In 2005, synthetic cannabinoid (SC) products marketed as “Spice” emerged as popular drugs of abuse in European countries. These products entered the US in 2009 and were commonly sold as “K2”.(1–12) They are now widely available worldwide and packaged with attractive labels that appeal to teens, young adults, and first-time drug users. “Not for human consumption” or “incense” are typical inscriptions found on product packaging. SC products contain varying concentrations of a number of potent SCs that when smoked in the same manner as marijuana produce similar, but often more intense, psychological and physical effects.(13) Referred to as “legal marijuana” by users because initially they fell outside existing controlled substance regulations, the SCs detected thus far are structurally unlike the classic cannabinoid Δ9-tetrahydrocannabinol.(14) Biochemically these compounds are commonly classified as aminoalkylindoles (i.e. AM-2201) and cyclohexophenols (i.e. CP-47,497).
Use of SCs is a matter of growing public health concern as reflected by the increasing number of telephone calls to US Poison Control Centers. Approximately 7000 calls regarding SC occurred in 2011, representing an exponential increase from the number of calls reported in 2009.(15, 16) Clinical reports involving SCs indicate tachycardia, extreme agitation, hallucinations, and syncope as the most prevalent symptoms of acute toxicity.(14, 17–21) Supraventricular tachycardia and generalized seizures (22) have also been reported in cases of severe toxicity, and severe psychosis and the development of dependence and tolerance can occur with chronic exposures.(19, 23–25) The long term health effects of these drugs are unknown.
Derivatives of SCs are considered to be highly potent cannabinoid receptor-1 (CB1) agonists. While twelve deaths involving SC use have been reported, (26, 27) confounding factors and the lack of adequate toxicological testing procedures have limited definitive diagnosis in these cases. The most publicized fatality is that of an 18 year old male who had severe hallucinations leading to suicide after smoking “K2”.
The following case report illustrates the severe toxic nature of SCs and provides a broad-based approach for confirming toxicity and death related to SC toxicity. Comprehensive forensic testing procedures were performed on the SC product, drug delivery device, and post-mortem blood. In addition, metabolism of AM-2201 in human blood and human liver microsomes is characterized.
Case Report
The decedent was a 23-year old white male with no known history of mental illness, seizure disorder, previous psychiatric care, or past or current use of illicit drugs or over-the-counter medication (OTC). In the hours preceding his death, he attended lunch with a family member and returned home where he appeared normal. A sibling, who found the decedent lying supine on the floor and covered in blood, reported hearing “stomping noises” in the decedent’s room for about thirty minutes. Emergency medical services and the coroner were notified of this case. No medical treatment was provided and the individual was pronounced dead on arrival. The decedent’s bedroom was noted by the coroner to be in complete disarray, a finding that was abnormal, according to the family. Significant damage to a wall and glass window was noted and a large volume of blood covered the floor, windows, and walls.
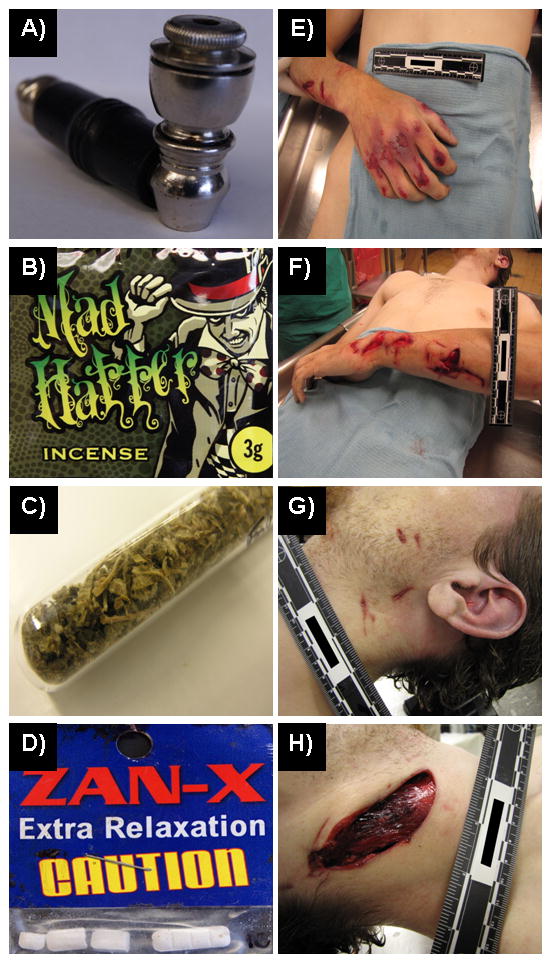
A smoking device containing a small amount of residue (Figure 1A), a foil package of green vegetable material labeled “Mad Hatter Incense” (Figures 1B & 1C), and a bag labeled “ZAN-X Extra Relaxation” containing scored rectangular white tablets (Figure 1D) were recovered from the decedent’s shirt pocket.
Figure 1.
Methods
Analysis of post-mortem samples was approved by the institutional review board. A comprehensive statewide surveillance system for SC product and exposure cases was used to identify this case for study.(28) Heart blood collected from the routine autopsy was tested for SCs using previously described testing procedures.(9, 12, 29) In brief, 100 μL of the heart blood, 400 μL of acetonitrile and 10 μL of 1 μg/mL deuterium-labeled internal standards were vortexed for 30 seconds. Protein was precipitated at −40°C for 30 minutes and pelleted by centrifugation (20817 RCF) for 5 min. Supernatant was collected, dried under a stream of N2 at 60°C, and reconstituted in 100 μL of dimethyl sulfoxide (DMSO) prior to LC-MS/MS analysis (AB Sciex: Framingham, Massachusetts). Analytes of interest were quantified using standard curves prepared using analytical standards purchased from Cayman Chemical (Ann Arbor, Michigan).
Interpretation of the metabolic patterns observed in blood was complemented with the use of human liver metabolism studies. The metabolism of JWH-018, a common SC, and AM-2201 were evaluated in vitro using pooled human liver microsomes (pHLM, 50 μg, BD Gentest, Woburn, MA). Substrates (JWH-018 and AM-2201, 5 μM, final concentration) were added in ethanol and allowed to dry at ambient temperature. Microsomes were added to a phosphate buffer (pH 7.4, 0.1 M KPO4) containing a NADPH regeneration system. Reactions were initiated by the addition of the protein, allowed to incubate at 37°C for 90 min, and quenched with an equal volume of ethanol. Before LC-MS/MS analysis, protein was precipitated by centrifugation at 20,817 RCF for 8 min.
Comprehensive drug testing was performed on the white tablets, green vegetable material contained in the “Mad Hatter Incense” package, and residue found in the smoking device found in the decedent’s pocket. The sample was examined microscopically, extracted with methanol, and analyzed using two gas chromatography mass spectroscopy (GC-MS) methods (Agilent: Santa Clara, California). Confirmation testing was performed with analytical standards purchased from Cayman Chemical (Ann Arbor, Michigan).
Results
Postmortem inspection of the body demonstrated blunt-force injuries to both hands, consistent with punching relatively hard, unyielding objects (Figure 1E). Sharp-force wounds were found on the head and upper extremities (Figures 1F & 1G). A large, chevron-shaped, complex stab wound to the right side of the neck is believed to be the fatal wound (Figure 1H).
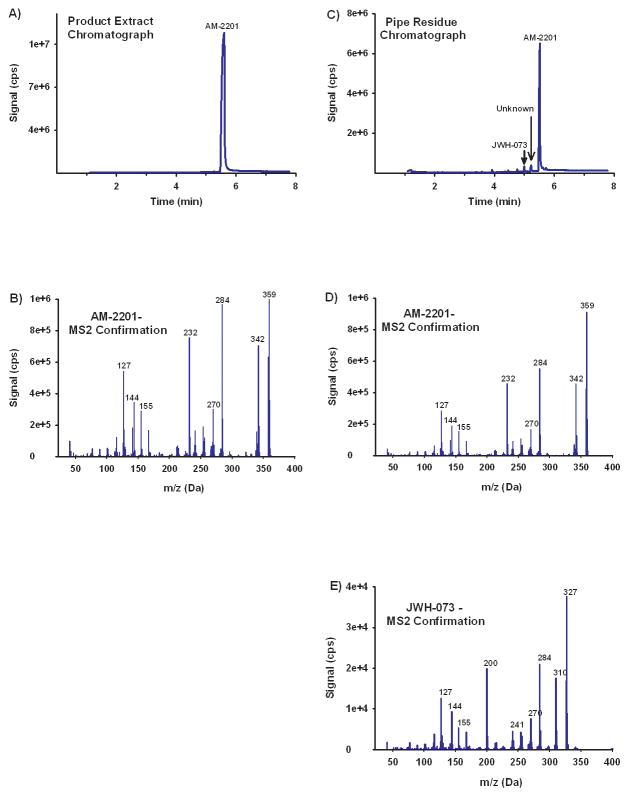
The recovered vegetable material, residue recovered from the smoking device, and several “ZAN-X Extra Relaxation” tablets were assayed for common OTCs and drugs of abuse (benzodiazepines, opiates, amphetamines, cocaine, THC, new designer stimulants (e.g. cathinones such as MDPV), and other commonly abused SCs like JWH-018, CP-47-497, etc.). GC-MS analysis of the green vegetable material and the residue contained in the smoking device detected relatively high concentrations of AM-2201 (Figures 2A – 2D), where as the “ZAN-X Extra Relaxation” tablets tested negative for both controlled substances and OTC. The vegetable material contained 0.8 ± 0.2% AM-2201 by weight. Although sufficient residue was not available for quantitative studies, the residue collected from the smoking device contained a relatively high concentration of AM-2201 and a trace amount of another common SC JWH-073 (Figures 2C – 2E). No other controlled substances or OTC medications were detected in any of the samples tested.
Figure 2.
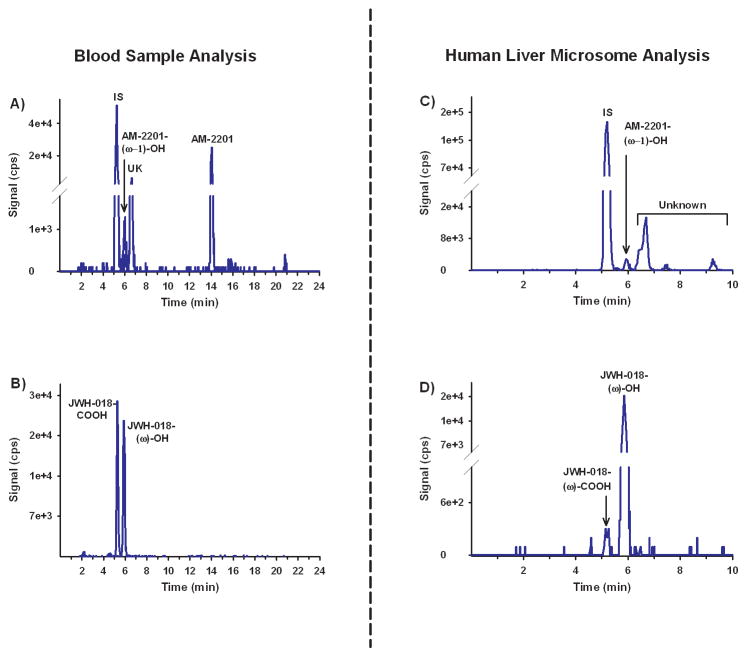
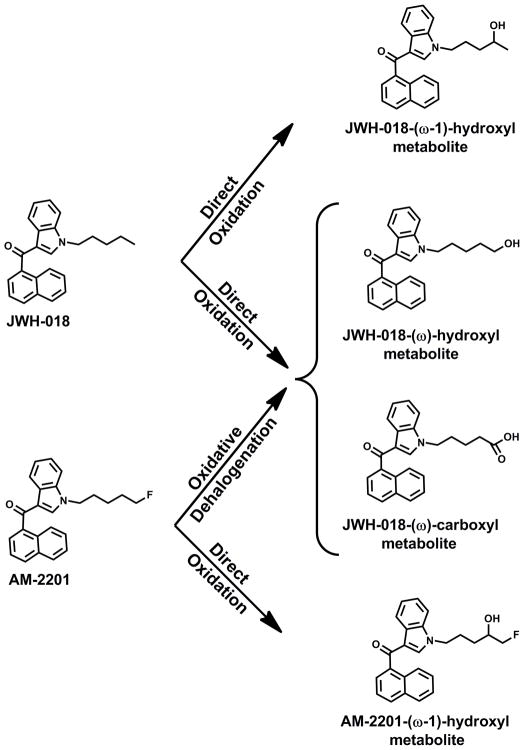
Comprehensive toxicological testing of blood was negative for opiates, benzodiazepines, amphetamines, cocaine, THC, and alcohol. The blood was subjected to further SC testing using LC-MS/MS procedures capable of detecting many common SCs and their metabolites (JWH-018, JWH-073, JWH-122, JWH-250, AM-2201, RCS-4, RCS-8, etc.). AM-2201 (12 ng/mL) was the only SC detected along with a known oxidized metabolite of AM-2201 (2.47 ng/mL) (Figure 3A). An unidentified fluoridated metabolite of AM-2201 was also detected (Figure 3A), but positional assignment and quantitative studies were not possible without an appropriate analytical standard. Both oxidized and carboxylated metabolites of JWH-018 were also observed in this analysis (123 ng/mL and 50.8 ng/mL, respectively) (Figure 3B). The presence of JWH-018 metabolites could be secondary to previous exposure to JWH-018 or due to oxidative dehalogenation of AM-2201. In vitro biochemical assays performed to further understand the metabolism of AM-2201 revealed that the metabolic pattern observed in the subject blood samples was consistent with recent AM-2201 exposure (Figures 3C & 3D). Human liver microsomal (HLM) metabolism of JWH-018 demonstrated the formation of three expected metabolites previously described in human urine,(29) while metabolism of AM-2201 produced a very similar metabolic profile to that of the decedent blood sample (Figures 3 & 4).
Figure 3.
Figure 4.
Additional investigation found no evidence of foul play and no evidence to indicate intent of self-harm; thus, the manner of death in this case appears to be related to psychiatric complications caused by AM-2201 use.
Discussion
Following an exposure to a product containing a high concentration of AM-2201, the decedent experienced an adverse physiological episode that resulted in a self-inflicted fatal wound. Many blunt- and sharp-force wounds were observed during post-mortem examination and the fatal wound was considered to be a self-inflicted stab wound to the right side of the neck. It is suspected that the decedent self-administered a portion of the green vegetable material utilizing the smoking device. Both the material and the smoking device primarily contained AM-2201; however, a trace of JWH-073 was confirmed to be present in residue collected from the smoking device. No other drugs were detected using analytical procedures capable of detecting trace levels of many OTC medications and controlled substances, including many cathinones and other SCs commonly present in a variety of “K2” products.(9, 12, 29)
The decedent’s blood indicated the presence of AM-2201 and known metabolites consistent with AM-2201 exposure. Metabolism studies indicated that AM-2201 undergoes both direct oxidation and oxidative dehalogenation.
Conclusion
The case described here indicates the potential mortality associated with the psychiatric complications of SC exposure. As exhibited in this case, SC exposure is not typical of THC and can induce adverse responses commonly observed with PCP, cocaine, and methamphetamine overdose. The broad-based approach detailed in this report demonstrates comprehensive human testing procedures for confirming cause of death cases associated with SC use and sets a new standard for assessing morbidity and mortality associated with emerging designer drugs posing significant public health concerns.
Supplementary Material
Acknowledgments
The Authors would like to thank Robert Hoffman, Jeff Lapoint, and Lewis Nelson for their technical review.
Footnotes
This work was supported, in part, by the Association of Public Health Laboratories [Grant Innovations in Quality Public health Laboratory Practice] (JHM), the Centers for Disease Control [Contract 200-2007-21729] (JHM), and an award from the University of Arkansas for Medical Sciences Translational Research Institute, which is funded by the National Center for Research Resources [1 UL 1RR029884, Curtis Lowery, PI] (LPJ).
The content is solely the responsibility of the authors and does not necessarily represent the official views of institutions.
All authors are without conflicts of interest, including financial interests, activities, relationships, and affiliations. In addition, all authors had full access to the data presented in this study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Specific Authorship Contributions:
All authors contributed equally in preparation of this manuscript. Amy Patton, Krishna Chimalakonda, Cindy Moran, and Jeffery Moran were responsible for conducting experiments and analytical testing. Keith McCain coordinated the experimental design and sample work flow that was coordinated by the Arkansas Poison and Drug Information Center. Laura James and Charles Kokes provided medical, toxicological and pathological expertise, while Anna Radominska-Pandya and Jeffery Moran provided expertise in drug metabolism and analytical analyses. Grants and contracts, listed above, were received by Jeffery Moran and Laura James and used to fund this work.
References
- 1.EMCDDA. Spice and Related Synthetic Cannabinoids. Lisbon: European Monitoring Centre for Drugs and Drug Addiction; 2009. Understanding the “Spice” phenomenon. [Google Scholar]
- 2.Auwarter V, Dresen S, Weinmann W, Muller M, Putz M, Ferreiros N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom. 2009 May;44(5):832–7. doi: 10.1002/jms.1558. [DOI] [PubMed] [Google Scholar]
- 3.Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, et al. Spice: a never ending story? Forensic Sci Int. 2009 Oct 30;191(1–3):58–63. doi: 10.1016/j.forsciint.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Hudson S, Ramsey J, King L, Timbers S, Maynard S, Dargan PI, et al. Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J Anal Toxicol. 34(5):252–60. doi: 10.1093/jat/34.5.252. [DOI] [PubMed] [Google Scholar]
- 5.Piggee C. Investigating a not-so-natural high. Anal Chem. 2009 May 1;81(9):3205–7. doi: 10.1021/ac900564u. [DOI] [PubMed] [Google Scholar]
- 6.Uchiyama N, Kikura-Hanajiri R, Kawahara N, Haishima Y, Goda Y. Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chem Pharm Bull (Tokyo) 2009 Apr;57(4):439–41. doi: 10.1248/cpb.57.439. [DOI] [PubMed] [Google Scholar]
- 7.Dresen S, Ferreiros N, Putz M, Westphal F, Zimmermann R, Auwarter V. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom. Oct;45(10):1186–94. doi: 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- 8.Moller I, Wintermeyer A, Bender K, Jubner M, Thomas A, Krug O, et al. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Anal. Sep;3(9):609–20. doi: 10.1002/dta.158. [DOI] [PubMed] [Google Scholar]
- 9.Moran CL, Le VH, Chimalakonda KC, Smedley AL, Lackey FD, Owen SN, et al. Quantitative Measurement of JWH-018 and JWH-073 Metabolites Excreted in Human Urine. Anal Chem. 2011 May 6;:6. doi: 10.1021/ac2005636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vardakou I, Pistos C, Spiliopoulou C. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. Sep 1;197(3):157–62. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Drug Enforcement Administration. Schedules of Controlled Substances: Temporary Placement of Five Synthetic Cannabinoids Into Schedule I. Microgram Bulletin. 2010 Jan;:1–5. [Google Scholar]
- 12.Moller I, Wintermeyer A, Bender K, Jubner M, Thomas A, Krug O, et al. Screening for the synthetic cannabinoid JWH-018 and its major metabolites in human doping controls. Drug Test Anal. 2010 Sep;3(9):609–20. doi: 10.1002/dta.158. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz E. K2/Spice: Much more than “fake marijuana”. Emergency Physicians Monthly. 2010 Sep [Google Scholar]
- 14.Lapoint J, Nelson LS. Synthetic cannabinoids: The newest, almost illicit drug of abuse. Emergency Medicine. 2011 Feb;:26–8. [Google Scholar]
- 15.American Association of Poison Control Centers A. Synthetic Marijuana Data. [January 12, 2012; cited]; Available from: http://www.aapcc.org/dnn/Portals/0/Synthetic%20Marijuana%20Data%20for%20Website%201.12.2012.pdf.
- 16.Hu X, Primack BA, Barnett TE, Cook RL. College students and use of K2: an emerging drug of abuse in young persons. Subst Abuse Treat Prev Policy. Jul 11;6(1):16. doi: 10.1186/1747-597X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller H, Sperling W, Kohrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res. May;118(1–3):309–10. doi: 10.1016/j.schres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Vearrier D, Osterhoudt KC. A teenager with agitation: higher than she should have climbed. Pediatr Emerg Care. Jun;26(6):462–5. doi: 10.1097/PEC.0b013e3181e4f416. [DOI] [PubMed] [Google Scholar]
- 19.Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. Oct;105(10):1859–60. doi: 10.1111/j.1360-0443.2010.03119.x. [DOI] [PubMed] [Google Scholar]
- 20.Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med. Mar;40(3):296–9. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Jaslow R. Synthetic marijuana sending more teens to hospital, study finds. CBS News. 2012 Mar 19; [Google Scholar]
- 22.Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011 Oct;49(8):760–4. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierre JM. Cannabis, synthetic cannabinoids, and psychosis risk: What the evidence says. Current Psychiatry. 2011 Sep;10(9):49–57. [Google Scholar]
- 24.Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. Sep 1;117(2–3):152–7. doi: 10.1016/j.drugalcdep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann US, Winkelmann PR, Pilhatsch M, Nees JA, Spanagel R, Schulz K. Withdrawal phenomena and dependence syndrome after the consumption of “spice gold”. Dtsch Arztebl Int. 2009 Jul;106(27):464–7. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattore L, Fratta W, Beyond THC. The New Generation of Cannabinoid Designer Drugs. Front Behav Neurosci. 5(60):60. doi: 10.3389/fnbeh.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanks KG, Dahn T, Terrell AR. Detection of JWH-018 and JWH-073 by UPLC-MS-MS in Postmortem Whole Blood Casework. J Anal Toxicol. Apr;36(3):145–52. doi: 10.1093/jat/bks013. [DOI] [PubMed] [Google Scholar]
- 28.Moran JH. Smart resource allocation needed to study ‘legal highs’. Nature medicine. 2011;17(11):1339. doi: 10.1038/nm1111-1339. [DOI] [PubMed] [Google Scholar]
- 29.Chimalakonda KC, Moran CL, Kennedy PD, Endres GW, Uzieblo A, Dobrowolski PJ, et al. Solid-phase extraction and quantitative measurement of omega and omega-1 metabolites of JWH-018 and JWH-073 in human urine. Anal Chem. Aug 15;83(16):6381–8. doi: 10.1021/ac201377m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.