Abstract
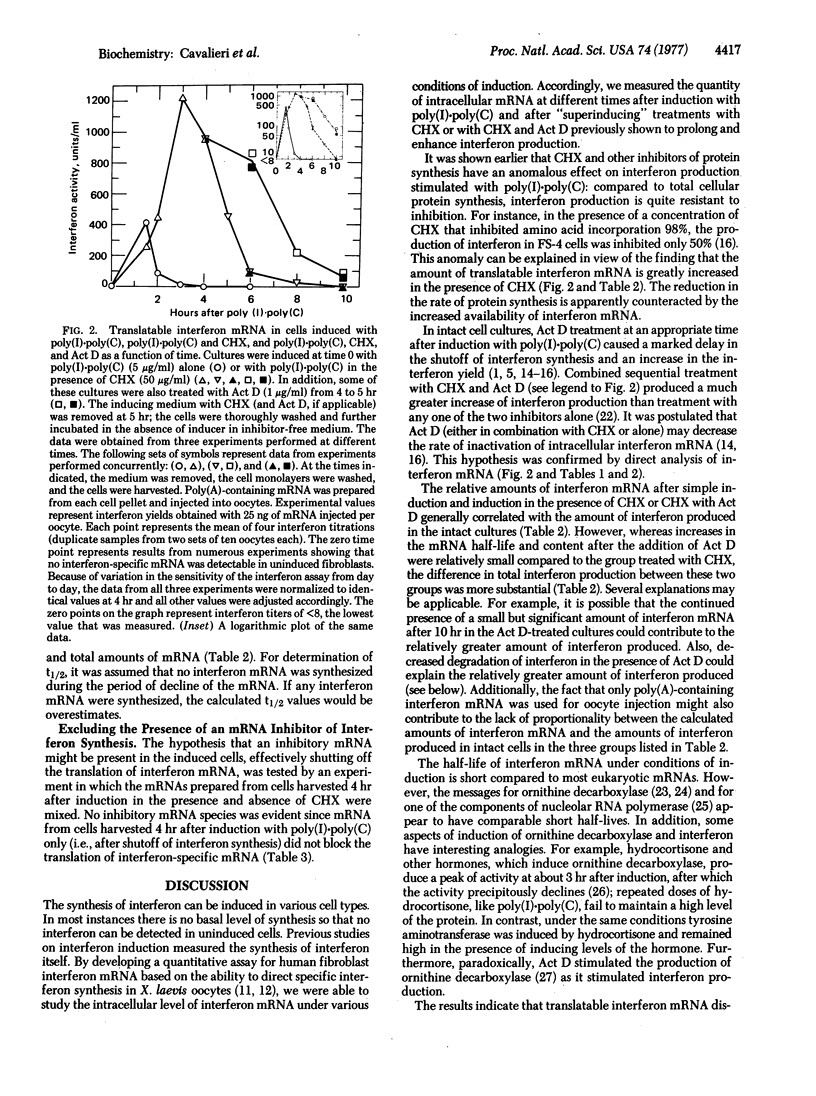
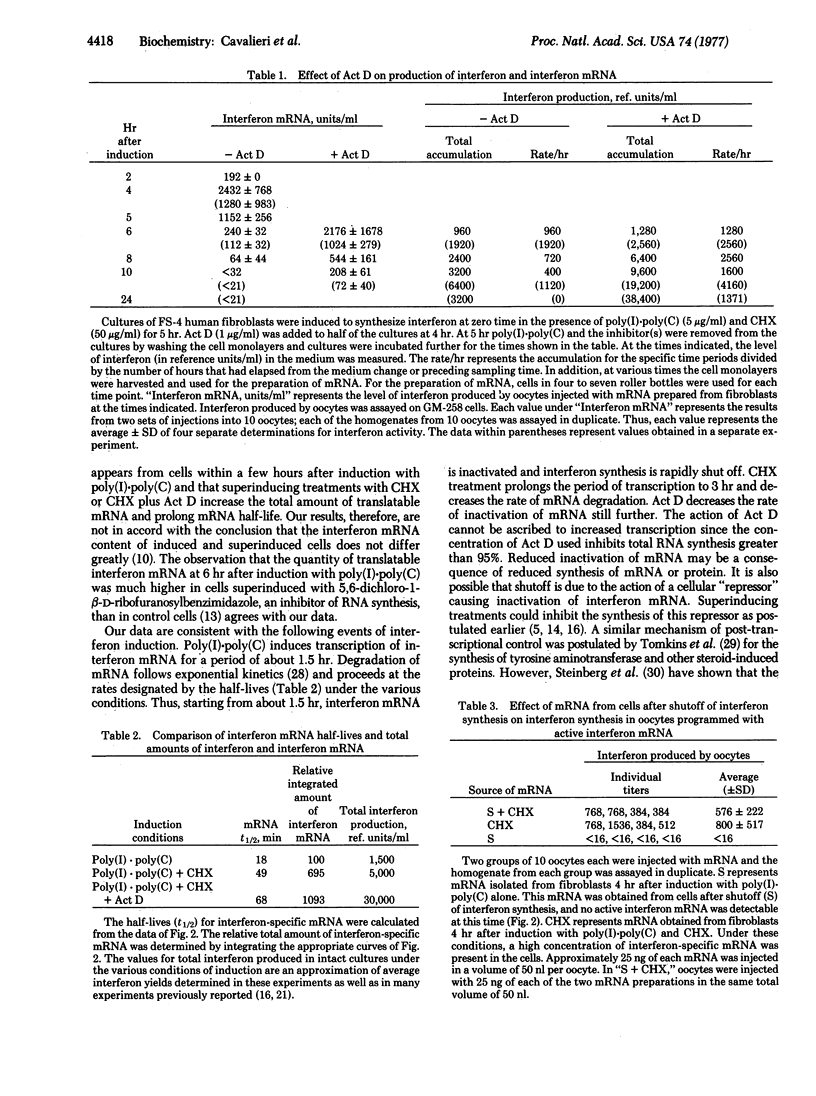
Polyadenylylated interferon mRNA, obtained from induced human fibroblasts, was quantitatively assayed by synthesis of biologically active human interferon in Xenopus laevis oocytes. The assay for interferon mRNA was used to distinguish between various hypotheses relating to interferon induction and biosynthesis. The data demonstrate that on induction with poly(I-poly(C) human fibroblasts accumulate interferon mRNA for 1-1.5 hr, after which time the mRNA is rapidly degraded with a half-life (t 1/2) of 18 min. Treatment of cells with cycloheximide prolongs the period of accumulation to 3 hr and decreases the rate of mRNA inactivation (t 1/2 = 49 min). Treatment with actinomycin D decreases the rate of inactivation still further (t 1/2 = 68 min). A comparison of cellular interferon synthesis with the relative amounts of interferon m RNA after simple induction or inductionin the presence of the inhibitors (superinduction) indicated a general correlation. Thus, on induction, the genes for interferon are activated to produce a transcript for a short time. The superinducing treatments prolong the period of accumulation and decrease the rate of degradation of this transcript.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalieri R. L., Havell E. A., Vilcek J., Pestka S. Synthesis of human interferon by Xenopus laevis oocytes: two structural genes for interferons in human cells. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3287–3291. doi: 10.1073/pnas.74.8.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. L., Pestka S. Synthesis of interferon in heterologous cells, cell-free extracts, and Xenopus laevis oocytes. Tex Rep Biol Med. 1977;35:117–125. [PubMed] [Google Scholar]
- Green M., Graves P. N., Zehavi-Willner T., McInnes J., Pestka S. Cell-free translation of immunoglobulin messenger RNA from MOPC-315 plasmacytoma and MOPC-315 NR, a variant synthesizing only light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):224–228. doi: 10.1073/pnas.72.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Zehavi-Willner T., Graves P. N., McInnes J., Pestka S. Isolation and cell-free translation of immunoglobulin messenger RNA. Arch Biochem Biophys. 1976 Jan;172(1):74–89. doi: 10.1016/0003-9861(76)90049-7. [DOI] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J., Falcoff E., Berman B. Suppression of human interferon production by inhibitors of glycosylation. Virology. 1975 Feb;63(2):475–483. doi: 10.1016/0042-6822(75)90320-7. [DOI] [PubMed] [Google Scholar]
- Manen C. A., Russell D. H. Relationship of ornithine decarboxylase to RNA polymerase i activity. Life Sci. 1975 Dec 15;17(12):1769–1775. doi: 10.1016/0024-3205(75)90459-2. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Mozes L. W., Vilcek J. Interferon induction in rabbit cells irradiated with UV light. J Virol. 1974 Mar;13(3):646–651. doi: 10.1128/jvi.13.3.646-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panko W. B., Kenney F. T. Hormonal stimulation of hepatic ornithine decarboxylase. Biochem Biophys Res Commun. 1971 Apr 16;43(2):346–350. doi: 10.1016/0006-291x(71)90759-5. [DOI] [PubMed] [Google Scholar]
- Pestka S., McInnes J., Havell E. A., Vilcek J. Cell-free synthesis of human interferon. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3898–3901. doi: 10.1073/pnas.72.10.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., McInnes J., Weiss D., Havell E. A., Vilcek J. De novo cell-free synthesis of human interferon. Ann N Y Acad Sci. 1977 Mar 4;284:697–702. doi: 10.1111/j.1749-6632.1977.tb22005.x. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Relationship between interferon production and interferon messenger RNA synthesis in human fibroblasts. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1483–1487. doi: 10.1073/pnas.74.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Premkumar E., Pitha P. M. Interferon activity produced by translation of human interferon messenger RNA in cell-free ribosomal systems and in Xenopus oöcytes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4881–4885. doi: 10.1073/pnas.72.12.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Dobberstein B., Tamm I. Interferon messenger RNA content of human fibroblasts during induction, shutoff, and superinduction of interferon production. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3409–3413. doi: 10.1073/pnas.74.8.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I. An evaluation of messenger RNA competition in the shutoff of human interferon production. Proc Natl Acad Sci U S A. 1976 May;73(5):1621–1625. doi: 10.1073/pnas.73.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Tamm I., Vilcek J. Human interferon production: superinduction by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Science. 1975 Oct 17;190(4211):282–284. doi: 10.1126/science.1179208. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. "Superinduction" of tyrosine aminotransferase by actinomycin D: a reevaluation. Cell. 1975 May;5(1):29–35. doi: 10.1016/0092-8674(75)90088-4. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Levinson B. B., Tomkins G. M. Kinetics of steroid induction and deinduction of tyrosine aminotransferase synthesis in cultured hepatoma cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2007–2011. doi: 10.1073/pnas.72.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y. H., Schneider E. L., Tischfield J., Epstein C. J., Ruddle F. H. Human chromosome 21 dosage: effect on the expression of the interferon induced antiviral state. Science. 1974 Oct 4;186(4158):61–63. doi: 10.1126/science.186.4158.61. [DOI] [PubMed] [Google Scholar]
- Thang M. N., Thang D. C., De Maeyer E., Montagnier L. Biosynthesis of mouse interferon by translation of its messenger RNA in a cell-free system. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3975–3977. doi: 10.1073/pnas.72.10.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A., Kohase M. Superinduction of interferon with metabolic inhibitors: possible mechanisms and practical applications. J Infect Dis. 1976 Jun;133 (Suppl):A22–A29. doi: 10.1093/infdis/133.supplement_2.a22. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Havell E. A. Stabilization of interferon messenger RNA activity by treatment of cells with metabolic inhibitors and lowering of the incubation temperature. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3909–3913. doi: 10.1073/pnas.70.12.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Ng M. H. Post-transcriptional control of interferon synthesis. J Virol. 1971 May;7(5):588–594. doi: 10.1128/jvi.7.5.588-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Rossman T. G., Varacalli F. Differential effects of actinomycin D and puromycin on the release of interferon induced by double stranded RNA. Nature. 1969 May 17;222(5194):682–683. doi: 10.1038/222682a0. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Ichihara A. Induction of ornithine decarboxylase in cultured mouse L cells. I. Effects of cellular growth, hormones, and actinomycin D. J Biochem. 1976 Sep;80(3):557–562. doi: 10.1093/oxfordjournals.jbchem.a131311. [DOI] [PubMed] [Google Scholar]
- Yu F. L., Feigelson P. The rapid turnover of RNA polymerase of rat liver nucleolus, and of its messenger RNA. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2833–2837. doi: 10.1073/pnas.69.10.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]


