Abstract
Purpose
Prematurely born children have affected visual functions at school age. Optical coherent tomography (OCT) has shown morphological changes in the retina, suggesting a disturbance in normal retinal development in these children. The aim of this study was to examine retinal function with fullfield electroretinogram (ffERG) in school-aged children born prematurely and compare with children born at term. A second aim was to correlate retinal function with visual acuity (VA), gestational age (GA), birth weight, and retinopathy of prematurity (ROP).
Methods
The study group consisted of 35 former preterm children born before GA of 32 weeks. A group of 42 children born at term acted as controls. All children were between 5- and 18-years old. FfERG was performed in both eyes. Best-corrected VA and refraction in cycloplegia was determined.
Results
The a-wave of the combined rod/cone responses was significantly reduced in the prematurely-born children compared with children born at term. There was a correlation between reduced a-wave amplitude in the combined rod/cone response and ROP and GA at birth.
Conclusion
Function of photoreceptors was affected in prematurely born children, possibly also in children without previous ROP. Whether immaturity per se affects the retinal function remains to be elucidated.
Translational Relevance
The present study illustrates that electrophysiological studies of the retinal function can help us understand visual dysfunctions in prematurely born children.
Keywords: fullfield ERG, prematurity, retinopathy of prematurity
Introduction
Retinal development starts early, and by mid gestation all retinal cells are present but very immature.1 Further development includes differentiation of the retinal cells, as well as migration and apoptosis of cells to form the adult retinal structure.2 This process continues gradually and is not fully complete until several years after birth. The development of retinal function follows the differentiation of the retinal cells and structures. Healthy fullterm infants have a more immature fullfield electroretinogram (ffERG) response from rods than from cones when measured at birth indicating that cones mature earlier than rods.3–5 A rapid development of the ERG response of both rods and cones takes place during the first 4 months of life, and continues slowly until early school age.6 The ERG response in prematurely born children is very immature when recorded at 30 weeks of gestational age (GA) with low amplitudes and long implicit times for both rod and cone responses.7,8 The ERG matures continuously and in healthy preterm children tested at 40 weeks of gestation the amplitudes reach the level of fullterms tested just after birth.4 ERG in former preterm schoolchildren has only been performed to a limited extent and mainly in children with previous retinopathy of prematurity (ROP).9
Prematurely born children have various visual dysfunctions when tested at school age, such as decreased visual acuity (VA), affected visual fields, reduced contrast vision, and increased prevalence of refractive errors and strabismus.10–14 The main reasons for their visual problems are ROP and neurological complications, such as periventricular leukomalacia (PVL) and intraventricular hemorrhages (IVH).15,16 However, preterm children with no ROP or only mild ROP and no evident neurological complications may also have affected visual functions, suggesting that other mechanisms are involved in this process.17,18
In a recent study, we showed increased macular thickness, measured with optical coherent tomography (OCT) in prematurely born children at school age.19 Further, increasing macular thickness was correlated with decreasing gestational age at birth, suggesting a disturbance in macular development due to the preterm birth.
The aim of the present study was to examine retinal function with ffERG in former preterm children and in children born at term. A second aim was to investigate if there was a correlation between retinal function and GA, VA, and ROP.
Materials and Methods
A study group of 35 children born before 32 weeks of gestation between 1996 and 2007 was recruited from the Department of Ophthalmology at Uppsala University Hospital, Sweden. All children had taken part in routine screening for ROP at the Neonatal Department at Uppsala University Hospital. Screening started in the fifth postnatal week and continued until the retina was fully vascularized, or in case of ROP, until it had fully resolved. ROP was classified according to the revised international classification.20 Treatment with cryopexy or laserphotocoagulation was performed if criteria for treatment were fulfilled: ROP stage 3 in at least four continuous clock hours with or without plus disease.
A control group of 42 children was recruited from Department of Ophthalmology at Uppsala University Hospital. They were born at term (GA > 37 weeks) with normal birth weights (BW > 2500 g).
Informed consent was obtained from the study children and their caregivers.
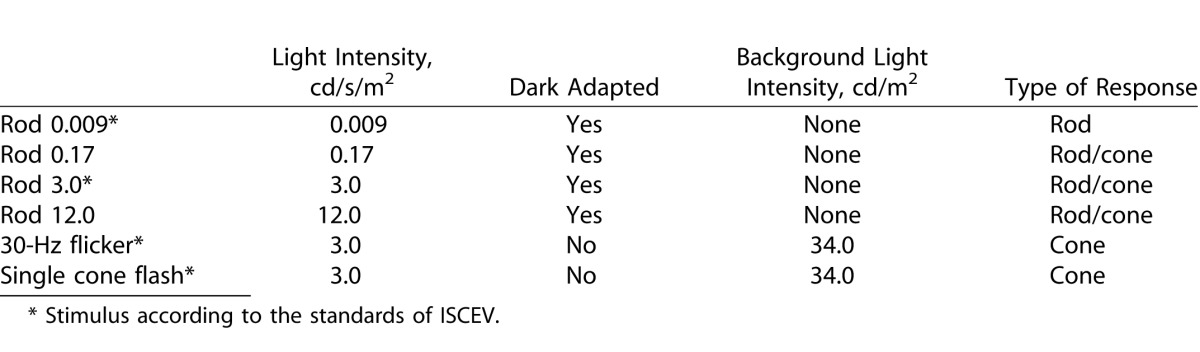
Best-corrected VA was assessed for each eye with linear logMar charts. Pupils were fully dilated using a mixture of phenylephrine 1.5% and cyclopentolate 0.85%. The pupil size was noted and extra dilating drops were given if the pupil size was less than 6 mm. FfERG was performed using the Espion system (Diagnosys, LCC, Lowell, MA) and stimuli were produced by white flash, light emitting diodes. Responses were obtained with a fixed-gain and a wide-band digital filter (0.3–300 Hz). Anesthetic eye drops were given and Dawson-Trick-Litzkow (DTL) electrodes, consisting of a thin silver thread, were applied along the lower eyelids. Reference electrodes were placed on both cheeks and a ground electrode on one hand. An impedance of 7 K was accepted. After dark adaption for 20 minutes the retina was stimulated in a Ganzfeld dome. A standard clinical protocol was used including scotopic rod response, scotopic rod/cone responses, and photopic cone responses. This protocol is mainly in accord with the standard of the International Society of Clinical physiology and Vision (ISCEV).21 An exception is the light-adaption time, which was excluded to make the procedure easier for the children.22 For details of the ERG-protocol see Table 1. The single-flash stimulations were performed six times and the interval between successive flashes in the rod responses was at least 5 seconds. Artifact rejection was active with response more than 1000 uV. An average of implicit times and amplitudes of the a- and b-waves with an amplitude greater than 1 uV was used for analysis. Fixation was continuously monitored with an infrared camera in the dome. After completion of the ffERG, automatic refraction in cyclopegia was performed.
Table 1.
Description of the Clinical Protocol for the Fullfield Electroretinogram Used in This Study, According to Light Intensity, Background Light, and Type of Response
The majority of the ERG assessments were performed by one of the authors (HÅ) and the others by two experienced technicians.
The study was approved by the ethics committee at Uppsala University, and adhered to the tenets of the Declaration of Helsinki.
Statistical Methods
Statistical calculations were performed using SPSS 21 (IBM Corporation, Armonk, NY). The preterm and fullterm groups were compared using the Mann-Whitney U test. Further, the fullterm group was compared with the preterm group divided into two groups, without ROP and with ROP, using Kruskal Wallis test and Dunn's multiple comparisons test. Correlations between ERG-responses and BW, VA, GA, ROP, and spherical equivalent were determined with the Spearman correlation test. P less than 0.05 was considered statistically significant.
Results
Data of the right eyes were used and descriptive data for the fullterm group and the preterm group are seen in Table 2. There was a difference in VA between the groups but not statistically significant. The spherical equivalent was significantly different between the groups using absolute values in the statistical analysis (P = 0.03). In the preterm group one child could only complete the Rod12.0, 30-Hz flicker and single cone flash due to poor cooperation. The result from the Rod 0.17 response in another child was excluded due to poor cooperation.
Table 2.
Descriptive Data (Mean and Range) of Right Eyes for the Fullterm and Preterm Groups
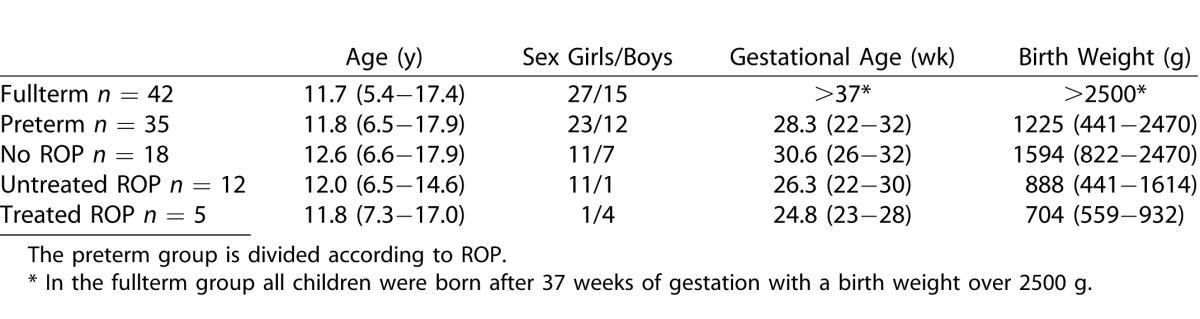
Table 2.
Extended
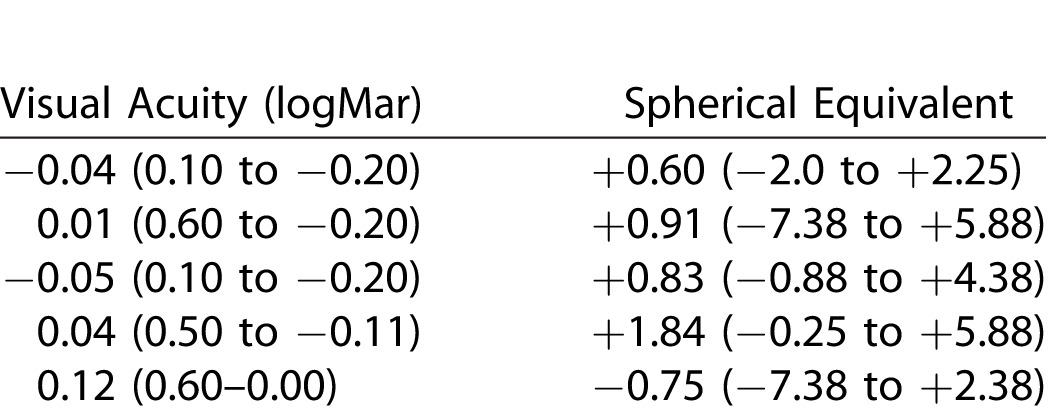
In the preterm group, 18 children had no ROP in the neonatal period, 12 had untreated ROP, and 5 children were treated for ROP with cryopexy or photocoagulation of the retina (Table 2).
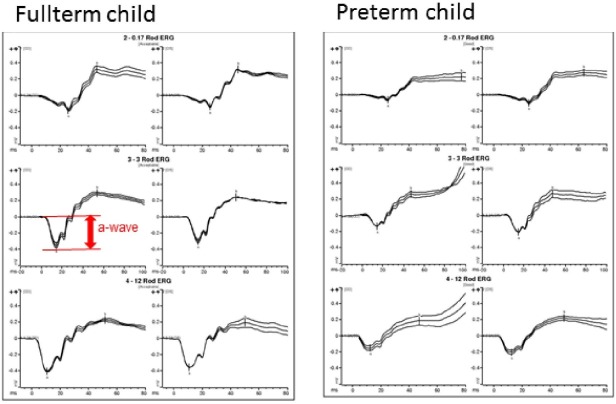
The results from the ERG protocols used from one fullterm and one preterm child are shown in Figure 1. The amplitudes and implicit times of the a-waves for the scotopic combined rod/cone responses in fullterms and preterms are seen in Table 3.
Figure 1.
Scotopic combined rod/cone responses (Rod 0.17, Rod 3.0, and Rod 12.0) of ffERG from one child in the fullterm group and one child in the preterm group of the same age at investigation. The a-wave in the scotopic rod/cone response (Rod 3.0) is marked for clarity.
Table 3.
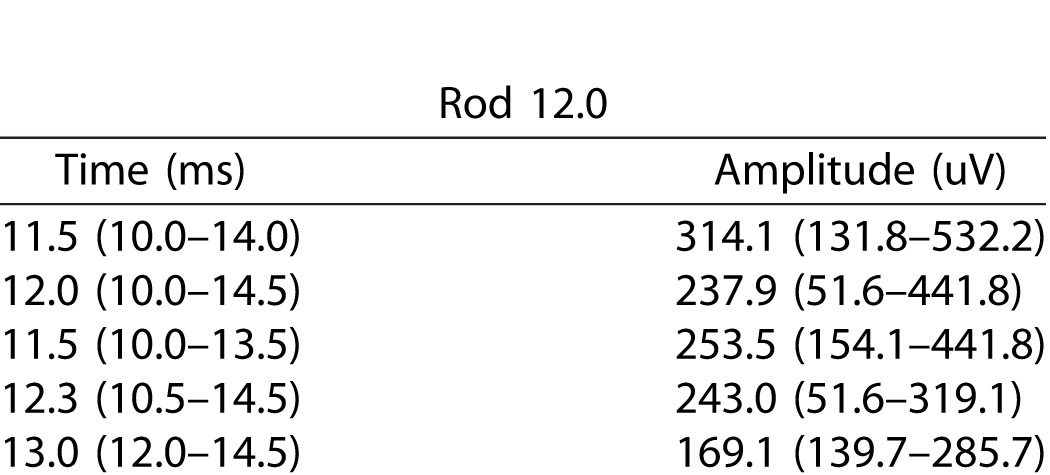
Median and Range of Implicit Times and Amplitudes of A-Waves in the Combined Rod/Cone ERG Responses
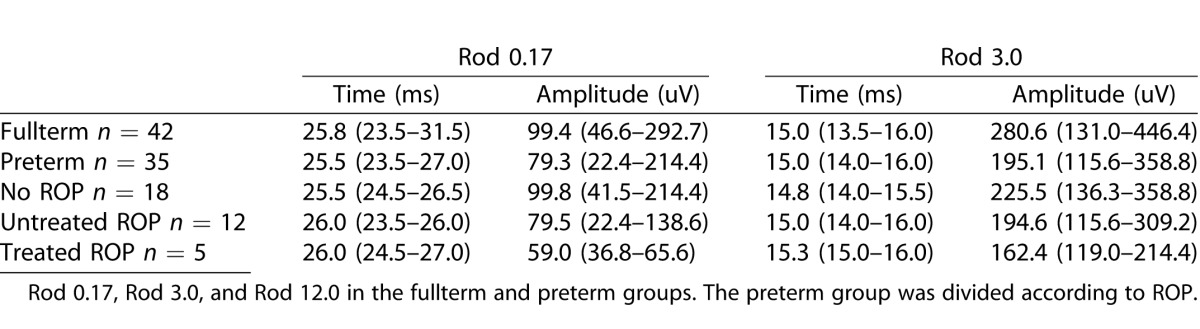
Table 3.
Extended
The a-wave amplitudes of the Rod 0.17, Rod 3.0, and Rod 12.0 responses were significantly lower in the preterm group compared with the fullterm group (Rod 0.17, P = 0.022; Rod 3.0, P = 0.003; Rod 12.0, P = 0.000). There were no differences between the groups regarding implicit times of the a-wave in any of the responses or regarding b-wave amplitude or implicit times. Finally, there were no differences between the preterm and fullterm groups regarding the 30-Hz flicker or single cone flash responses, neither regarding implicit times nor amplitudes.
All ERG-values for the fullterm group are presented in Supplementary Table S1 (36KB, docx) .
Relation to ROP
A-wave amplitudes and implicit times of the different ERG responses of the three subgroups of preterm children, children without ROP, with untreated ROP, and with treated ROP respectively, are illustrated in Table 3.
When dividing the preterm group in two groups, preterm without ROP and preterm with ROP, multiple comparisons showed that the difference between fullterm and preterm children was mainly due to the difference between the fullterms and the preterms with ROP, Rod 0.17 (P < 0.01), Rod 3.0 (P < 0.01), and Rod 12.0 (P < 0.001). There was a difference between the fullterm group and the preterms without ROP and between preterm with and without ROP as described in the boxplot in Figure 2, but the differences were not statistically significant.
Figure 2.
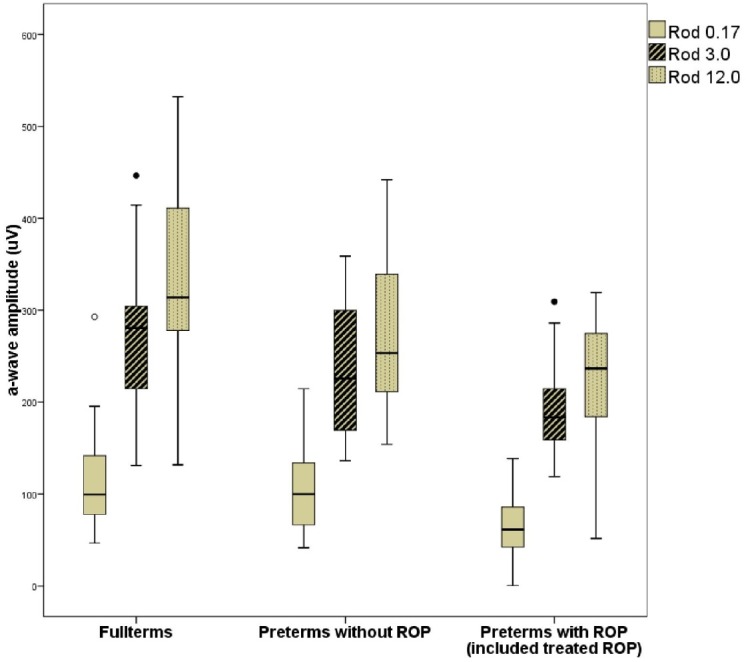
A-wave amplitude in Rod 0.17, Rod 3.0, and Rod 12.0 responses in right eyes of the fullterm group, the preterms without ROP and the preterms with ROP. Each box show the median and interquartile range, bars show range, and circles outliers.
Relation to GA
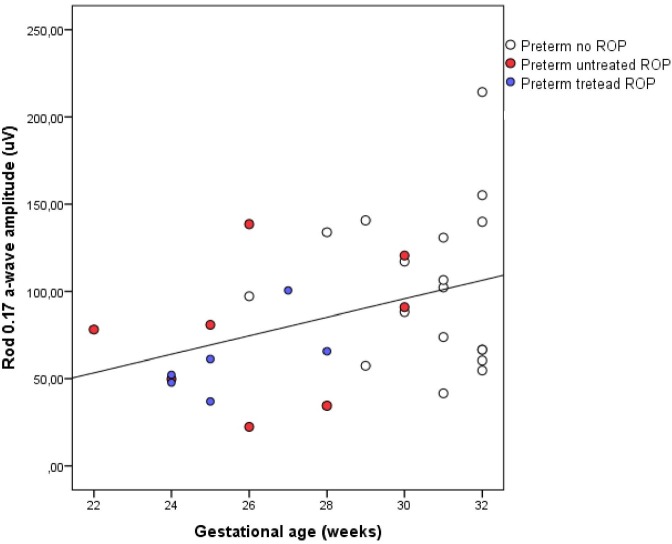
In the preterm group there were significant correlations between the a-wave amplitude of the Rod 0.17 response and GA (correlation coefficient = 0.38, P = 0.036), the Rod 3.0 response (correlation coefficient = 0.33, P = 0.039), and the Rod 12.0 response (correlation coefficient = 0.40, P = 0.029). All correlations became weaker and nonsignificant if children with ROP were excluded. In the other responses, there were no significant correlations with GA at birth. For illustration, the correlation between Rod 0.17 a-wave amplitude and GA is presented in Figure 3.
Figure 3.
Correlation between Rod 0.17 a-wave amplitude and gestational age at birth in the right eyes of the preterm group. Cases with untreated ROP and treated ROP are marked for clarity.
There were no correlations between a-wave amplitude and birth weight, VA, or spherical equivalent for any response.
Discussion
In the present study, the a-wave of the dark adapted rod/cone responses was reduced in prematurely born children compared with children born at term.
The a-wave of the ffERG represents the photoreceptor function, whereas the b-wave reflects the function of the postreceptor cells.23 The different ffERG responses include the contribution of rods and cones in various degree depending on stimulus parameters and adaption.24 Additionally, it has been suggested that early components of the post receptor response may affect the a-wave.25 In the ISCEV protocol, the scotopic Rod 0.17, Rod 3.0, and Rod 12.0 provide combined responses from both rods and cones.21
The a-wave amplitude in the combined rod/cone responses was reduced in the prematurely born children compared with children born at term in the present study. The b-wave amplitude was not affected when compared with the fullterm group. These results are in line with previous studies where preterm children with ROP in the neonatal period were found to have a reduced rod function compared with fullterm children.9,26 In those studies, the rod function was determined by repeated recordings of the responses to brief blue stimuli with different intensities, after which the sensitivity and saturation of the rod photoreceptors were calculated. The present study uses a “clinical protocol” mainly in accordance with the standards of ISCEV,21 which makes it possible to correlate the results to ERGs of known retinal diseases, and for others to repeat our trials.
The cone photoreceptor function in former preterm children seems to be affected to a lesser extent than the rod photoreceptor function.27 The photoreceptors in the peripheral retina consist of both rods and cones, but rods outnumber cones by 20 to 1. Differentiation of the peripheral photoreceptors differs and rods mature later than cones during the second part of gestation. It has been suggested that this makes the rods more vulnerable to effects of preterm birth, and may thus explain why cones are less affected than rods.8 This accords with the result of the present study, in which the cone response was not affected in the preterm group when compared with the fullterm group.
In the present study, ROP affected the rod photoreceptor function in former preterm children, also if children treated for ROP were excluded. This was in line with previous studies of Fulton et al.26 who discussed the role of ROP in the etiology of reduced rod photoreceptor function, explained by the hypoxic environment in the retina causing ROP. Our data point to an effect on rod function also in preterm children without ROP, but extended studies with larger groups of prematurely born children without ROP are needed to possibly show this more clearly.
There was a correlation between a-wave amplitude and GA in the preterm group of our study, but the correlation became weaker if the children with ROP were removed. In a previous study, we found a correlation between central macular thickness measured with OCT and GA, suggesting that prematurity per se affects the retinal development.19 Possibly a study with a larger number of children without ROP in the neonatal period would show if immaturity per se also affects the retinal function.
To conclude, prematurely born children in the present study had a reduced photoreceptor function at school age, possibly even if they did not have ROP during the neonatal period. The photoreceptor function is more reduced in the children with the lowest gestational ages at birth. Whether this is explained by ROP, maturity per se or a combination of both, remains to be elucidated.
Acknowledgments
The authors thank Eva Nuija, Department of Ophthalmology, and Margareta Grindlund and Berit Lindgren, Department of Neurophysiology, Uppsala University hospital, for helping with the examinations of the children in this study. They also thank Asa Vernby, Statistician, for advice concerning the statistical evaluation.
Supported by grants from the Carmen and Bertil Regner's fund and Crown princess Margareta's fund.
Disclosure: H. Åkerblom, None; S. Andreasson, None; E. Larsson, None; G. Holmström, None
References
- 1.Hendrickson A, Drucker D. The development of parafoveal and mid-peripheral human retina. Behav Brain Res. 1992;49:21–31. doi: 10.1016/s0166-4328(05)80191-3. [DOI] [PubMed] [Google Scholar]
- 2.Provis JM, van Driel D, Billson FA, Russell P. Development of the human retina: patterns of cell distribution and redistribution in the ganglion cell layer. J Comp Neurol. 1985;233:429–451. doi: 10.1002/cne.902330403. [DOI] [PubMed] [Google Scholar]
- 3.Fulton AB, Hansen RM, Westall CA. Development of ERG responses: the ISCEV rod, maximal and cone responses in normal subjects. Doc Ophthalmol. 2003;107:235–241. doi: 10.1023/b:doop.0000005332.88367.b8. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X, Huang X, Chen H, Zhao P. Comparison of electroretinogram between healthy preterm and term infants. Doc Ophthalmol. 2010;121:205–213. doi: 10.1007/s10633-010-9248-8. [DOI] [PubMed] [Google Scholar]
- 5.Hansen RM, Fulton AB. Development of the cone ERG in infants. Invest Ophthalmol Vis Sci. 2005;46:3458–3462. doi: 10.1167/iovs.05-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birch DG, Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;110:1571–1576. doi: 10.1001/archopht.1992.01080230071024. [DOI] [PubMed] [Google Scholar]
- 7.Berezovsky A, Moraes NS, Nusinowitz S, Salomao SR. Standard full-field electroretinography in healthy preterm infants. Doc Ophthalmol. 2003;107:243–249. doi: 10.1023/b:doop.0000005333.76622.c2. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton R, Bradnam MS, Dudgeon J, Mactier H. Maturation of rod function in preterm infants with and without retinopathy of prematurity. J Pediatr. 2008;153:605–611. doi: 10.1016/j.jpeds.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Harris ME, Moskowitz A, Fulton AB, Hansen RM. Long-term effects of retinopathy of prematurity (ROP) on rod and rod-driven function. Doc Ophthalmol. 2011;122:19–27. doi: 10.1007/s10633-010-9251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson EK, Rydberg AC, Holmstrom GE. A population-based study on the visual outcome in 10-year-old preterm and full-term children. Arch Ophthalmol. 2005;123:825–832. doi: 10.1001/archopht.123.6.825. [DOI] [PubMed] [Google Scholar]
- 11.Larsson EK, Rydberg AC, Holmstrom GE. A population-based study of the refractive outcome in 10-year-old preterm and full-term children. Arch Ophthalmol. 2003;121:1430–1436. doi: 10.1001/archopht.121.10.1430. [DOI] [PubMed] [Google Scholar]
- 12.Larsson E, Rydberg A, Holmstrom G. Contrast sensitivity in 10 year old preterm and full term children: a population based study. Br J Ophthalmol. 2006;90:87–90. doi: 10.1136/bjo.2005.081653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmstrom G, Rydberg A, Larsson E. Prevalence and development of strabismus in 10-year-old premature children: a population-based study. J Pediatr Ophthalmol Strabismus. 2006;43:346–352. doi: 10.3928/01913913-20061101-04. [DOI] [PubMed] [Google Scholar]
- 14.Larsson E, Martin L, Holmstrom G. Peripheral and central visual fields in 11-year-old children who had been born prematurely and at term. J Pediatr Ophthalmol Strabismus. 2004;41:39–45. doi: 10.3928/0191-3913-20040101-10. [DOI] [PubMed] [Google Scholar]
- 15.Hellgren K, Hellstrom A, Jacobson L, Flodmark O, Wadsby M, Visual Martin L. and cerebral sequelae of very low birth weight in adolescents. Arch Dis Child Fetal Neonatal Ed. 2007;92:F259–F264. doi: 10.1136/adc.2006.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Connor AR, Spencer R, Birch EE. Predicting long-term visual outcome in children with birth weight under 1001 g. J AAPOS. 2007;11:541–545. doi: 10.1016/j.jaapos.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Holmstrom G, Larsson E. Long-term follow-up of visual functions in prematurely born children–a prospective population-based study up to 10 years of age. J AAPOS. 2008;12:157–162. doi: 10.1016/j.jaapos.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor AR, Stewart CE, Singh J, Fielder AR. Do infants of birth weight less than 1500 g require additional long term ophthalmic follow up? Br J Ophthalmol. 2006;90:451–455. doi: 10.1136/bjo.2005.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akerblom H, Larsson E, Eriksson U, Holmstrom G. Central macular thickness is correlated with gestational age at birth in prematurely born children. Br J Ophthalmol. 2011;95:799–803. doi: 10.1136/bjo.2010.184747. [DOI] [PubMed] [Google Scholar]
- 20.International Committee for the Classification of Retinopathy of P. The International Classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 21.Marmor MF, Fulton AB, Holder GE, et al. ISCEV Standard for full-field clinical electroretinography (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 22.Malm E, Ponjavic V, Moller C, Kimberling WJ, Stone ES, Andreasson S. Alteration of rod and cone function in children with Usher syndrome. Eur J Ophthalmol. 2011;21:30–38. doi: 10.5301/ejo.2010.5433. [DOI] [PubMed] [Google Scholar]
- 23.Hood DC, Birch DG. The A-wave of the human electroretinogram and rod receptor function. Invest Ophthalmol Vis Sci. 1990;31:2070–2081. [PubMed] [Google Scholar]
- 24.Robson JG, Frishman LJ. Dissecting the dark-adapted electroretinogram. Doc Ophthalmol. 1998;95:187–215. doi: 10.1023/a:1001891904176. [DOI] [PubMed] [Google Scholar]
- 25.Jamison JA, Bush RA, Lei B, Sieving PA. Characterization of the rod photoresponse isolated from the dark-adapted primate ERG. Vis Neurosci. 2001;18:445–455. doi: 10.1017/s0952523801183112. [DOI] [PubMed] [Google Scholar]
- 26.Fulton AB, Hansen RM, Petersen RA, Vanderveen DK. The rod photoreceptors in retinopathy of prematurity: an electroretinographic study. Arch Ophthalmol. 2001;119:499–505. doi: 10.1001/archopht.119.4.499. [DOI] [PubMed] [Google Scholar]
- 27.Fulton AB, Hansen RM, Moskowitz A. The cone electroretinogram in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2008;49:814–819. doi: 10.1167/iovs.07-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]