cooling but limited generator power; and (c) third-generation systems incorporate antenna cooling and high-power generators. Each system is characterized by a different combination of antenna diameter, number of antennae, frequency and phase control, generated power, and power loss between the generator and applicator tip. System performance can vary widely, so it is critical that physicians understand the ablation-zone shapes and sizes created by different time and power combinations with a particular system.
The volume of microwave ablation zones depends on the power applied, antenna design, number and orientation of antennae, and microwave frequency.
Microwave antenna design is a critical factor that affects the size and shape of the ablation zone, and dozens of antennae have been described to control energy delivery (Fig 2) (24,25). Many antennae produce an elongated ablation zone (as long as 6 cm) that may increase the risk for burns to the body wall and other structures, so newer designs aim for more rounded and forward-weighted heating that is amenable to treating smaller tumors (Fig 3) (26,27). Some systems also include antenna designs to transfer energy more optimally to different organ types, such as the lungs (28). As with other ablation modalities, multiple antennae can also be used in concert to create larger, more conformal and confluent ablation zones (29,30). In addition, the driving phase of each antenna can be controlled to create zones of “constructive” and “destructive” wave interaction that can have a profound effect on the ablation result (31–33).
Figure 2.
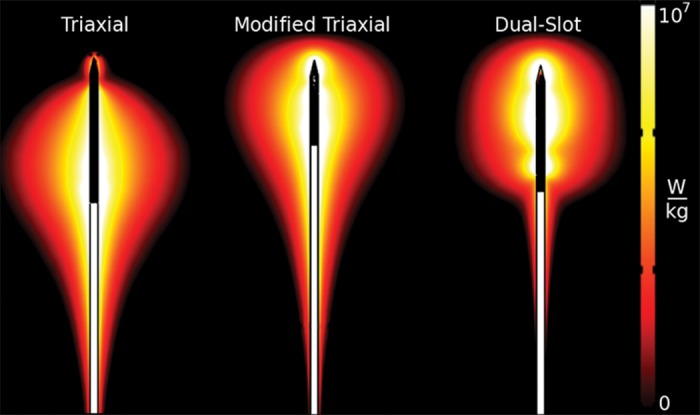
Diagram compares the electromagnetic fields induced by three different microwave ablation antenna designs. The differences in electromagnetic fields directly affect the size and shape of the resulting ablation zone. (Scale is in watts per kilogram.)
Figure 3a.
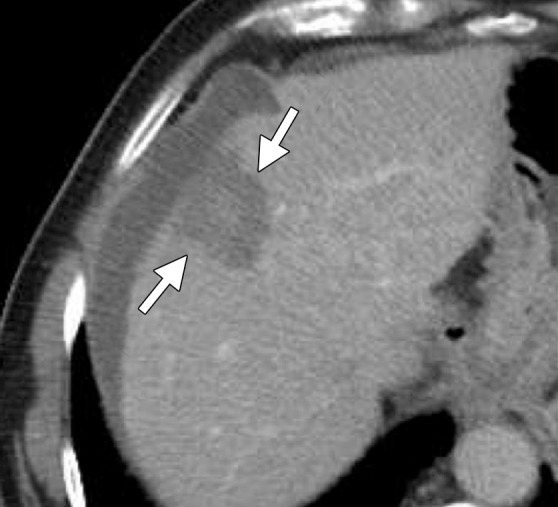
Computed tomographic (CT) images in a 77-year-old man with cirrhosis and a 2.6-cm hepatocellular carcinoma (HCC) in the right hepatic lobe. The HCC was targeted for microwave ablation with a single, specially designed “precision” 17-gauge gas-cooled antenna at 65 W for 7.5 minutes. The resulting ablation zone (arrows) shown on axial (a) and coronal (b) contrast material–enhanced postablation CT images is round and measures approximately 3.4 cm in all dimensions. Microwave ablation allowed destruction of the HCC with minimal associated damage to the hepatic parenchyma. Note the artificial ascites used to protect the diaphragm (arrowhead in b).
Figure 3b.
Computed tomographic (CT) images in a 77-year-old man with cirrhosis and a 2.6-cm hepatocellular carcinoma (HCC) in the right hepatic lobe. The HCC was targeted for microwave ablation with a single, specially designed “precision” 17-gauge gas-cooled antenna at 65 W for 7.5 minutes. The resulting ablation zone (arrows) shown on axial (a) and coronal (b) contrast material–enhanced postablation CT images is round and measures approximately 3.4 cm in all dimensions. Microwave ablation allowed destruction of the HCC with minimal associated damage to the hepatic parenchyma. Note the artificial ascites used to protect the diaphragm (arrowhead in b).
Frequency is another issue that differentiates systems. Although investigators in previous studies have suggested that 915 MHz may create larger ablation zones than can 2.45 GHz (because of increased energy penetration), several acknowledged limitations make those conclusions suspect (34). Most notably, these investigators did not control for power delivered to the tissue, and the assumption that penetration is greater at lower frequencies is not valid in the near-field of microwave antennae (35). Adequately powered 915-MHz and 2.45-GHz systems can create relatively large ablation zones, but the longer wavelength at 915 MHz typically results in a longer ablation zone (28,34,36–38). In general, the greatest heating from any system occurs within 1 cm of the antenna.
Overall, the potential advantages of microwave over RF ablation include faster ablations (typically 2–8 minutes with high-powered systems), higher temperatures without the limitations related to electric impedance, less sensitivity to tissue type with more consistent results (which may be beneficial in treating some tumors that are resistant to treatment with RF, such as sarcomas and hemangiopericytomas), a relative insensitivity to “heat sinks” compared with RF, and the ability to create much larger ablation zones if needed (ablation zones as long as 8 cm have been achieved without applicator repositioning) (22,25,33,39,40). The continued challenges of microwave ablation are as follows: (a) Because microwave ablation is relatively new, clinical data and experience are minimal; (b) a learning curve is associated with using microwave ablation safely because of the potentially larger ablation zones compared with RF ablation; and (c) the clinical systems are heterogeneous in terms of antenna design, wavelength, frequency, power, and cooling, which leads to different performance characteristics and confusion regarding the interpretation of clinical results and the predictability of results between systems from different manufacturers.
Cryoablation
The mechanism of cell death with cold temperatures is different from that with heat. The freezing process results in both intracellular and extracellular ice formation, both of which can result in cellular death, but by different mechanisms. The location of ice formation and, therefore, the mechanism of cell death vary with the freezing rate and final tissue temperature. Faster freezing to lower temperatures promotes the formation of intracellular ice crystals, which results in cell death because of direct damage to the cell membrane and organelles (41). Slower rates of freezing favor the formation of extracellular ice crystals, which leads to a change of osmolality within the extracellular space and, in turn, to cell dehydration and death (42). In addition, apoptosis induced by freezing contributes to this process, a role that is poorly understood (43). The mechanism of cell death with freezing is associated with cell membrane disruption and an associated release of intracellular contents. Because the ablation zone is reperfused after the ice ball melts, the result is a rapid release of cellular debris into the systemic circulation. This probably explains the systemic complications that are seen with cryoablation (ie, cryoshock) but are rare with heat-based ablation (44,45). The temperature necessary to cause reliable cellular necrosis depends on the cell type and thermal history of the tissue, with estimates of −35°C to −20°C (46,47). The lower estimates suggest that cell death may only occur approximately 8 mm deep to the edge of the visualized ice ball; although there is a phase change from liquid to solid at the 0°C isotherm along the edge of the ice ball, this phase change is not necessarily associated with cell death (48). One of the biggest benefits of cryoablation is that the expanding ice ball (predictive of the ablation zone) is well visualized at US, CT, and magnetic resonance imaging, thus allowing relatively more precise monitoring of the ablation zone than is possible with many heat-based systems (48,49).
Cryoablation systems use the Joule-Thomson theory of expanding gases within a needlelike cryoprobe. As the cryogen (typically argon) moves from an internal feed line into an internal expansion chamber, it produces a heat sink near the antenna tip that cools the probe to temperatures of −160°C or colder. Heat transfer from the tissue into the cryoprobe is governed by passive thermal diffusion; unlike RF and microwave ablation devices, cryoablation devices provide no zone of direct or active cooling. As a result, the surface area of the cryoprobe limits cooling efficiency; smaller cryoprobe diameters are associated with lower cooling capacity and, consequently, smaller ablation zones. Therefore, several cryoprobes are required to treat most tumors in clinical practice, and ablation times are typically on the order of 25–30 minutes, much longer than the average times seen with microwave ablation (approximately 5 minutes) or RF ablation (12–30 minutes) (50).
Clinical Decision Making
Arguably the most critical component for a successful ablation practice is the operator. The importance of excellent training and a thorough understanding of the indications, imaging options, techniques, complications, ablation modalities, and specific system being used cannot be stressed enough. In addition, the ability to accurately place needles by using US and CT guidance is paramount, as is a thorough knowledge of the expected appearance of successful and unsuccessful ablations and complications at immediate postprocedural imaging.
In deciding which ablation device to use for a specific patient, many factors should be considered, not the least of which is availability. The unfortunate reality is that many institutions have only one ablation system available. Nonetheless, it is optimal to be familiar with and have available both a heat- and a cold-based system. Herein, we discuss a variety of algorithms for determining which modality may be optimal for a given patient, but some rules of thumb to keep in mind include the following: (a) The preponderance of the data suggests that current microwave systems have inherent advantages over RF ablation and that these advantages can give similar or superior results in the clinical setting in less time (note that the increased power does come with the potential for increased complications related to collateral damage as well, but thus far, this has not proven to be the case in clinical practice [51,52]); (b) the organ involved, tumor type, and underlying comorbid conditions can affect the safety and effectiveness of different modalities; (c) the precision that is possible with cryoablation can be important for treatment of tumors adjacent to vulnerable structures (49,53); and (d) vascular mediated cooling can dramatically affect the size and shape of the ablation zone and should be considered in determining both the modality and the positioning and number of applicators (54–56).
In determining the optimal ablation modality for a particular case, the decision usually comes down to “heat versus cold.” If heat is selected, then our group generally favors microwave ablation because of the associated benefits of decreased procedure time, larger ablation zones (leading to use of fewer applicators), higher temperatures, less influence from vascular heat sinks, and increased predictability (23,57). If microwave ablation is not available, or if the available system is suboptimal, then RF ablation can be used effectively; however, its relative limitations should be understood. The decision of whether to use a heat- or a cold-based ablation modality can be a complex one, and in our practice, we base the decision on organ-specific considerations, tumor type, tumor location (especially proximity to vulnerable structures), and patient factors (eg, comorbid conditions). We describe these considerations according to organ system.
Organ-specific Considerations
Liver.—In the liver, the primary indications for image-guided tumor ablation are HCC and metastatic disease. In addition, ablation modalities are increasingly used to treat benign hepatic masses, including giant hemangiomas, hepatic adenomas, and cysts (58–62). The indications for treatment of benign tumors include controlling mass-related symptoms and, in the case of hepatic adenomas, preventing tumoral hemorrhage and potential malignant degeneration (63,64). The option of treating benign hepatic tumors with percutaneous ablation rather than with open or laparoscopic surgical resection (procedures with increased morbidity compared with ablation) should be presented to patients when applicable.
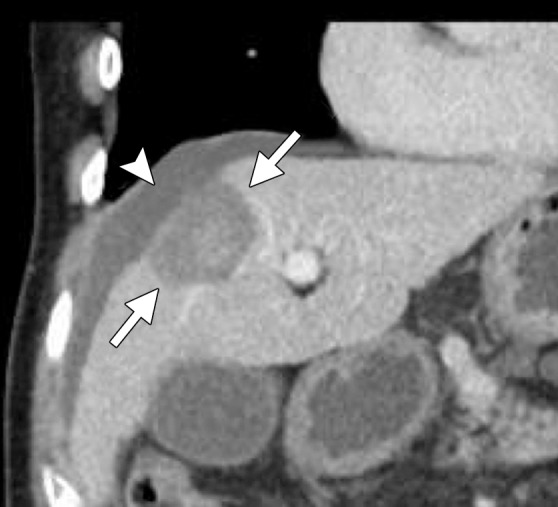
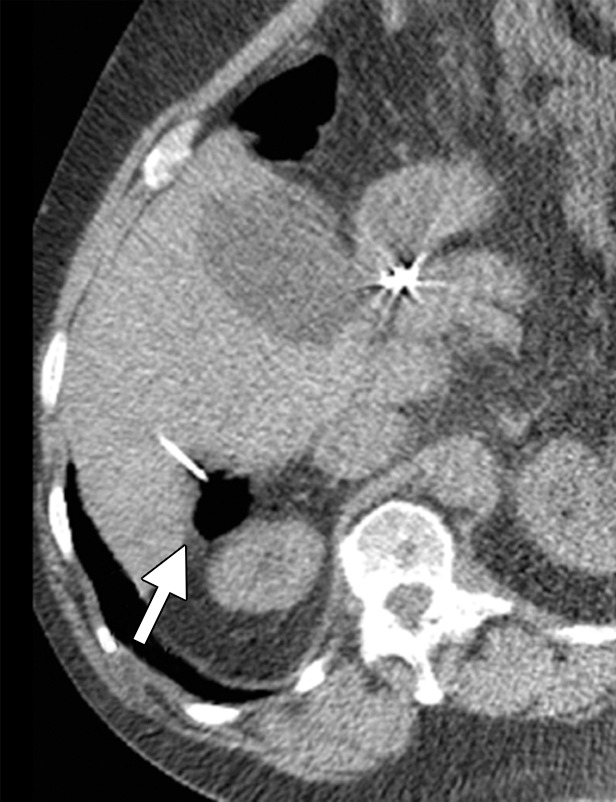
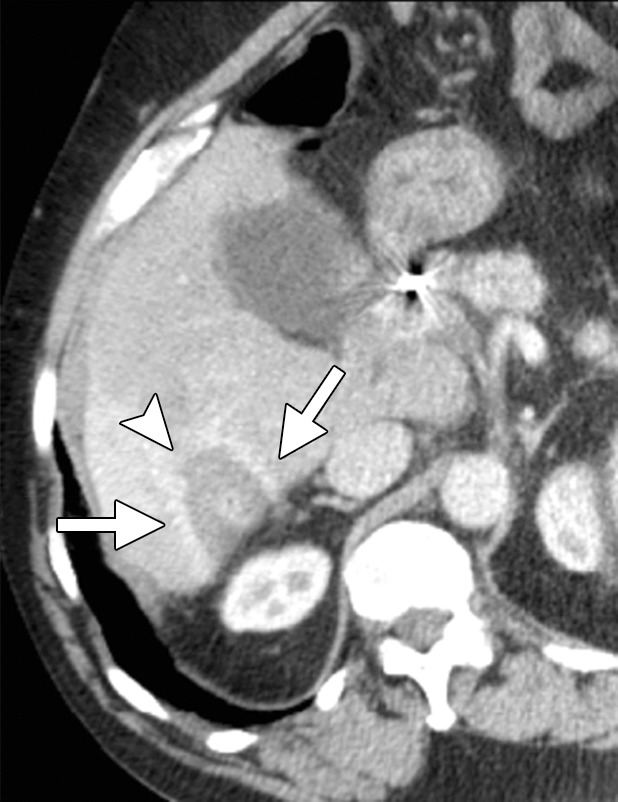
Heat is the preferred method for treating HCC in patients with cirrhosis because of the increased bleeding risk and disseminated intravascular coagulation-like reaction, called cryoshock, associated with cryoablation (44,65,66). Heat-based ablation provides improved hemostasis compared with cryoablation and essentially no risk for cryoshock (67). Therefore, although many studies have shown that small-volume cryoablation is feasible in patients with cirrhosis and HCC, it is difficult to justify the additional risk of cryoablation in these patients when viable heat-based alternatives are available (44). Small HCCs (up to 2–3 cm in diameter) are effectively treated with RF or microwave ablation alone, depending on tumor location. Treatment of larger tumors (>3.0 cm) with RF or microwave monotherapy is associated with high rates of local recurrence (68). Combination treatment of large HCCs (>3.0 cm in diameter) with transarterial chemoembolization followed by RF or microwave ablation results in fewer local failures and is well tolerated, with few serious adverse effects (Figs 4, 5) (69,70).
Figure 4a.
CT images in a 64-year-old male patient with cirrhosis and HCC. (a) Axial arterial phase biphasic CT image shows a 1.8-cm HCC in the right hepatic lobe (arrows). This was targeted for microwave ablation with a single 17-gauge gas-cooled antenna. A 3-minute ablation was performed at 90 W. (b) Axial nonenhanced CT image obtained during ablation shows that the mass has essentially been replaced by gas (arrow). (c) Axial CT image obtained immediately after ablation shows a 3.0 × 2.8-cm ablation zone (arrows), with complete ablation of the tumor and only the expected benign periablational enhancement (arrowhead). The high attenuation seen centrally in the ablation zone is desiccated dense tissue, which is more prominent with microwave ablation than with RF ablation.
Figure 5a.
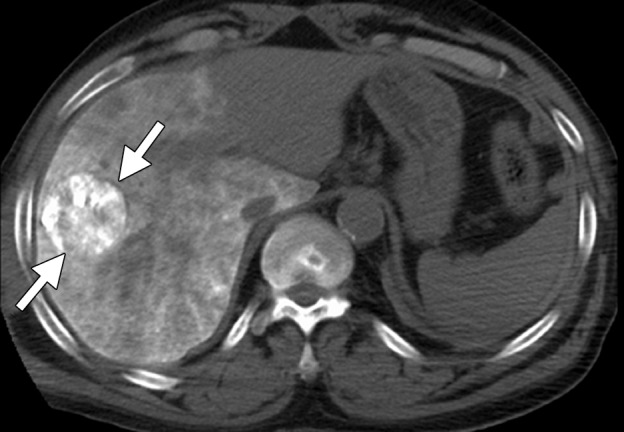
CT images in a 55-year-old male patient with cirrhosis and HCC. (a) Axial nonenhanced CT image obtained during transarterial chemoembolization shows a 5.4 × 5.2-cm HCC in the right hepatic lobe, with intense uptake of ethiodized oil (arrows) and less intense nontarget uptake in the remainder of the liver. The tumor was targeted for microwave ablation by using US guidance. Three 17-gauge gas-cooled microwave antennae were positioned in a triangular configuration within the tumor, and 65 W was applied to all antennae simultaneously, for a total ablation time of 10 minutes. (b) Sagittal reconstructed contrast-enhanced CT image obtained immediately after ablation shows that the ablation was technically successful, with an adequate circumferential ablative margin (arrows). Note the necrosis in the nontarget liver related to transarterial chemoembolization (arrowhead).
Figure 4b.
CT images in a 64-year-old male patient with cirrhosis and HCC. (a) Axial arterial phase biphasic CT image shows a 1.8-cm HCC in the right hepatic lobe (arrows). This was targeted for microwave ablation with a single 17-gauge gas-cooled antenna. A 3-minute ablation was performed at 90 W. (b) Axial nonenhanced CT image obtained during ablation shows that the mass has essentially been replaced by gas (arrow). (c) Axial CT image obtained immediately after ablation shows a 3.0 × 2.8-cm ablation zone (arrows), with complete ablation of the tumor and only the expected benign periablational enhancement (arrowhead). The high attenuation seen centrally in the ablation zone is desiccated dense tissue, which is more prominent with microwave ablation than with RF ablation.
Figure 4c.
CT images in a 64-year-old male patient with cirrhosis and HCC. (a) Axial arterial phase biphasic CT image shows a 1.8-cm HCC in the right hepatic lobe (arrows). This was targeted for microwave ablation with a single 17-gauge gas-cooled antenna. A 3-minute ablation was performed at 90 W. (b) Axial nonenhanced CT image obtained during ablation shows that the mass has essentially been replaced by gas (arrow). (c) Axial CT image obtained immediately after ablation shows a 3.0 × 2.8-cm ablation zone (arrows), with complete ablation of the tumor and only the expected benign periablational enhancement (arrowhead). The high attenuation seen centrally in the ablation zone is desiccated dense tissue, which is more prominent with microwave ablation than with RF ablation.
Figure 5b.
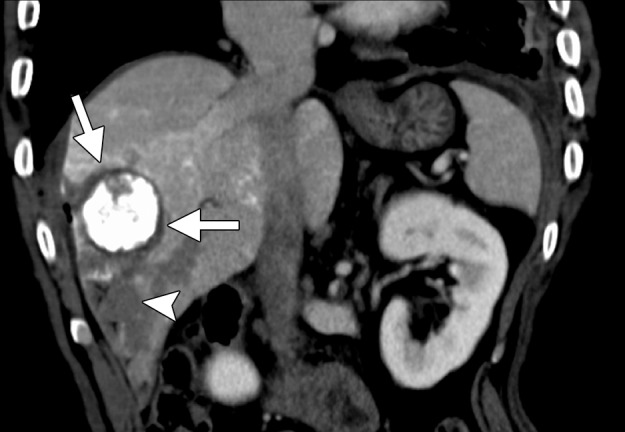
CT images in a 55-year-old male patient with cirrhosis and HCC. (a) Axial nonenhanced CT image obtained during transarterial chemoembolization shows a 5.4 × 5.2-cm HCC in the right hepatic lobe, with intense uptake of ethiodized oil (arrows) and less intense nontarget uptake in the remainder of the liver. The tumor was targeted for microwave ablation by using US guidance. Three 17-gauge gas-cooled microwave antennae were positioned in a triangular configuration within the tumor, and 65 W was applied to all antennae simultaneously, for a total ablation time of 10 minutes. (b) Sagittal reconstructed contrast-enhanced CT image obtained immediately after ablation shows that the ablation was technically successful, with an adequate circumferential ablative margin (arrows). Note the necrosis in the nontarget liver related to transarterial chemoembolization (arrowhead).
For patients with normal background liver, heat-based ablation or cryoablation can be considered. The results in terms of tumor control and survival are expected to be similar, but cryoablation is associated with an increased risk for complications (67). In our experience, cryoablation is rarely needed in the liver and is limited to central tumors for which precision is critical because of proximity to important bile ducts that cannot be sacrificed. Some data indicate that cryoablation is less damaging to ducts than are heat-based therapies (71,72). In general, larger tumors that may have previously been treated only with cryoablation (because of the ability to place numerous cryoprobes simultaneously) can now reasonably be treated with microwave ablation because third-generation systems can produce large confluent ablation zones (Fig 6).
Figure 6a.
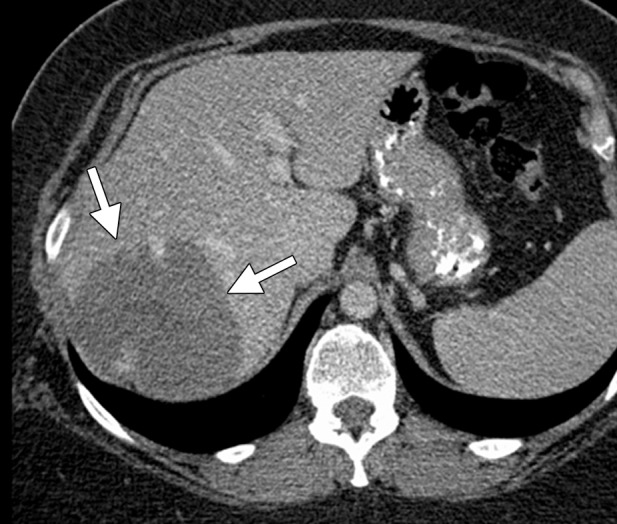
CT images in a symptomatic 37-year-old woman. (a) Axial contrast-enhanced portal venous phase CT image shows an 8.8 × 8.7-cm giant hemangioma in the right hepatic lobe (arrows). (b) Sagittal unenhanced CT image shows the hemangioma targeted for microwave ablation, with three 17-gauge gas-cooled microwave antennae distributed evenly in the hemangioma (arrows). (c) Axial contrast-enhanced CT image obtained after 15 minutes of ablation at 65 W shows complete ablation of the tumor, with a small margin of normal hepatic tissue (arrow). Note the hydrodissection fluid (arrowhead), which was used to protect the abdominal wall and diaphragm.
Figure 6b.
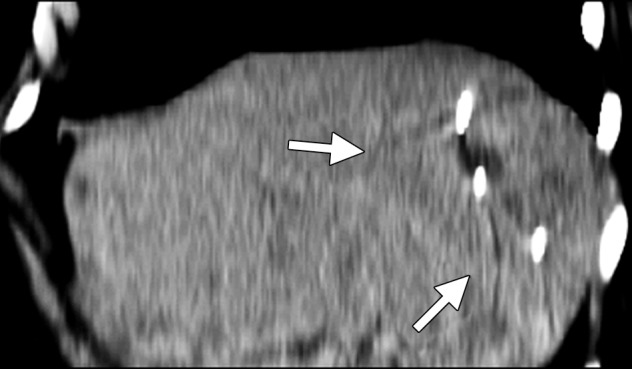
CT images in a symptomatic 37-year-old woman. (a) Axial contrast-enhanced portal venous phase CT image shows an 8.8 × 8.7-cm giant hemangioma in the right hepatic lobe (arrows). (b) Sagittal unenhanced CT image shows the hemangioma targeted for microwave ablation, with three 17-gauge gas-cooled microwave antennae distributed evenly in the hemangioma (arrows). (c) Axial contrast-enhanced CT image obtained after 15 minutes of ablation at 65 W shows complete ablation of the tumor, with a small margin of normal hepatic tissue (arrow). Note the hydrodissection fluid (arrowhead), which was used to protect the abdominal wall and diaphragm.
Figure 6c.
CT images in a symptomatic 37-year-old woman. (a) Axial contrast-enhanced portal venous phase CT image shows an 8.8 × 8.7-cm giant hemangioma in the right hepatic lobe (arrows). (b) Sagittal unenhanced CT image shows the hemangioma targeted for microwave ablation, with three 17-gauge gas-cooled microwave antennae distributed evenly in the hemangioma (arrows). (c) Axial contrast-enhanced CT image obtained after 15 minutes of ablation at 65 W shows complete ablation of the tumor, with a small margin of normal hepatic tissue (arrow). Note the hydrodissection fluid (arrowhead), which was used to protect the abdominal wall and diaphragm.
Of note, the absence of a specific Current Procedural Terminology code for microwave ablation somewhat limited early adoption of this modality. However, both the American College of Radiology and the Society of Interventional Radiology have subsequently addressed this issue and specifically do not recommend the use of unlisted procedure codes. Rather, because microwaves are part of the RF spectrum, they recommend the use of the appropriate RF ablation code based on anatomic location.
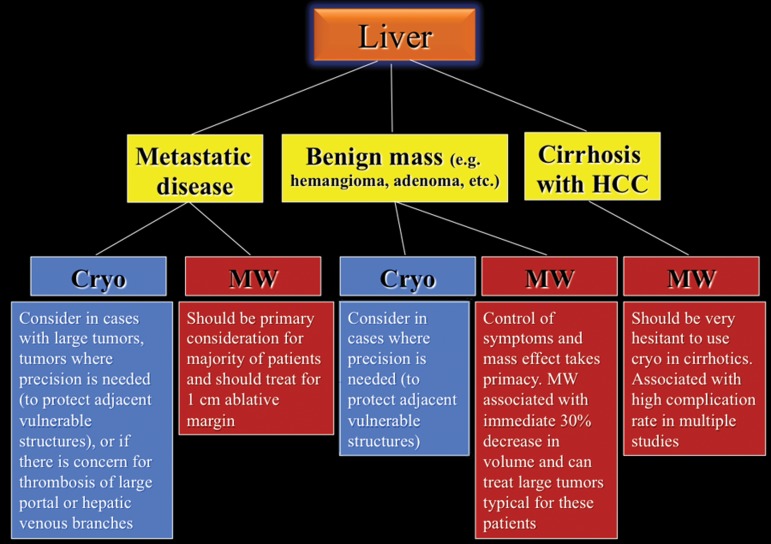
A proposed decision-making algorithm for liver ablation is shown in Figure 7.
Figure 7.
Flowchart shows the recommended modality choices for liver ablation. Cryo = cryoablation, MW = microwave.
Kidney.—Most tumors treated with ablation are renal cell carcinomas (RCCs), although a subset of patients with benign disease, such as oncocytoma and angiomyolipoma, may benefit from ablation for symptom control and prevention of complications (eg, mass effect and tumor-related hemorrhage) (73,74).
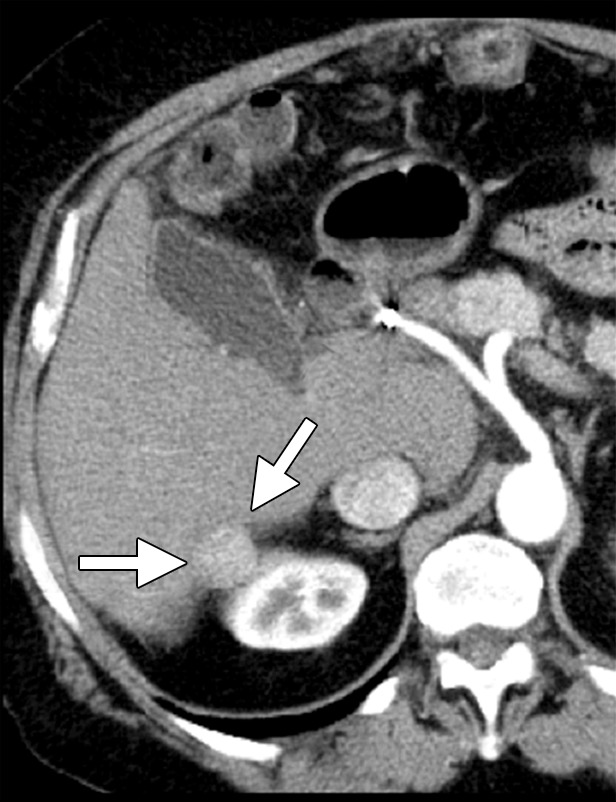
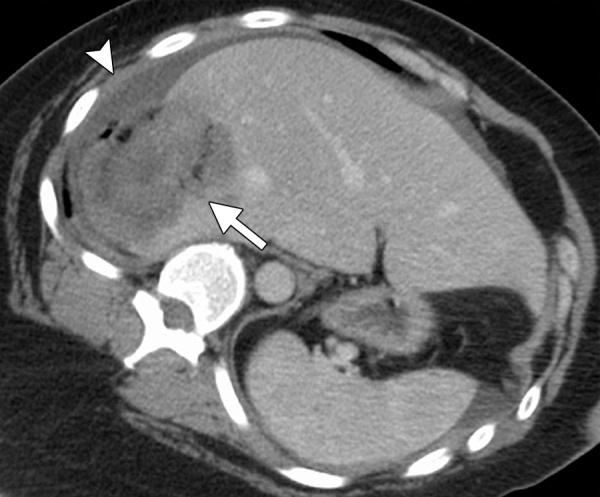
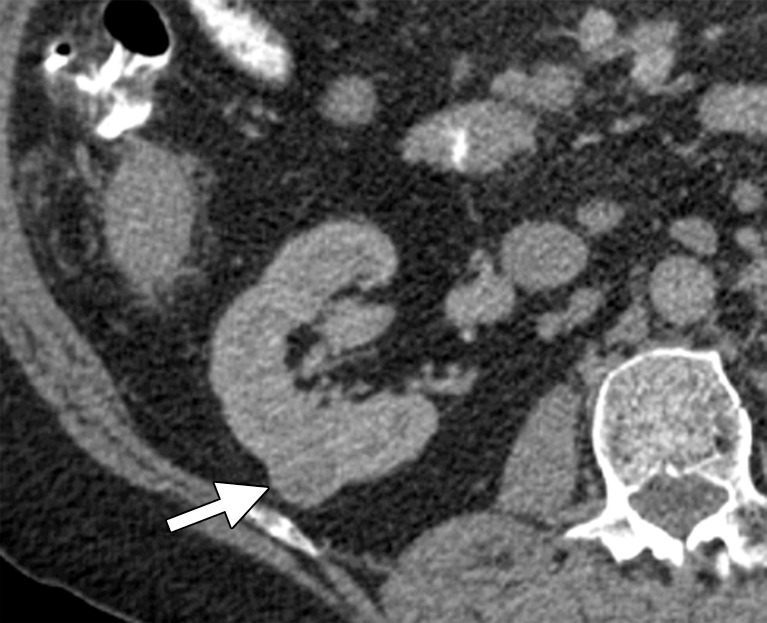
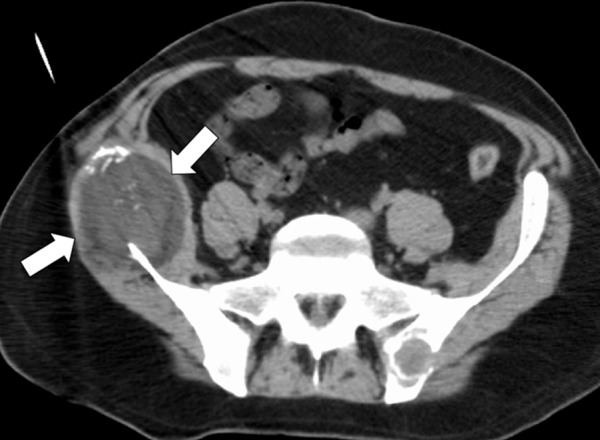
There are many ways to successfully ablate a renal tumor, and both RF ablation and cryoablation have proven successful for renal ablation (75). However, the most appropriate modality to use in the kidney depends on tumor size, tumor location, and proximity of vulnerable structures. As seen with the liver, microwave ablation has several theoretical advantages that make it our modality of choice for many renal tumors (57). With small exophytic masses, microwave ablation has the advantage of speed and the need for fewer applicators (Fig 8). However, central tumors and tumors adjacent to vulnerable structures may benefit from the precision and relative sparing of the collecting system and ureter that are associated with cryoablation (71,72,76). However, recall that the zone of cellular necrosis does not extend to the margin of the ice ball and, depending on multiple factors, may extend to 8 mm inside of the edge of the ice ball (50). Therefore, defining margins and performing a complete treatment may be more challenging than is generally appreciated. Tumors larger than 3–4 cm can be difficult to completely treat with ablation. These tumors may benefit from the larger ablation zones that are possible with both microwave ablation and cryoablation; therefore, the choice of modality may hinge on the ability to perform the ablation safely based on the proximity of adjacent structures or the ability to hydrodissect effectively (Fig 9). Note that hydrodissection plays a much larger role in renal ablations than in hepatic ablations because of the relatively close proximity of the bowel, pancreas, and ureter. In fact, more than 50% of our renal ablations require hydrodissection to allow safe ablation (77). The treatment of tumors adjacent to the ureter may benefit from the placement of a ureteral stent and pyeloperfusion in addition to hydrodissection.
Figure 8a.
CT and US images in a 63-year-old male patient with RCC. (a) Axial contrast-enhanced nephrographic phase CT image shows a 2.9-cm posterior exophytic RCC (arrow) that was confirmed at biopsy. The lesion was targeted for microwave ablation by using a single 17-gauge gas-cooled microwave antenna with US guidance. (b) Transverse US image during a 3-minute ablation with 90 W shows complete replacement of the tumor by hyperechoic gas bubbles (arrow). (c) Axial contrast-enhanced nephrographic phase postablation CT image shows complete ablation of the lesion, with an adequate ablative margin (arrows). Note the nonenhancing “tumor ghost” in the ablation zone (arrowhead).
Figure 9a.
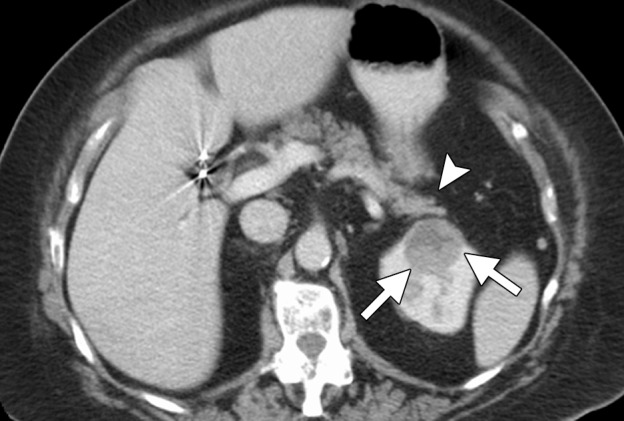
CT images in a 69-year-old woman with biopsy-proven RCC. (a, b) Axial CT images obtained with the patient in the supine (a) and decubitis (b) positions show a 4.0 × 3.5-cm RCC in the left anterior kidney (arrows). The pancreas (arrowhead) is closely adjacent to the RCC in a, which increases the risk associated with heat-based ablation, and the relationship does not change in b with the patient in the decubitus position. The RCC was targeted for cryoablation with US guidance. (c) Axial contrast-enhanced CT image shows that hydrodissection (high-attenuation fluid) was used to displace the pancreas (arrowhead), and the RCC was ablated successfully with three 15-gauge cryoprobes and a 10-, 5-, 10-minute freeze-thaw-freeze protocol. Note that the visibility of the ice ball (arrows) makes the ablation precise. (d) On an axial 6-month follow-up CT image, the RCC (arrows) shows no substantial enhancement and has decreased in size, as expected. The patient had no evidence of pancreatitis or other complications.
Figure 8b.
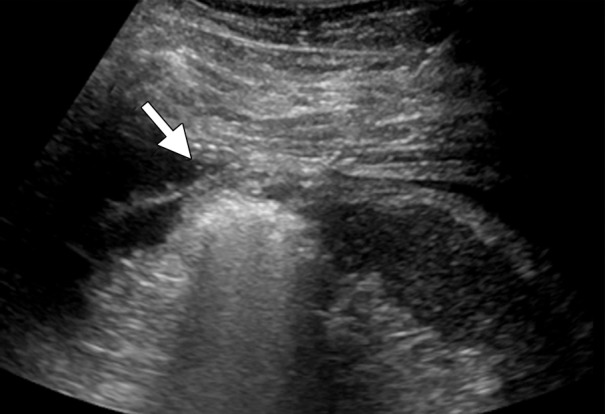
CT and US images in a 63-year-old male patient with RCC. (a) Axial contrast-enhanced nephrographic phase CT image shows a 2.9-cm posterior exophytic RCC (arrow) that was confirmed at biopsy. The lesion was targeted for microwave ablation by using a single 17-gauge gas-cooled microwave antenna with US guidance. (b) Transverse US image during a 3-minute ablation with 90 W shows complete replacement of the tumor by hyperechoic gas bubbles (arrow). (c) Axial contrast-enhanced nephrographic phase postablation CT image shows complete ablation of the lesion, with an adequate ablative margin (arrows). Note the nonenhancing “tumor ghost” in the ablation zone (arrowhead).
Figure 8c.
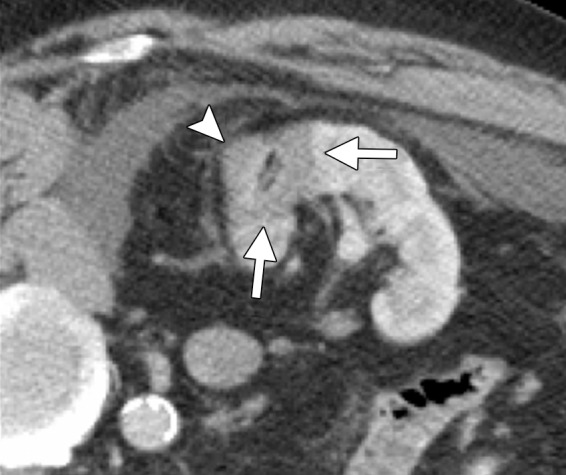
CT and US images in a 63-year-old male patient with RCC. (a) Axial contrast-enhanced nephrographic phase CT image shows a 2.9-cm posterior exophytic RCC (arrow) that was confirmed at biopsy. The lesion was targeted for microwave ablation by using a single 17-gauge gas-cooled microwave antenna with US guidance. (b) Transverse US image during a 3-minute ablation with 90 W shows complete replacement of the tumor by hyperechoic gas bubbles (arrow). (c) Axial contrast-enhanced nephrographic phase postablation CT image shows complete ablation of the lesion, with an adequate ablative margin (arrows). Note the nonenhancing “tumor ghost” in the ablation zone (arrowhead).
Figure 9b.
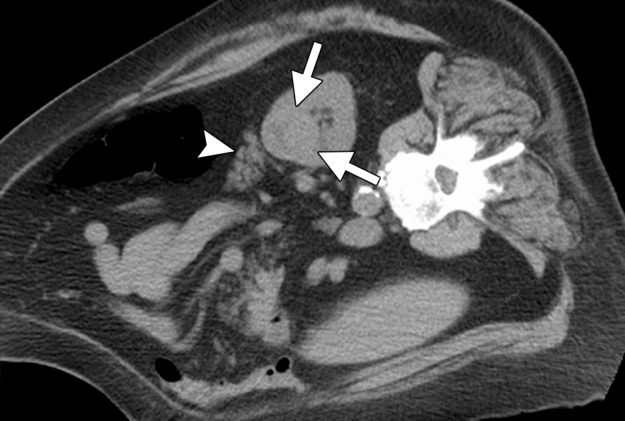
CT images in a 69-year-old woman with biopsy-proven RCC. (a, b) Axial CT images obtained with the patient in the supine (a) and decubitis (b) positions show a 4.0 × 3.5-cm RCC in the left anterior kidney (arrows). The pancreas (arrowhead) is closely adjacent to the RCC in a, which increases the risk associated with heat-based ablation, and the relationship does not change in b with the patient in the decubitus position. The RCC was targeted for cryoablation with US guidance. (c) Axial contrast-enhanced CT image shows that hydrodissection (high-attenuation fluid) was used to displace the pancreas (arrowhead), and the RCC was ablated successfully with three 15-gauge cryoprobes and a 10-, 5-, 10-minute freeze-thaw-freeze protocol. Note that the visibility of the ice ball (arrows) makes the ablation precise. (d) On an axial 6-month follow-up CT image, the RCC (arrows) shows no substantial enhancement and has decreased in size, as expected. The patient had no evidence of pancreatitis or other complications.
Figure 9c.
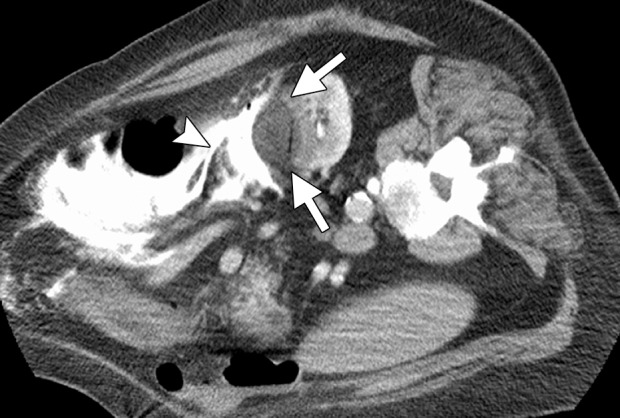
CT images in a 69-year-old woman with biopsy-proven RCC. (a, b) Axial CT images obtained with the patient in the supine (a) and decubitis (b) positions show a 4.0 × 3.5-cm RCC in the left anterior kidney (arrows). The pancreas (arrowhead) is closely adjacent to the RCC in a, which increases the risk associated with heat-based ablation, and the relationship does not change in b with the patient in the decubitus position. The RCC was targeted for cryoablation with US guidance. (c) Axial contrast-enhanced CT image shows that hydrodissection (high-attenuation fluid) was used to displace the pancreas (arrowhead), and the RCC was ablated successfully with three 15-gauge cryoprobes and a 10-, 5-, 10-minute freeze-thaw-freeze protocol. Note that the visibility of the ice ball (arrows) makes the ablation precise. (d) On an axial 6-month follow-up CT image, the RCC (arrows) shows no substantial enhancement and has decreased in size, as expected. The patient had no evidence of pancreatitis or other complications.
Figure 9d.
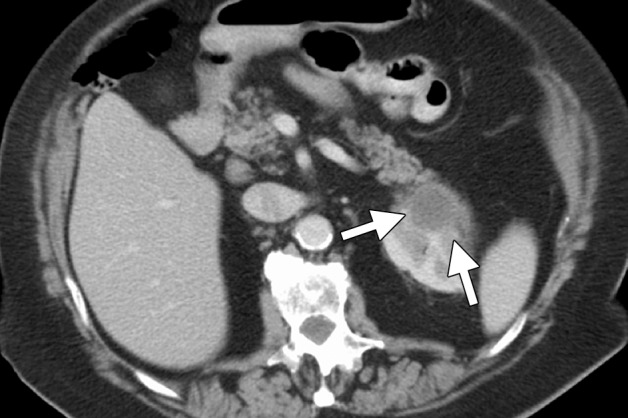
CT images in a 69-year-old woman with biopsy-proven RCC. (a, b) Axial CT images obtained with the patient in the supine (a) and decubitis (b) positions show a 4.0 × 3.5-cm RCC in the left anterior kidney (arrows). The pancreas (arrowhead) is closely adjacent to the RCC in a, which increases the risk associated with heat-based ablation, and the relationship does not change in b with the patient in the decubitus position. The RCC was targeted for cryoablation with US guidance. (c) Axial contrast-enhanced CT image shows that hydrodissection (high-attenuation fluid) was used to displace the pancreas (arrowhead), and the RCC was ablated successfully with three 15-gauge cryoprobes and a 10-, 5-, 10-minute freeze-thaw-freeze protocol. Note that the visibility of the ice ball (arrows) makes the ablation precise. (d) On an axial 6-month follow-up CT image, the RCC (arrows) shows no substantial enhancement and has decreased in size, as expected. The patient had no evidence of pancreatitis or other complications.
The primary indication for the treatment of renal angiomyolipomas is to decrease the associated risk for future hemorrhage (73,74). In this setting, we prefer heat-based ablation because of the associated cautery effect, even with relatively large vessels. In our experience, RF is limited by the high impedance associated with these tumors, presumably related to the insulative effects of fat. When ablation of angiomyolipomas is performed, the solid soft-tissue nodules and larger feeding vessels can be specifically targeted and ablated, markedly reducing the risk for future hemorrhage (Fig 10).
Figure 10a.
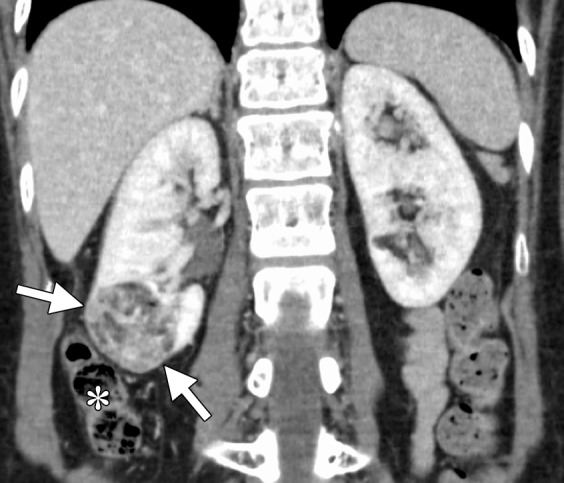
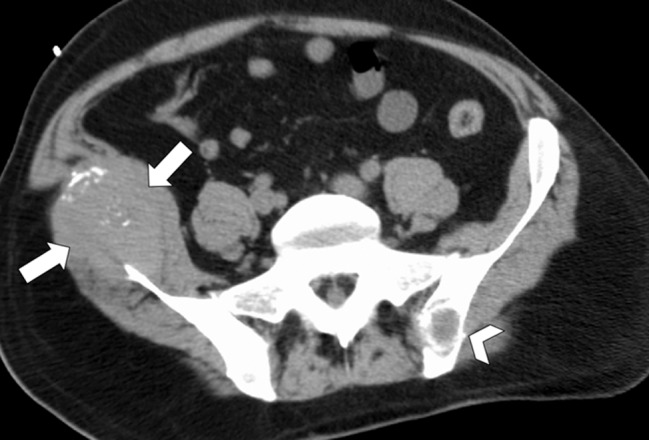
CT images in a 55-year-old woman with a history of right renal angiomyolipoma with associated massive retroperitoneal hemorrhage that required immediate embolization. (a) Coronal reconstructed image from follow-up contrast-enhanced CT shows continued avid enhancement of the lesion (arrows). Note the closely adjacent colon (*). After successful hydrodissection for displacement of the colon, the lesion was targeted for microwave ablation with three 17-gauge gas-cooled antennae at 65 W for 5 minutes. (b) Coronal reconstructed image from contrast-enhanced CT 4 months after ablation shows minimal, if any, residual enhancement of the lesion (arrows). The patient has remained asymptomatic.
Figure 10b.
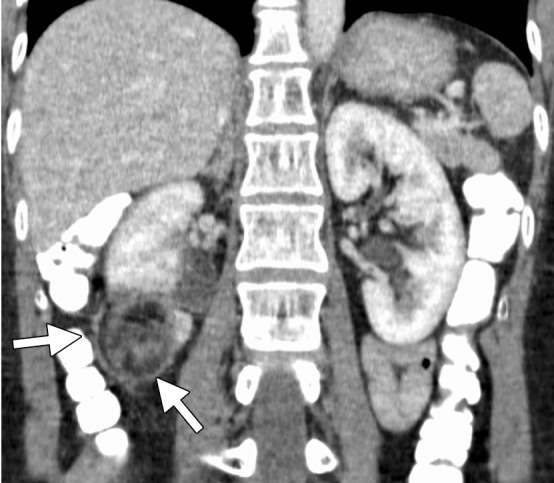
CT images in a 55-year-old woman with a history of right renal angiomyolipoma with associated massive retroperitoneal hemorrhage that required immediate embolization. (a) Coronal reconstructed image from follow-up contrast-enhanced CT shows continued avid enhancement of the lesion (arrows). Note the closely adjacent colon (*). After successful hydrodissection for displacement of the colon, the lesion was targeted for microwave ablation with three 17-gauge gas-cooled antennae at 65 W for 5 minutes. (b) Coronal reconstructed image from contrast-enhanced CT 4 months after ablation shows minimal, if any, residual enhancement of the lesion (arrows). The patient has remained asymptomatic.
In summary, renal tumors smaller than 3 cm in diameter can be ablated successfully with both RF ablation and cryoablation. Emerging data on microwave ablation for RCC is promising and is the preferred method in our practice for treating peripheral renal tumors because of speed, high temperatures, and cautery. For central renal tumors, we favor cryoablation because of the theoretical decreased risk for injury to the collecting system and ureter.
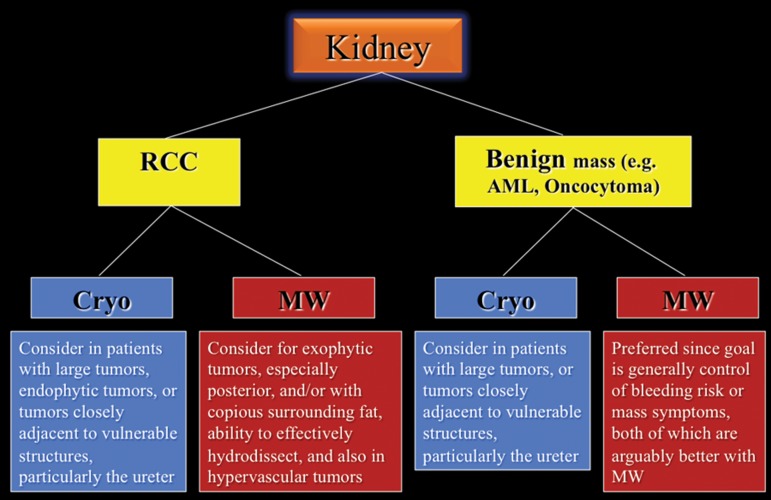
A proposed decision-making algorithm for kidney ablation is shown in Figure 11.
Figure 11.
Flowchart shows the recommended modality choices for kidney ablation. AML = angiomyolipoma, Cryo = cryoablation, MW = microwave.
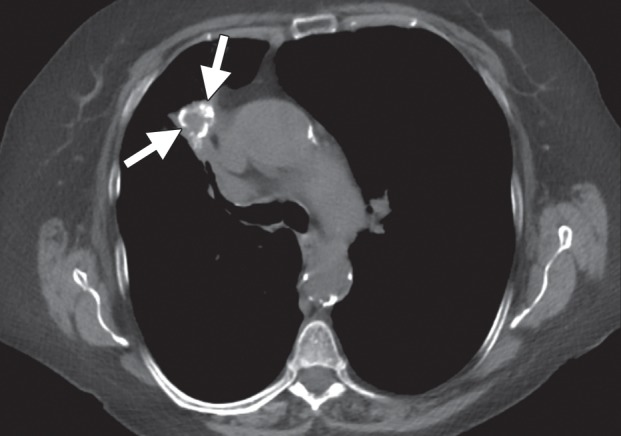
Lung.—Most patients are referred for image-guided ablation of nonoperable non–small-cell lung carcinoma or metastases to the lung, wherein tumor eradication would increase survival or control symptoms. The choice between stereotactic body radiation therapy and tumor ablation for T1a non–small-cell lung carcinoma remains controversial, and few investigators have compared these two modalities (78). Patients with non–small-cell lung carcinoma often have associated cardiopulmonary disease due to a long smoking history, and percutaneous procedures in patients with emphysema are associated with a higher complication rate than that seen in patients with normal background lung tissue. However, most studies of percutaneous RF ablation, microwave ablation, and cryoablation in patients with non–small-cell lung carcinoma demonstrate low rates of serious complications (79,80).
Most studies of tumor ablation in the lung have concerned RF ablation, although several investigators have assessed microwave ablation and cryoablation (4,79,81). The results with RF ablation in the lung have been mixed, perhaps because of the inherent limitations to the conduction of electric current in the presence of aerated lung tissue. Air is an insulator, resulting in high electric impedance that limits current flow and tissue heating. These physical limitations can produce poor clinical results, even with correctly placed electrodes in small tumors (Fig 12) (82). Cryoablation can effectively ablate tumors in the lung and has the advantage of being relatively resistant to the cold-sink effects of ventilation, compared with RF ablation (83,84). The effect has not been studied in microwave ablation as of yet, but according to animal studies, microwave ablation seems to be less susceptible to these effects and creates a larger and more confluent zone of ablation compared with RF ablation and cryoablation (28). Another advantage of cryoablation is seen with tumors that are relatively central or closely adjacent to or invasive into the mediastinum or chest wall (Fig 13). Although patients who have undergone previous radiation therapy and experience recurrent or new disease in the radiation field are part of an interesting potential patient population for this procedure, the ablation zone may be larger than expected in these patients (probably because of abnormal perfusion), and the complication rate may be significantly higher (because of disordered vasculature and poor tissue healing). Therefore, although these patients may benefit from ablation, it is important to be aware of the increased risk. Cryoablation is associated with minimal, if any, postprocedural pain, even when the ablation extends into the chest wall. The relative rate of complications and the oncologic effectiveness of the various modalities have not been established definitively.
Figure 12a.
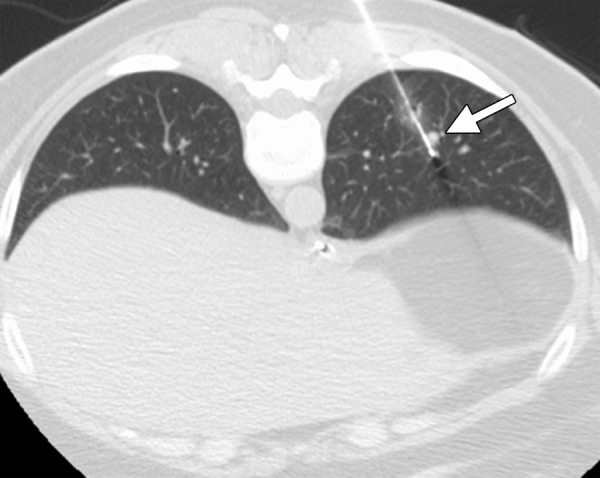
CT images in a 54-year-old woman with a history of metastatic colorectal cancer. (a) Axial CT image shows a 6-mm biopsy-proven metastatic focus in the left lower lobe of the lung (arrow) that was targeted for RF ablation. A single 17-gauge water-cooled electrode was positioned in the medial aspect of the nodule, and a 12-minute ablation was performed. (b) Axial CT image obtained 6 months after ablation shows rapid local tumor progression, with the metastatic focus now measuring 1.6 cm. This area was targeted for repeat RF ablation by using a water-cooled cluster electrode. The repeat ablation resulted in long-term local control but was complicated by a large hemothorax that ultimately required surgical intervention. The hemothorax was at least partially related to the increased invasiveness of the cluster electrode.
Figure 13a.
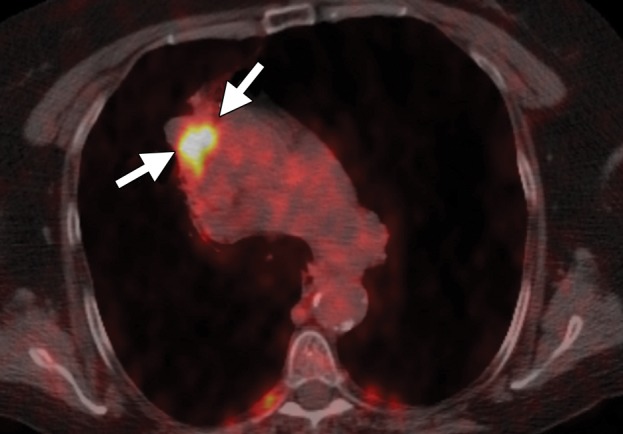
Imaging findings in a 71-year-old woman with a history of non–small-cell lung carcinoma who had undergone radiation therapy 2 years previously. (a) Axial positron emission tomography (PET)/CT image shows recurrent disease in the radiation field (arrows). The patient was referred for ablation. Cryoablation was chosen because of the close proximity of the mass to the mediastinum and the associated risk for injury to the phrenic nerve. (b) Axial CT image shows the four cryoprobes (arrow) used to perform the ablation, which involved a 3-, 5-, 7-, 5-, 5-minute freeze-thaw protocol. This resulted in excellent coverage with the ice ball (arrowhead). Phrenic nerve palsy developed, with associated diaphragmatic paralysis. Combined with poor baseline lung function, this complication resulted in a prolonged postablation hospitalization. Within 5 weeks, however, both diaphragmatic function and respiratory status returned to baseline levels. (c) Axial nonenhanced CT image obtained 18 months after ablation shows calcification of the treated mass (arrows), with no evidence of local or distant recurrence.
Figure 12b.
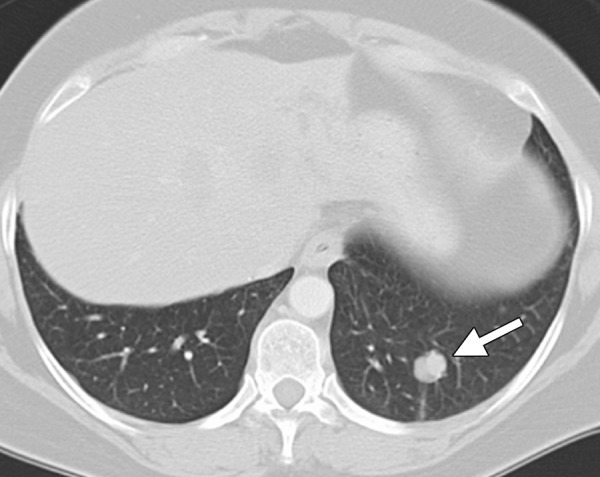
CT images in a 54-year-old woman with a history of metastatic colorectal cancer. (a) Axial CT image shows a 6-mm biopsy-proven metastatic focus in the left lower lobe of the lung (arrow) that was targeted for RF ablation. A single 17-gauge water-cooled electrode was positioned in the medial aspect of the nodule, and a 12-minute ablation was performed. (b) Axial CT image obtained 6 months after ablation shows rapid local tumor progression, with the metastatic focus now measuring 1.6 cm. This area was targeted for repeat RF ablation by using a water-cooled cluster electrode. The repeat ablation resulted in long-term local control but was complicated by a large hemothorax that ultimately required surgical intervention. The hemothorax was at least partially related to the increased invasiveness of the cluster electrode.
Figure 13b.
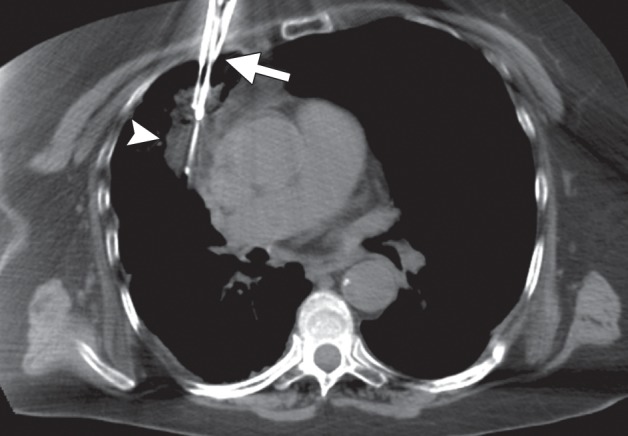
Imaging findings in a 71-year-old woman with a history of non–small-cell lung carcinoma who had undergone radiation therapy 2 years previously. (a) Axial positron emission tomography (PET)/CT image shows recurrent disease in the radiation field (arrows). The patient was referred for ablation. Cryoablation was chosen because of the close proximity of the mass to the mediastinum and the associated risk for injury to the phrenic nerve. (b) Axial CT image shows the four cryoprobes (arrow) used to perform the ablation, which involved a 3-, 5-, 7-, 5-, 5-minute freeze-thaw protocol. This resulted in excellent coverage with the ice ball (arrowhead). Phrenic nerve palsy developed, with associated diaphragmatic paralysis. Combined with poor baseline lung function, this complication resulted in a prolonged postablation hospitalization. Within 5 weeks, however, both diaphragmatic function and respiratory status returned to baseline levels. (c) Axial nonenhanced CT image obtained 18 months after ablation shows calcification of the treated mass (arrows), with no evidence of local or distant recurrence.
Figure 13c.
Imaging findings in a 71-year-old woman with a history of non–small-cell lung carcinoma who had undergone radiation therapy 2 years previously. (a) Axial positron emission tomography (PET)/CT image shows recurrent disease in the radiation field (arrows). The patient was referred for ablation. Cryoablation was chosen because of the close proximity of the mass to the mediastinum and the associated risk for injury to the phrenic nerve. (b) Axial CT image shows the four cryoprobes (arrow) used to perform the ablation, which involved a 3-, 5-, 7-, 5-, 5-minute freeze-thaw protocol. This resulted in excellent coverage with the ice ball (arrowhead). Phrenic nerve palsy developed, with associated diaphragmatic paralysis. Combined with poor baseline lung function, this complication resulted in a prolonged postablation hospitalization. Within 5 weeks, however, both diaphragmatic function and respiratory status returned to baseline levels. (c) Axial nonenhanced CT image obtained 18 months after ablation shows calcification of the treated mass (arrows), with no evidence of local or distant recurrence.
Microwave ablation has the advantage of creating predictable and large ablation zones, even in the setting of aerated lung (28). The advantage of a faster procedure is also true in the lungs—most ablations require approximately 5 minutes after antenna placement. Physicians should keep in mind several important considerations when performing RF and microwave ablation of the lungs. First, unlike standard lung biopsy technique, in which the shortest possible path to the tumor is often desirable, a longer approach that traverses normal lung is better for two reasons: (a) The relatively long ablation zone associated with certain microwave ablation devices (as long as 6 cm, depending on the antenna design) means that a direct puncture can be associated with ablation zones that extend into the chest wall, resulting in pain and potential skin burns, and (b) the ablated tumor or lung becomes dehydrated and noncompliant, which, in combination with volume loss, can result in retraction of the antenna or electrode insertion site from the pleural surface, potentially resulting in persistent air leaks and bronchopleural fistulas (85). Therefore, an indirect approach that leaves an unablated tract of at least 2 cm of aerated lung is preferred (Fig 14). Note that tract cautery should not be performed in the lung because of the risk for persistent air leak. Another important consideration is to recognize the large ablation zone that is possible with microwave ablation in the lung. Because third-generation microwave systems (we recommend using only third-generation systems) can create larger ablation zones, one can experience complications when higher powers or longer times are used for the ablation (Fig 15).
Figure 14a.
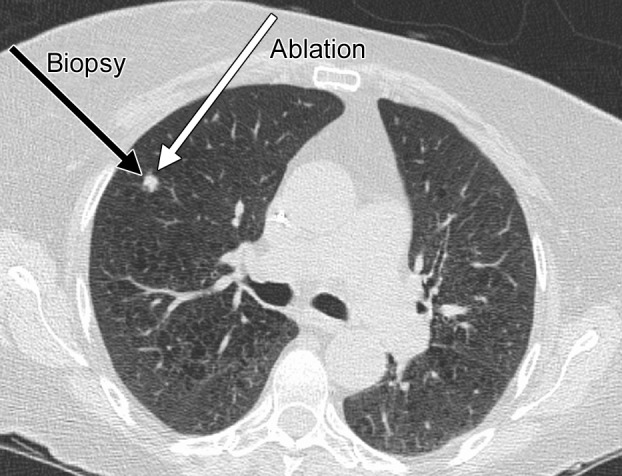
CT images in a 65-year-old female patient with a history of non–small-cell lung cancer. (a) Axial nonenhanced CT image shows an 8-mm focus of recurrent disease in the right upper lobe of the lung. Although it would be reasonable to perform a biopsy of the lesion with an approach perpendicular to the pleural surface (black arrow), thus minimizing the lung parenchyma traversed, or with a more tangential approach, it is important with microwave ablation to use an approach that allows for an adequate tract within the lung parenchyma (white arrow). This minimizes the likelihood of a persistent air leak or bronchopleural fistula. (b) Axial nonenhanced CT image shows the area of recurrent disease that was targeted for microwave ablation with CT guidance. A single 17-gauge gas-cooled microwave ablation antenna was positioned in the nodule (arrow), and a 5-minute ablation at 40–55 W was performed. Note the area of scarring from a microwave ablation performed 11 months previously (arrowhead). (c) Axial nonenhanced CT image shows that the procedure resulted in technically successful ablation, with complete coverage of the nodule by ground-glass opacity (arrow). A pneumothorax that developed at ablation was treated successfully during the procedure with a pleural blood patch. A chest tube was not required.
Figure 15a.
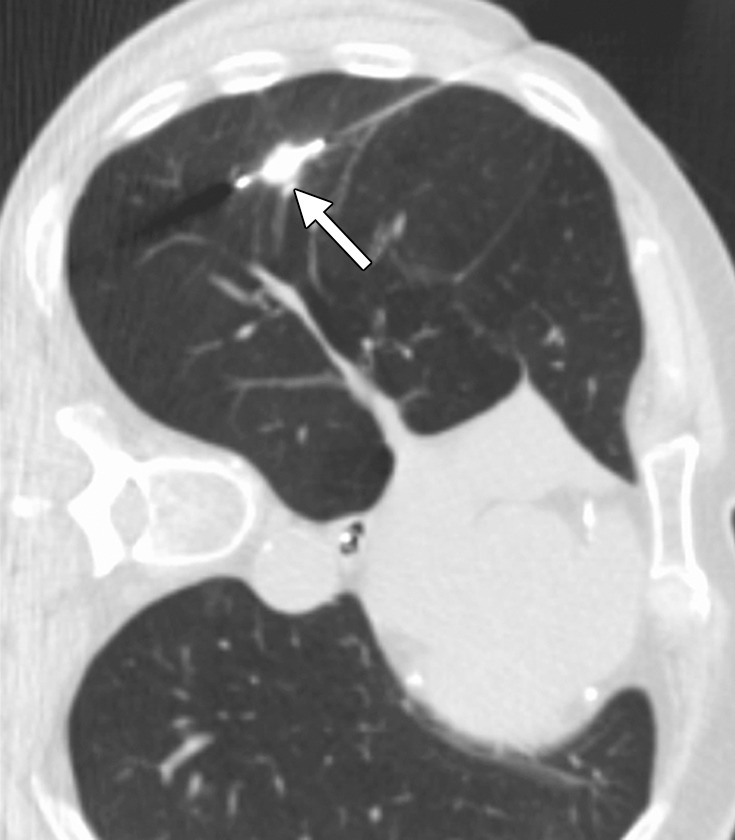
CT images in a 73-year-old man with a history of non–small-cell lung carcinoma who had undergone several resections. (a) Axial CT image shows a 2.0 × 1.2-cm PET-positive, biopsy-proven focus of recurrent disease (arrow). The area was targeted for microwave ablation with a single 17-gauge gas-cooled antenna at 140 W for 10 minutes. The longer time and high power were used because of previous difficulties with achieving complete ablation, even with small tumors, by using RF ablation. (b) Axial CT image shows the resulting near-complete vaporization of the small tumor and a 6.4 × 3.5-cm ablation zone (arrowheads). (c) Axial CT image obtained 5 months after ablation shows no evidence of local tumor progression, but the aggressive ablation led to the formation of a large area of parenchymal cavitation (arrows). This was associated with hemoptysis that lasted more than 6 months. As a result of this experience and subsequent animal studies, we have decreased the power and time used for microwave ablation of the lung.
Figure 14b.
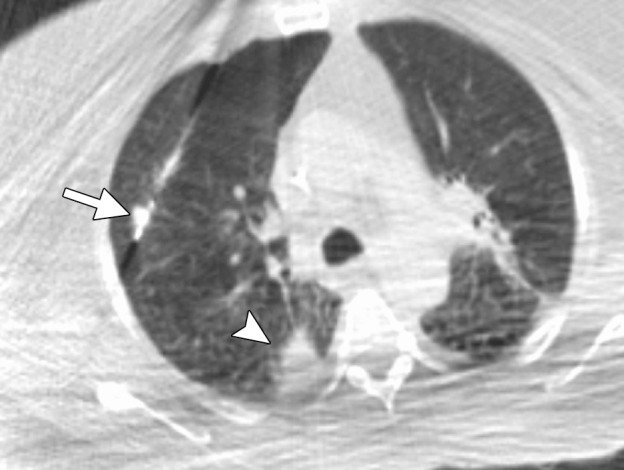
CT images in a 65-year-old female patient with a history of non–small-cell lung cancer. (a) Axial nonenhanced CT image shows an 8-mm focus of recurrent disease in the right upper lobe of the lung. Although it would be reasonable to perform a biopsy of the lesion with an approach perpendicular to the pleural surface (black arrow), thus minimizing the lung parenchyma traversed, or with a more tangential approach, it is important with microwave ablation to use an approach that allows for an adequate tract within the lung parenchyma (white arrow). This minimizes the likelihood of a persistent air leak or bronchopleural fistula. (b) Axial nonenhanced CT image shows the area of recurrent disease that was targeted for microwave ablation with CT guidance. A single 17-gauge gas-cooled microwave ablation antenna was positioned in the nodule (arrow), and a 5-minute ablation at 40–55 W was performed. Note the area of scarring from a microwave ablation performed 11 months previously (arrowhead). (c) Axial nonenhanced CT image shows that the procedure resulted in technically successful ablation, with complete coverage of the nodule by ground-glass opacity (arrow). A pneumothorax that developed at ablation was treated successfully during the procedure with a pleural blood patch. A chest tube was not required.
Figure 14c.
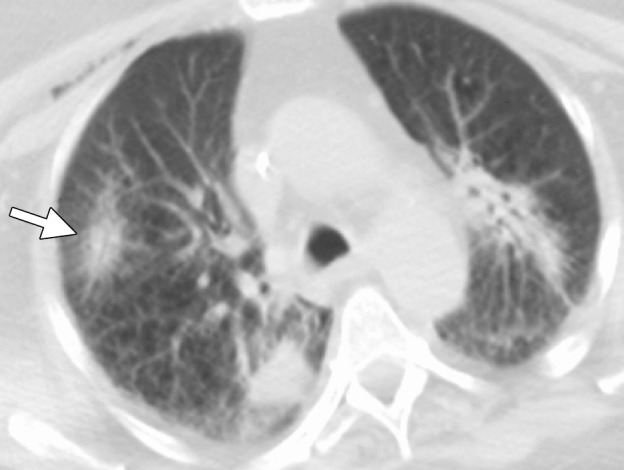
CT images in a 65-year-old female patient with a history of non–small-cell lung cancer. (a) Axial nonenhanced CT image shows an 8-mm focus of recurrent disease in the right upper lobe of the lung. Although it would be reasonable to perform a biopsy of the lesion with an approach perpendicular to the pleural surface (black arrow), thus minimizing the lung parenchyma traversed, or with a more tangential approach, it is important with microwave ablation to use an approach that allows for an adequate tract within the lung parenchyma (white arrow). This minimizes the likelihood of a persistent air leak or bronchopleural fistula. (b) Axial nonenhanced CT image shows the area of recurrent disease that was targeted for microwave ablation with CT guidance. A single 17-gauge gas-cooled microwave ablation antenna was positioned in the nodule (arrow), and a 5-minute ablation at 40–55 W was performed. Note the area of scarring from a microwave ablation performed 11 months previously (arrowhead). (c) Axial nonenhanced CT image shows that the procedure resulted in technically successful ablation, with complete coverage of the nodule by ground-glass opacity (arrow). A pneumothorax that developed at ablation was treated successfully during the procedure with a pleural blood patch. A chest tube was not required.
Figure 15b.
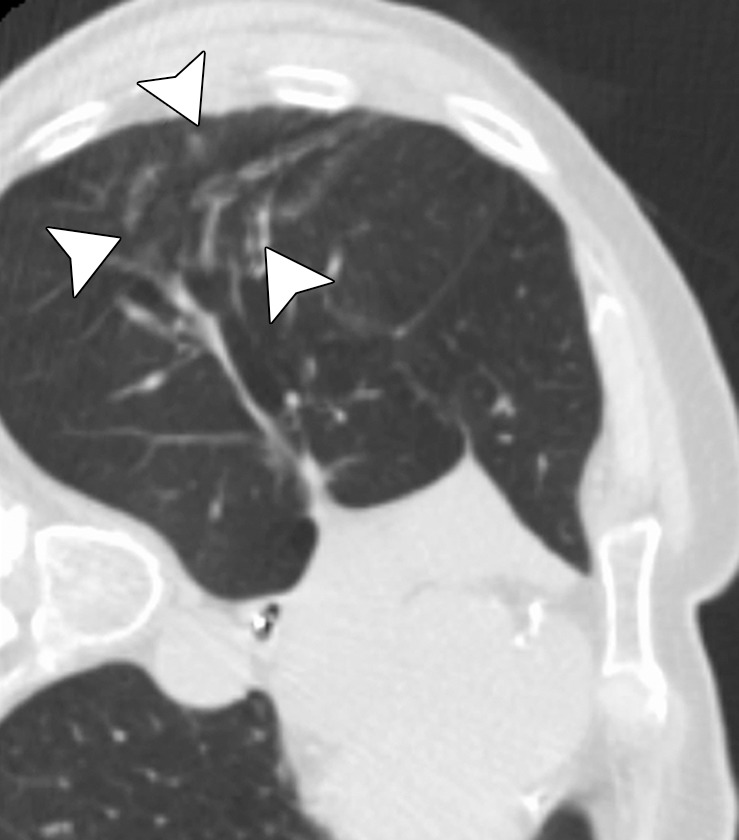
CT images in a 73-year-old man with a history of non–small-cell lung carcinoma who had undergone several resections. (a) Axial CT image shows a 2.0 × 1.2-cm PET-positive, biopsy-proven focus of recurrent disease (arrow). The area was targeted for microwave ablation with a single 17-gauge gas-cooled antenna at 140 W for 10 minutes. The longer time and high power were used because of previous difficulties with achieving complete ablation, even with small tumors, by using RF ablation. (b) Axial CT image shows the resulting near-complete vaporization of the small tumor and a 6.4 × 3.5-cm ablation zone (arrowheads). (c) Axial CT image obtained 5 months after ablation shows no evidence of local tumor progression, but the aggressive ablation led to the formation of a large area of parenchymal cavitation (arrows). This was associated with hemoptysis that lasted more than 6 months. As a result of this experience and subsequent animal studies, we have decreased the power and time used for microwave ablation of the lung.
Figure 15c.
CT images in a 73-year-old man with a history of non–small-cell lung carcinoma who had undergone several resections. (a) Axial CT image shows a 2.0 × 1.2-cm PET-positive, biopsy-proven focus of recurrent disease (arrow). The area was targeted for microwave ablation with a single 17-gauge gas-cooled antenna at 140 W for 10 minutes. The longer time and high power were used because of previous difficulties with achieving complete ablation, even with small tumors, by using RF ablation. (b) Axial CT image shows the resulting near-complete vaporization of the small tumor and a 6.4 × 3.5-cm ablation zone (arrowheads). (c) Axial CT image obtained 5 months after ablation shows no evidence of local tumor progression, but the aggressive ablation led to the formation of a large area of parenchymal cavitation (arrows). This was associated with hemoptysis that lasted more than 6 months. As a result of this experience and subsequent animal studies, we have decreased the power and time used for microwave ablation of the lung.
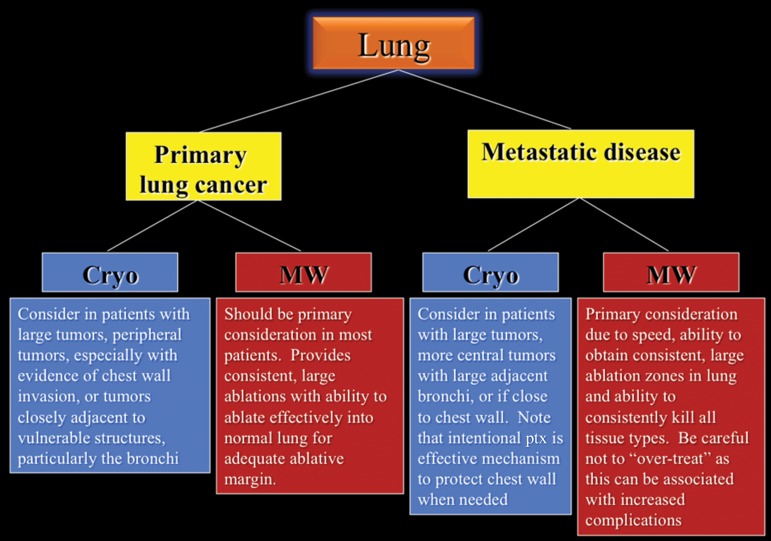
A proposed decision-making algorithm for lung ablation is shown in Figure 16.
Figure 16.
Flowchart shows the recommended modality choices for lung ablation. Cryo = cryoablation, MW = microwave, ptx = pneuomothorax.
Musculoskeletal System.—Pain is the most common indication for percutaneous ablation of musculoskeletal tumors. The use of ablation is widely accepted for both painful disease metastatic to bone and osteoid osteomas (5,86,87). Soft-tissue ablation is most frequently performed for metastatic disease and for palliative or curative intent, depending on the scenario (86).
Metastatic disease to the bone with associated intractable pain is regrettably common. Although radiation treatment is often considered the first-line therapy, it is expensive, inconvenient (requiring numerous treatments), and associated with a delay in onset of effect. In addition, repeat treatment with radiation is often limited by dose, whereas all ablation modalities can be repeated as needed. This and other factors make ablation an attractive alternative, and its effectiveness has been evaluated in many trials. The results of these trials suggest that although both heat-based ablation and cryoablation can be effective, cryoablation is associated with more rapid and durable pain relief. Therefore, cryoablation should be preferred in this setting and can be associated with exceptional results (Fig 17) (86,87).
Figure 17a.
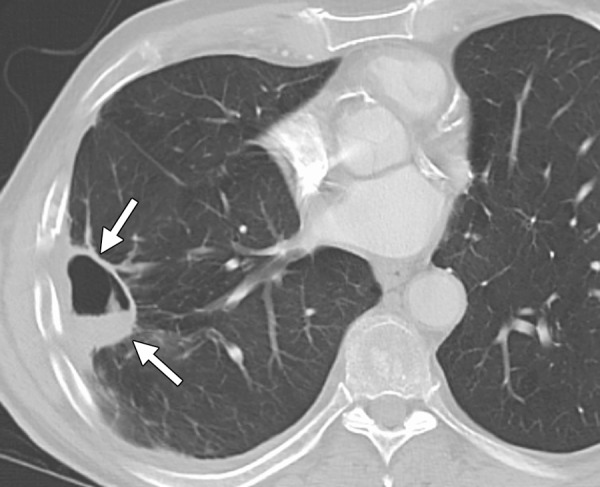
CT images in a 48-year-old man with a history of non–small-cell carcinoma of the lung who presented with a painful area of metastasis to the right iliac wing. (a) Axial nonenhanced CT image obtained before ablation shows a lytic expansile mass in the right iliac wing (arrows). Note the metastasis in the left posterior iliac wing (arrowhead), which was also targeted for cryoablation. Four 15-gauge cryoprobes were placed in the lesion with CT fluoroscopic guidance, and a 10-, 5-, 10-minute double freeze-thaw cycle was performed. (b) Axial nonenhanced CT image obtained during ablation shows the tumor completely enveloped in the ice ball (arrows). The patient’s pain rapidly decreased from a score of 5 of 10 on a visual analogue scale to a score of 2 of 10, and the patient survived another 18 months relatively free of pain from this site of disease. (Case courtesy of Kirkland Davis, MD, Department of Radiology, University of Wisconsin School of Medicine and Public Health, Madison, Wis.)
Figure 17b.
CT images in a 48-year-old man with a history of non–small-cell carcinoma of the lung who presented with a painful area of metastasis to the right iliac wing. (a) Axial nonenhanced CT image obtained before ablation shows a lytic expansile mass in the right iliac wing (arrows). Note the metastasis in the left posterior iliac wing (arrowhead), which was also targeted for cryoablation. Four 15-gauge cryoprobes were placed in the lesion with CT fluoroscopic guidance, and a 10-, 5-, 10-minute double freeze-thaw cycle was performed. (b) Axial nonenhanced CT image obtained during ablation shows the tumor completely enveloped in the ice ball (arrows). The patient’s pain rapidly decreased from a score of 5 of 10 on a visual analogue scale to a score of 2 of 10, and the patient survived another 18 months relatively free of pain from this site of disease. (Case courtesy of Kirkland Davis, MD, Department of Radiology, University of Wisconsin School of Medicine and Public Health, Madison, Wis.)
Percutaneous ablation of osteoid osteomas is now considered standard therapy because of a high success rate with minimal complications (5). Treatment of osteoid osteomas is almost always performed with RF ablation because of the ability to create a small and short ablation zone (approximately 1 cm), which is adequate for optimal outcomes and results in almost no associated collateral damage. Percutaneous ablation for osteoid osteoma can even be performed in difficult-to-access locations with minimal morbidity (Fig 18). Microwave ablation may have a role in the future as antennae with shorter ablation zones become available.
Figure 18a.
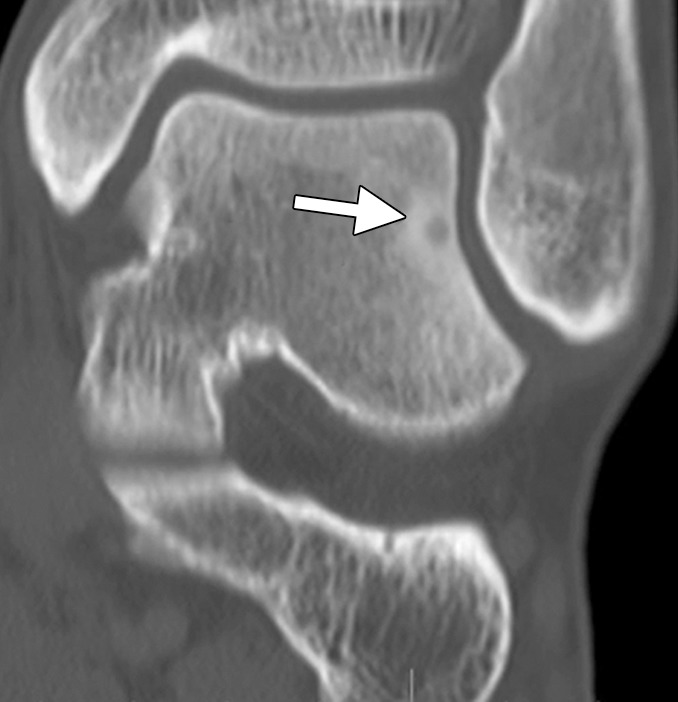
CT images in a 16-year-old male patient with lateral ankle pain. (a) Coronal nonenhanced CT image shows an osteoid osteoma of the lateral talar dome (arrow). (b) Because a surgical approach would be associated with substantial potential morbidity, the osteoid osteoma was targeted for treatment with RF ablation. As shown on an axial CT image, a single 17-gauge electrode with a 1-cm active tip (arrow) was placed in the lesion with CT fluoroscopic guidance, and a 6-minute ablation was performed. The symptoms completely resolved, and the patient remains asymptomatic. (Case courtesy of Kirkland Davis, MD.)
Figure 18b.
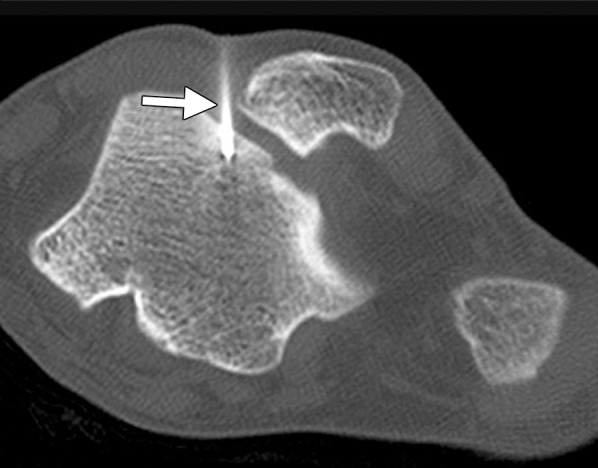
CT images in a 16-year-old male patient with lateral ankle pain. (a) Coronal nonenhanced CT image shows an osteoid osteoma of the lateral talar dome (arrow). (b) Because a surgical approach would be associated with substantial potential morbidity, the osteoid osteoma was targeted for treatment with RF ablation. As shown on an axial CT image, a single 17-gauge electrode with a 1-cm active tip (arrow) was placed in the lesion with CT fluoroscopic guidance, and a 6-minute ablation was performed. The symptoms completely resolved, and the patient remains asymptomatic. (Case courtesy of Kirkland Davis, MD.)
For soft-tissue ablation, the decision about which modality to use primarily depends on the location of the tumor and adjacent structures. Encompassing a nerve in a cryoablation zone is associated with regeneration of nerve function over time (88). As is seen with ablation in other tissues, the visibility of the ice ball can be an advantage in certain anatomic situations. Heat-based ablation does not allow as definitive a delineation of the ablation zone and can therefore be associated with damage to adjacent vulnerable structures (Fig 19). The advantages of microwave ablation for the treatment of soft-tissue tumors include speed, the ability to create a large ablation zone, and the ability to heat and ablate nearly every tumor type. This characteristic of microwave ablation may be particularly important in the treatment of fibrous tumors, such as sarcomas, which may be resistant to cryoablation, and tumors with closely adjacent bone or vascular heat sinks (Fig 20).
Figure 19a.
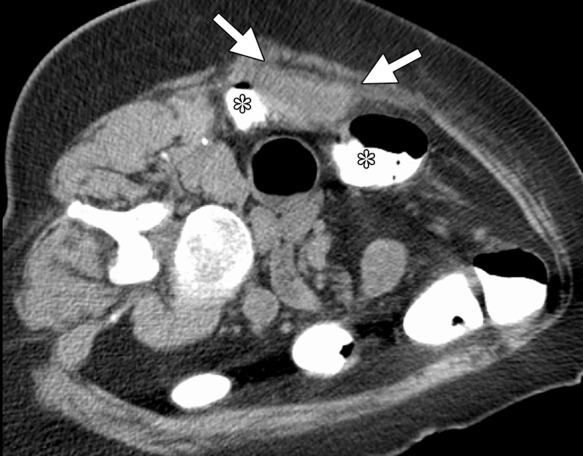
CT images in a 62-year-old woman with a history of multiple episodes of locally recurrent RCC and two previous surgical resections. (a) Axial nonenhanced CT image obtained before image-guided tumor ablation shows a 4.5 × 2.6-cm site of recurrent disease (arrows) positioned immediately between two loops of colon filled with positive oral contrast material (*) near the hepatic flexure. (b) Axial CT image shows that aggressive hydrodissection with two separate needles (arrows) increased the distance between the mass (arrowhead) and the adjacent loops of colon (*). The tumor was targeted for microwave ablation with two 17-gauge gas-cooled antennae at 65 W for 3 minutes. Other retroperitoneal sites of disease were also treated during the same session. Fever, flank pain, and erythema developed in the days after the procedure. (c) Planar fluoroscopic spot image obtained during an enema examination with Gastrografin (Bracco Diagnostic, Princeton, NJ) helped confirm a contained colon leak (arrows). This was treated successfully with percutaneous drainage.
Figure 20a.
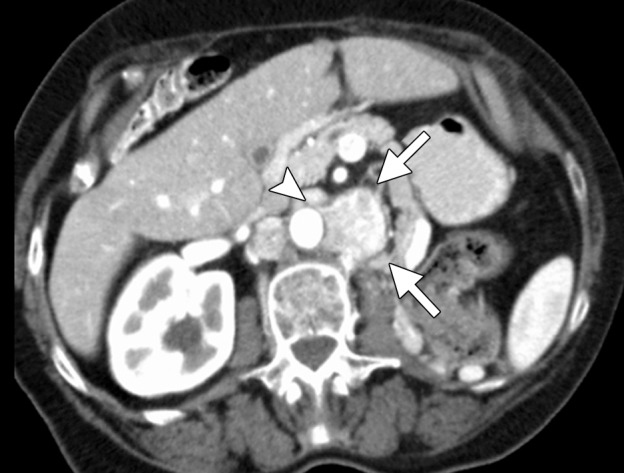
CT images in a 71-year-old woman with a history of RCC of the left kidney that was resected 13 years previously. (a) Axial contrast-enhanced CT image shows recurrent retroperitoneal disease (arrows). The tumor is partially encasing and wrapping anterior to the aorta (arrowhead). This would be problematic with RF ablation because of heat-sink effects and associated tumor sparing. The tumor was targeted for ablation with three microwave antennae for 5 minutes at 50 W. (b) Axial contrast-enhanced CT image obtained at 1-month follow-up shows complete ablation of the tumor, with an adequate margin (arrows). In addition, the tumor that was encasing and anterior to the aorta has been stripped off the aorta and completely ablated (arrowhead).
Figure 19b.
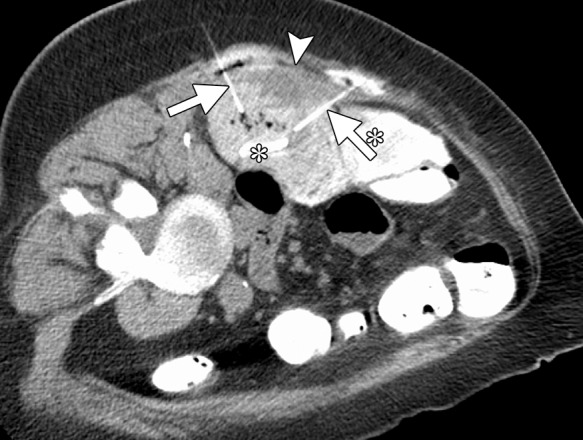
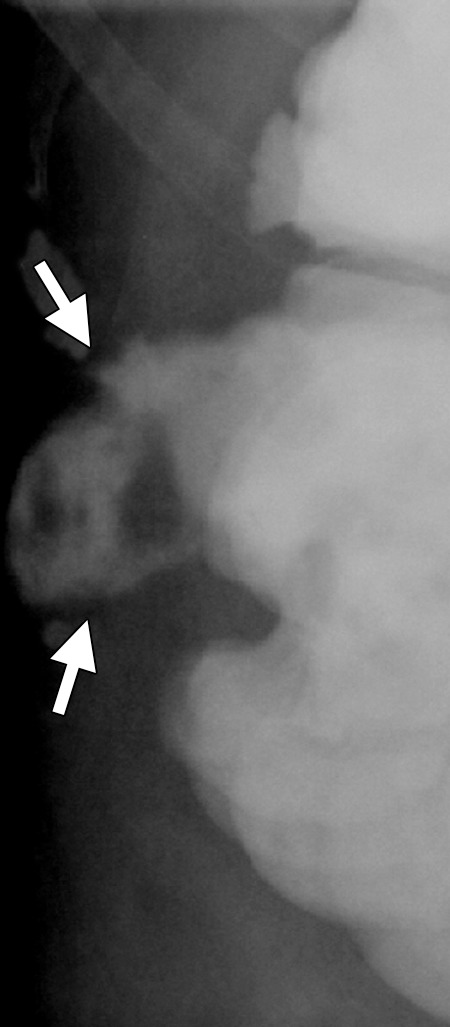
CT images in a 62-year-old woman with a history of multiple episodes of locally recurrent RCC and two previous surgical resections. (a) Axial nonenhanced CT image obtained before image-guided tumor ablation shows a 4.5 × 2.6-cm site of recurrent disease (arrows) positioned immediately between two loops of colon filled with positive oral contrast material (*) near the hepatic flexure. (b) Axial CT image shows that aggressive hydrodissection with two separate needles (arrows) increased the distance between the mass (arrowhead) and the adjacent loops of colon (*). The tumor was targeted for microwave ablation with two 17-gauge gas-cooled antennae at 65 W for 3 minutes. Other retroperitoneal sites of disease were also treated during the same session. Fever, flank pain, and erythema developed in the days after the procedure. (c) Planar fluoroscopic spot image obtained during an enema examination with Gastrografin (Bracco Diagnostic, Princeton, NJ) helped confirm a contained colon leak (arrows). This was treated successfully with percutaneous drainage.
Figure 19c.
CT images in a 62-year-old woman with a history of multiple episodes of locally recurrent RCC and two previous surgical resections. (a) Axial nonenhanced CT image obtained before image-guided tumor ablation shows a 4.5 × 2.6-cm site of recurrent disease (arrows) positioned immediately between two loops of colon filled with positive oral contrast material (*) near the hepatic flexure. (b) Axial CT image shows that aggressive hydrodissection with two separate needles (arrows) increased the distance between the mass (arrowhead) and the adjacent loops of colon (*). The tumor was targeted for microwave ablation with two 17-gauge gas-cooled antennae at 65 W for 3 minutes. Other retroperitoneal sites of disease were also treated during the same session. Fever, flank pain, and erythema developed in the days after the procedure. (c) Planar fluoroscopic spot image obtained during an enema examination with Gastrografin (Bracco Diagnostic, Princeton, NJ) helped confirm a contained colon leak (arrows). This was treated successfully with percutaneous drainage.
Figure 20b.
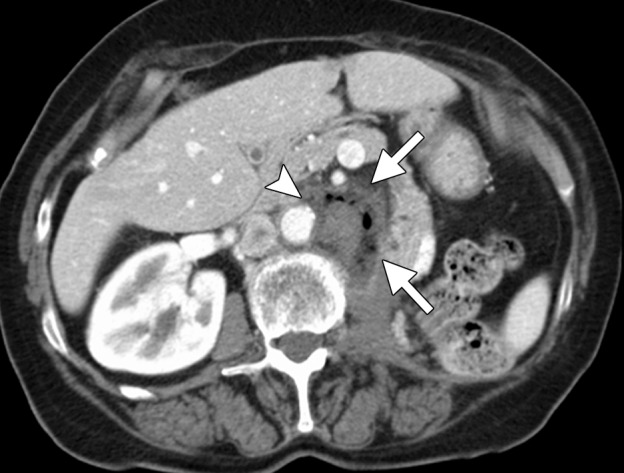
CT images in a 71-year-old woman with a history of RCC of the left kidney that was resected 13 years previously. (a) Axial contrast-enhanced CT image shows recurrent retroperitoneal disease (arrows). The tumor is partially encasing and wrapping anterior to the aorta (arrowhead). This would be problematic with RF ablation because of heat-sink effects and associated tumor sparing. The tumor was targeted for ablation with three microwave antennae for 5 minutes at 50 W. (b) Axial contrast-enhanced CT image obtained at 1-month follow-up shows complete ablation of the tumor, with an adequate margin (arrows). In addition, the tumor that was encasing and anterior to the aorta has been stripped off the aorta and completely ablated (arrowhead).
Conclusion
Image-guided tumor ablation is becoming increasingly accepted for treatment of both benign and malignant tumors in a variety of organs. However, the large number of percutaneous ablation tools that are now clinically available has led to confusion about which device should be used to treat particular tumors. In general, we favor the use of high-powered microwave ablation for treatment of tumors in the liver, periphery of the kidney, and lung. The use of any heat-based ablation modality in the lung can be associated with prolonged air leaks or bronchopleural fistulas, and, thus, the operator must avoid both tract cautery and direct puncture of peripheral tumors. RF ablation has substantial physical limitations compared with microwave ablation for tissue heating and is thus reserved for treating osteoid osteomas and for other situations that require a short ablation zone. We use cryoablation to treat central renal tumors because of the theoretical risk for damage to the central collecting system and ureter, although we recognize that this is not the experience in all practices. Cryoablation can also be useful for treating some lung tumors close to the chest wall and mediastinum and musculoskeletal tumors, for which pain palliation is the primary objective.
Acknowledgments
Acknowledgment
The authors thank Kirkland Davis, MD, for providing two of the cases featured in the article.
Recipient of a Cum Laude award for an education exhibit at the 2012 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors J.L.H, M.G.L, F.T.L, and C.L.B have provided disclosures (see “Disclosures of Conflicts of Interest”); the other author, editor, and reviewers have disclosed no relevant relationships.
Funding: The research was supported by the National Institutes of Health [grant numbers R01CA149379 and R01CA142737].
Disclosures of Conflicts of Interest.—J.L.H.: Activities related to the present article: shareholder in NeuWave Medical. Activities not related to the present article: disclosed no relevant relationships. Other: disclosed no relevant relationships. M.G.L.: Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: grant from GE Medical. Other: disclosed no relevant relationships. F.T.L.: Activities related to the present article: shareholder, patent holder, and board of directors member for NeuWave Medical; patent holder with and royalties received from Covidien. Activities not related to the present article: disclosed no relevant relationships. Other: disclosed no relevant relationships. C.L.B.: Activities related to the present article: stock in and consulting fees from NeuWave Medical, patent pending for energy delivery systems and uses. Activities not related to the present article: disclosed no relevant relationships. Other: disclosed no relevant relationships.
Abbreviations:
- HCC
- hepatocellular carcinoma
- RCC
- renal cell carcinoma
- RF
- radiofrequency
References
- 1.Atwell TD, Farrell MA, Callstrom MR, et al. Percutaneous cryoablation of 40 solid renal tumors with US guidance and CT monitoring: initial experience. Radiology 2007;243(1):276–283. [DOI] [PubMed] [Google Scholar]
- 2.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 2012;265(3):958–968. [DOI] [PubMed] [Google Scholar]
- 3.Kim SR, Han HJ, Park SJ, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol 2012;81(2):395–399. [DOI] [PubMed] [Google Scholar]
- 4.Belfiore G, Ronza F, Belfiore MP, et al. Patients’ survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol 2013; 82(1):177–181. [DOI] [PubMed] [Google Scholar]
- 5.Woertler K, Vestring T, Boettner F, Winkelmann W, Heindel W, Lindner N. Osteoid osteoma: CT-guided percutaneous radiofrequency ablation and follow-up in 47 patients. J Vasc Interv Radiol 2001;12(6): 717−722. [DOI] [PubMed] [Google Scholar]
- 6.Kurup AN, Callstrom MR. Image-guided percutaneous ablation of bone and soft tissue tumors. Semin Intervent Radiol 2010;27(3):276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res 2005;127(2):208–223. [DOI] [PubMed] [Google Scholar]
- 8.Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol 2010;21(suppl 8):S179–186. [DOI] [PubMed] [Google Scholar]
- 9.Schramm W, Yang D, Wood BJ, Rattay F, Haemmerich D. Contribution of direct heating, thermal conduction and perfusion during radiofrequency and microwave ablation. Open Biomed Eng J 2007;1:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed M, Liu Z, Humphries S, Goldberg SN. Computer modeling of the combined effects of perfusion, electrical conductivity, and thermal conductivity on tissue heating patterns in radiofrequency tumor ablation. Int J Hyperthermia 2008;24(7):577–588. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Ahmed M, Weinstein Y, Yi M, Mahajan RL, Goldberg SN. Characterization of the RF ablation-induced “oven effect”: the importance of background tissue thermal conductivity on tissue heating. Int J Hyperthermia 2006;22(4):327–342. [DOI] [PubMed] [Google Scholar]
- 12.Solazzo SA, Liu Z, Lobo SM, et al. Radiofrequency ablation: importance of background tissue electrical conductivity—an agar phantom and computer modeling study. Radiology 2005;236(2):495–502. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg SN, Gazelle GS, Compton CC, McLoud TC. Radiofrequency tissue ablation in the rabbit lung: efficacy and complications. Acad Radiol 1995;2(9): 776–784. [DOI] [PubMed] [Google Scholar]
- 14.Morrison PR, vanSonnenberg E, Shankar S, et al. Radiofrequency ablation of thoracic lesions. I. Experiments in the normal porcine thorax. AJR Am J Roentgenol 2005;184(2):375–380. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg SN, Gazelle GS, Solbiati L, Rittman WJ, Mueller PR. Radiofrequency tissue ablation: increased lesion diameter with a perfusion electrode. Acad Radiol 1996;3(8):636–644. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SN, Solbiati L, Hahn PF, et al. Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: laboratory and clinical experience in liver metastases. Radiology 1998;209(2):371–379. [DOI] [PubMed] [Google Scholar]
- 17.Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation creates confluent areas of necrosis: in vivo porcine liver results. Radiology 2006;241(1):116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laeseke PF, Sampson LA, Haemmerich D, et al. Multiple-electrode radiofrequency ablation: simultaneous production of separate zones of coagulation in an in vivo porcine liver model. J Vasc Interv Radiol 2005;16(12):1727–1735. [DOI] [PubMed] [Google Scholar]
- 19.Laeseke PF, Sampson LA, Frey TM, et al. Multiple-electrode radiofrequency ablation: comparison with a conventional cluster electrode in an in vivo porcine kidney model. J Vasc Interv Radiol 2007;18(8): 1005–1010. [DOI] [PubMed] [Google Scholar]
- 20.Brace CL, Laeseke PF, Sampson LA, Frey TM, Mukherjee R, Lee FT, Jr. Radiofrequency ablation with a high-power generator: device efficacy in an in vivo porcine liver model. Int J Hyperthermia 2007;23(4): 387–394. [DOI] [PubMed] [Google Scholar]
- 21.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT, Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236(1): 132–139. [DOI] [PubMed] [Google Scholar]
- 22.Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38(3):135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andreano A, Brace CL. A comparison of direct heating during radiofrequency and microwave ablation in ex vivo liver. Cardiovasc Intervent Radiol 2013;36(2): 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertram JM, Yang D, Converse MC, Webster JG, Mahvi DM. A review of coaxial-based interstitial antennas for hepatic microwave ablation. Crit Rev Biomed Eng 2006;34(3):187–213. [DOI] [PubMed] [Google Scholar]
- 25.Brace CL. Microwave tissue ablation: biophysics, technology, and applications. Crit Rev Biomed Eng 2010;38(1):65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Sun Y, Feng L, Gao Y, Ni X, Liang P. Internally cooled antenna for microwave ablation: results in ex vivo and in vivo porcine livers. Eur J Radiol 2008;67(2): 357–361. [DOI] [PubMed] [Google Scholar]
- 27.Brace CL. Dual-slot antennas for microwave tissue heating: parametric design analysis and experimental validation. Med Phys 2011;38(7):4232–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durick NA, Laeseke PF, Broderick LS, et al. Microwave ablation with triaxial antennas tuned for lung: results in an in vivo porcine model. Radiology 2008;247(1):80–87. [DOI] [PubMed] [Google Scholar]
- 29.Laeseke PF, Lee FT, Jr, van der Weide DW, Brace CL. Multiple-antenna microwave ablation: spatially distributing power improves thermal profiles and reduces invasiveness. J Interv Oncol 2009;2(2):65–72. [PMC free article] [PubMed] [Google Scholar]
- 30.Oshima F, Yamakado K, Nakatsuka A, Takaki H, Makita M, Takeda K. Simultaneous microwave ablation using multiple antennas in explanted bovine livers: relationship between ablative zone and antenna. Radiat Med 2008;26(7):408–414. [DOI] [PubMed] [Google Scholar]
- 31.Trembly BS, Douple EB, Ryan TP, Hoopes PJ. Effect of phase modulation on the temperature distribution of a microwave hyperthermia antenna array in vivo. Int J Hyperthermia 1994;10(5):691–705. [DOI] [PubMed] [Google Scholar]
- 32.Jones KM, Mechling JA, Trembly BS, Strohbehn JW. SAR distributions for 915 MHz interstitial microwave antennas used in hyperthermia for cancer therapy. IEEE Trans Biomed Eng 1988;35(10):851–857. [DOI] [PubMed] [Google Scholar]
- 33.Lubner MG, Brace CL, Hinshaw JL, Lee FT, Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21(suppl 8):S192–S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun Y, Wang Y, Ni X, et al. Comparison of ablation zone between 915- and 2,450-MHz cooled-shaft microwave antenna: results in in vivo porcine livers. AJR Am J Roentgenol 2009;192(2):511–514. [DOI] [PubMed] [Google Scholar]
- 35.Johnson RH, Andrasic G, Smith DL, James JR. Field penetration of arrays of compact applicators in localized hyperthermia. Int J Hyperthermia 1985;1(4):321–336. [DOI] [PubMed] [Google Scholar]
- 36.Cavagnaro M, Amabile C, Bernardi P, Pisa S, Tosoratti N. A minimally invasive antenna for microwave ablation therapies: design, performances, and experimental assessment. IEEE Trans Biomed Eng 2011;58(4): 949–959. [DOI] [PubMed] [Google Scholar]
- 37.Castle SM, Salas N, Leveillee RJ. Initial experience using microwave ablation therapy for renal tumor treatment: 18-month follow-up. Urology 2011;77(4):792–797. [DOI] [PubMed] [Google Scholar]
- 38.Strickland AD, Clegg PJ, Cronin NJ, et al. Experimental study of large-volume microwave ablation in the liver. Br J Surg 2002;89(8):1003–1007. [DOI] [PubMed] [Google Scholar]
- 39.Knavel EM, Hinshaw JL, Lubner MG, et al. High-powered gas-cooled microwave ablation: shaft cooling creates an effective stick function without altering the ablation zone. AJR Am J Roentgenol 2012;198(3): W260–W265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Zhang L, Fan W, et al. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: results in ex vivo porcine livers. Int J Hyperthermia 2011;27(3):240–248. [DOI] [PubMed] [Google Scholar]
- 41.Rui J, Tatsutani KN, Dahiya R, Rubinsky B. Effect of thermal variables on human breast cancer in cryosurgery. Breast Cancer Res Treat 1999;53(2):185–192. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology 2002;60(2 suppl 1):S40–S49. [DOI] [PubMed] [Google Scholar]
- 43.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998;37(3):171−186. [DOI] [PubMed] [Google Scholar]
- 44.Jansen MC, van Hillegersberg R, Schoots IG, et al. Cryoablation induces greater inflammatory and coagulative responses than radiofrequency ablation or laser induced thermotherapy in a rat liver model. Surgery 2010;147(5):686−695. [DOI] [PubMed] [Google Scholar]
- 45.Chapman WC, Debelak JP, Wright Pinson C, et al. Hepatic cryoablation, but not radiofrequency ablation, results in lung inflammation. Ann Surg 2000;231(5): 752−761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubinsky B. Cryosurgery. Annu Rev Biomed Eng 2000;2:157−187. [DOI] [PubMed] [Google Scholar]
- 47.Rubinsky B, Lee CY, Bastacky J, Onik G. The process of freezing and the mechanism of damage during hepatic cryosurgery. Cryobiology 1990;27(1):85−97. [DOI] [PubMed] [Google Scholar]
- 48.Weber SM, Lee FT, Jr, Warner TF, Chosy SG, Mahvi DM. Hepatic cryoablation: US monitoring of extent of necrosis in normal pig liver. Radiology 1998;207(1): 73−77. [DOI] [PubMed] [Google Scholar]
- 49.Lee FT, Jr, Chosy SG, Littrup PJ, Warner TF, Kuhlman JE, Mahvi DM. CT-monitored percutaneous cryoablation in a pig liver model: pilot study. Radiology 1999; 211(3):687−692. [DOI] [PubMed] [Google Scholar]
- 50.Littrup PJ, Jallad B, Vorugu V, et al. Lethal isotherms of cryoablation in a phantom study: effects of heat load, probe size, and number. J Vasc Interv Radiol 2009;20(10):1343−1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding J, Jing X, Liu J, et al. Complications of thermal ablation of hepatic tumours: comparison of radiofrequency and microwave ablative techniques. Clin Radiol 2013;68(6):608−615. [DOI] [PubMed] [Google Scholar]
- 52.Livraghi T, Meloni F, Solbiati L, Zanus G; Collaborative Italian Group using AMICA system. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Intervent Radiol 2012;35(4):868−874. [DOI] [PubMed] [Google Scholar]
- 53.Lee FT, Jr, Mahvi DM, Chosy SG, et al. Hepatic cryosurgery with intraoperative US guidance. Radiology 1997;202(3):624−632. [DOI] [PubMed] [Google Scholar]
- 54.Chiang J, Hynes K, Brace CL. Flow-dependent vascular heat transfer during microwave thermal ablation. Conf Proc IEEE Eng Med Biol Soc 2012;2012: 5582–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thanos L, Mylona S, Galani P, Pomoni M, Pomoni A, Koskinas I. Overcoming the heat-sink phenomenon: successful radiofrequency thermal ablation of liver tumors in contact with blood vessels. Diagn Interv Radiol 2008;14(1):51–56. [PubMed] [Google Scholar]
- 56.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol 2002;178(1):47–51. [DOI] [PubMed] [Google Scholar]
- 57.Laeseke PF, Lee FT, Jr, Sampson LA, van der Weide DW, Brace CL. Microwave ablation versus radiofrequency ablation in the kidney: high-power triaxial antennas create larger ablation zones than similarly sized internally cooled electrodes. J Vasc Interv Radiol 2009;20(9):1224–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhim H, Kim YS, Heo JN, et al. Radiofrequency thermal ablation of hepatic cyst. J Vasc Interv Radiol 2004; 15(1 Pt 1):95–96. [DOI] [PubMed] [Google Scholar]
- 59.Rocourt DV, Shiels WE, Hammond S, Besner GE. Contemporary management of benign hepatic adenoma using percutaneous radiofrequency ablation. J Pediatr Surg 2006;41(6):1149–1152. [DOI] [PubMed] [Google Scholar]
- 60.Hinshaw JL, Laeseke PJ, Weber SM, Lee FT, Jr. Multiple-electrode radiofrequency ablation of symptomatic hepatic cavernous hemangioma. AJR Am J Roentgenol 2007;189(3):W146–W149. [DOI] [PubMed] [Google Scholar]
- 61.Zagoria RJ, Roth TJ, Levine EA, Kavanagh PV. Radiofrequency ablation of a symptomatic hepatic cavernous hemangioma. AJR Am J Roentgenol 2004;182(1): 210–212. [DOI] [PubMed] [Google Scholar]
- 62.Cui Y, Zhou LY, Dong MK, et al. Ultrasonography guided percutaneous radiofrequency ablation for hepatic cavernous hemangioma. World J Gastroenterol 2003;9(9):2132–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fink AS, Appelman HD, Thompson NW. Hemorrhage into a hepatic adenoma and type Ia glycogen storage disease: a case report and review of the literature. Surgery 1985;97(1):117–124. [PubMed] [Google Scholar]
- 64.Pimparkar BD, Bhalerao RA, Deodhar KP, Nerurkar MG, Mehta JM. Hepatic adenoma with malignant change: a case report. J Assoc Physicians India 1972;20(5):391–396. [PubMed] [Google Scholar]
- 65.Kerkar S, Carlin AM, Sohn RL, et al. Long-term follow up and prognostic factors for cryotherapy of malignant liver tumors. Surgery 2004;136(4):770–779. [DOI] [PubMed] [Google Scholar]
- 66.Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg 2004;239(4): 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg 1999;23(2):109–113; discussion 113–114. [DOI] [PubMed] [Google Scholar]
- 68.Morimoto M, Numata K, Kondou M, Nozaki A, Morita S, Tanaka K. Midterm outcomes in patients with intermediate-sized hepatocellular carcinoma: a randomized controlled trial for determining the efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization. Cancer 2010;116(23): 5452–5460. [DOI] [PubMed] [Google Scholar]
- 69.Lu Z, Wen F, Guo Q, Liang H, Mao X, Sun H. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol 2013;25(2):187–194. [DOI] [PubMed] [Google Scholar]
- 70.Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31(4):426–432. [DOI] [PubMed] [Google Scholar]
- 71.Warlick CA, Lima GC, Allaf ME, et al. Clinical sequelae of radiographic iceball involvement of collecting system during computed tomography–guided percutaneous renal tumor cryoablation. Urology 2006;67(5): 918–922. [DOI] [PubMed] [Google Scholar]
- 72.Janzen NK, Perry KT, Han KR, et al. The effects of intentional cryoablation and radio frequency ablation of renal tissue involving the collecting system in a porcine model. J Urol 2005;173(4):1368–1374. [DOI] [PubMed] [Google Scholar]
- 73.Gregory SM, Anderson CJ, Patel U. Radiofrequency ablation of large renal angiomyolipoma: median-term follow-up. Cardiovasc Intervent Radiol 2013;36(3): 682–689. [DOI] [PubMed] [Google Scholar]
- 74.Castle SM, Gorbatiy V, Ekwenna O, Young E, Leveillee RJ. Radiofrequency ablation (RFA) therapy for renal angiomyolipoma (AML): an alternative to angio-embolization and nephron-sparing surgery. BJU Int 2012;109(3):384–387. [DOI] [PubMed] [Google Scholar]
- 75.Atwell TD, Schmit GD, Boorjian SA, et al. Percutaneous ablation of renal masses measuring 3.0 cm and smaller: comparative local control and complications after radiofrequency ablation and cryoablation. AJR Am J Roentgenol 2013;200(2):461–466. [DOI] [PubMed] [Google Scholar]
- 76.Rosenberg MD, Kim CY, Tsivian M, et al. Percutaneous cryoablation of renal lesions with radiographic ice ball involvement of the renal sinus: analysis of hemorrhagic and collecting system complications. AJR Am J Roentgenol 2011;196(4):935–939. [DOI] [PubMed] [Google Scholar]
- 77.Patel SR, Hinshaw JL, Lubner MG, Lee FT, Jr, Nakada SY, Hedican SP. Hydrodissection using an iodinated contrast medium during percutaneous renal cryoablation. J Endourol 2012;26(5):463–466. [DOI] [PubMed] [Google Scholar]
- 78.Crabtree T, Puri V, Timmerman R, et al. Treatment of stage I lung cancer in high-risk and inoperable patients: comparison of prospective clinical trials using stereotactic body radiotherapy (RTOG 0236), sublobar resection (ACOSOG Z4032), and radiofrequency ablation (ACOSOG Z4033). J Thorac Cardiovasc Surg 2013;145(3):692–699. [DOI] [PubMed] [Google Scholar]
- 79.Bang HJ, Littrup PJ, Currier BP, et al. Percutaneous cryoablation of metastatic lesions from non–small-cell lung carcinoma: initial survival, local control, and cost observations. J Vasc Interv Radiol 2012;23(6):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carrafiello G, Mangini M, Fontana F, et al. Complications of microwave and radiofrequency lung ablation: personal experience and review of the literature. Radiol Med (Torino) 2012;117(2):201–213. [DOI] [PubMed] [Google Scholar]
- 81.Alexander ES, Machan JT, Ng T, Breen LD, DiPetrillo TA, Dupuy DE. Cost and effectiveness of radiofrequency ablation versus limited surgical resection for stage I non–small-cell lung cancer in elderly patients: is less more? J Vasc Interv Radiol 2013;24(4):476–482. [DOI] [PubMed] [Google Scholar]
- 82.Beland MD, Wasser EJ, Mayo-Smith WW, Dupuy DE. Primary non–small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology 2010;254(1):301–307. [DOI] [PubMed] [Google Scholar]
- 83.Anai H, Uchida BT, Pavcnik D, et al. Effects of blood flow and/or ventilation restriction on radiofrequency coagulation size in the lung: an experimental study in swine. Cardiovasc Intervent Radiol 2006;29(5): 838–845. [DOI] [PubMed] [Google Scholar]
- 84.Hinshaw JL, Sampson L, Lee FT, Jr, Laeseke PF, Brace CL. Does selective intubation increase ablation zone size during pulmonary cryoablation? J Vasc Interv Radiol 2008;19(10):1497–1501. [DOI] [PubMed] [Google Scholar]
- 85.Sakurai J, Hiraki T, Mukai T, et al. Intractable pneumothorax due to bronchopleural fistula after radiofrequency ablation of lung tumors. J Vasc Interv Radiol 2007;18(1 Pt 1):141–145. [DOI] [PubMed] [Google Scholar]
- 86.Callstrom MR, Kurup AN. Percutaneous ablation for bone and soft tissue metastases: why cryoablation? Skeletal Radiol 2009;38(9):835–839. [DOI] [PubMed] [Google Scholar]
- 87.Thacker PG, Callstrom MR, Curry TB, et al. Palliation of painful metastatic disease involving bone with imaging-guided treatment: comparison of patients’ immediate response to radiofrequency ablation and cryoablation. AJR Am J Roentgenol 2011;197(2): 510–515. [DOI] [PubMed] [Google Scholar]
- 88.Leiria TL, Glavinovic T, Armour JA, Cardinal R, de Lima GG, Kus T. Longterm effects of cardiac mediastinal nerve cryoablation on neural inducibility of atrial fibrillation in canines. Auton Neurosci 2011;161(1-2): 68–74. [DOI] [PubMed] [Google Scholar]