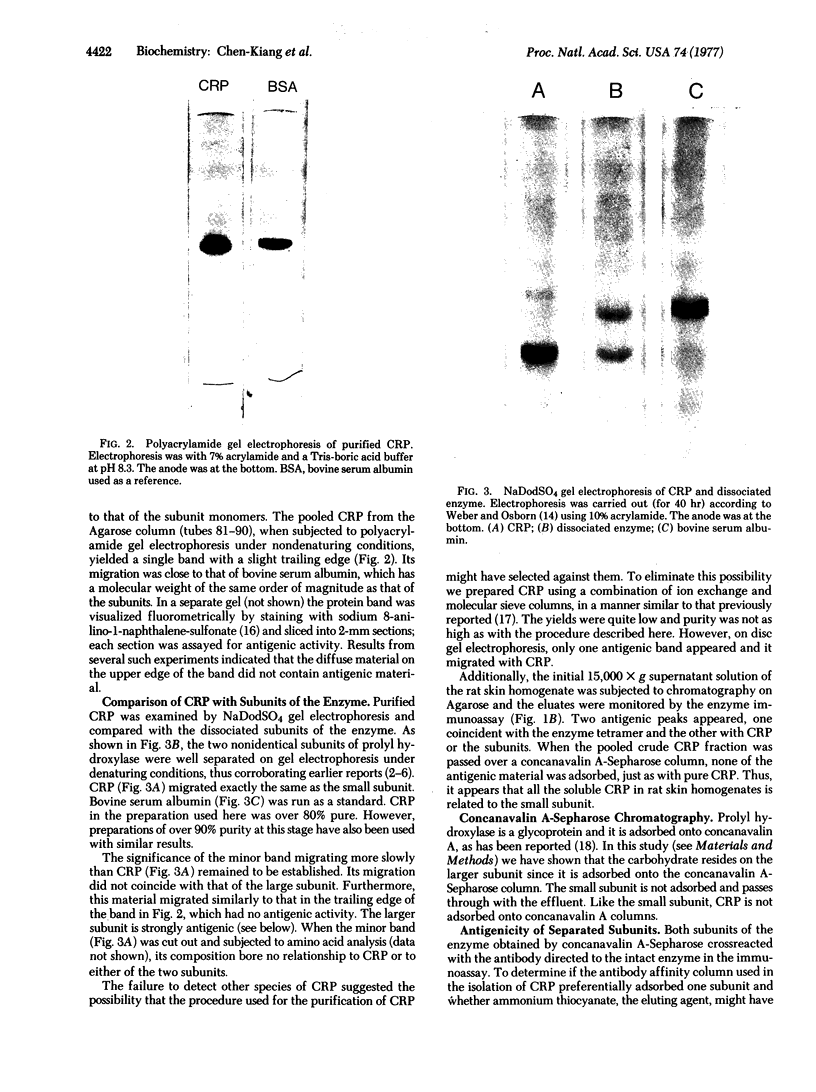
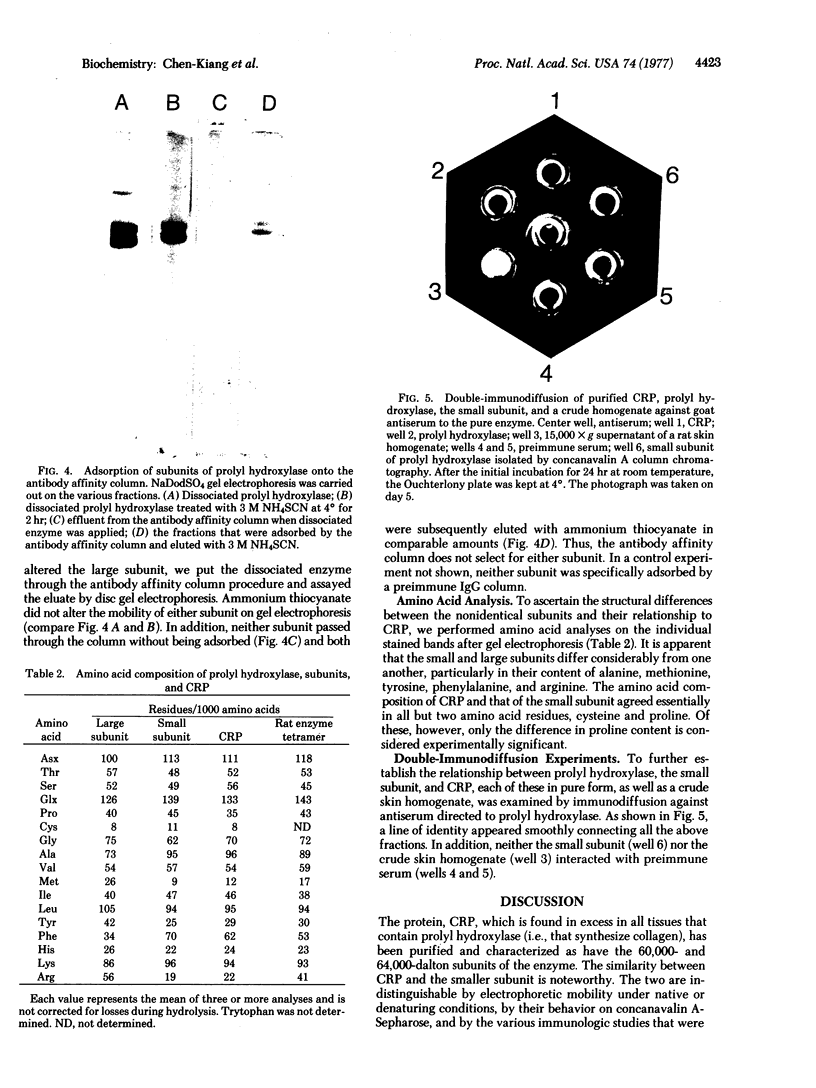
Abstract
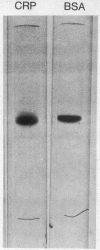
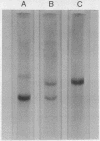
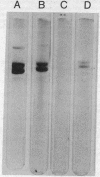
A protein, enzymatically inactive but immunologically related to prolyl hydroxylase (prolyl-glycyl-peptide, 2-oxoglutarate:oxygen oxidoreductase; EC 1.14.11.2) (cross-reacting protein), has been purified to near homogeneity from skin of newborn rats. The purified protein has a molecular weight of 60,000 on gel filtration and sodium dodecyl sulfate gel electrophoresis, corresponding to that of the smaller of the two dissimilar subunits of the enzyme. The two subunits of prolyl hydroxylase differ markedly from one another in their amino acid compositions, but crossreating protein and the smaller subunit are very similar in composition. On antibody-affinity chromatography both subunits reacted with the antibody developed against the intact enzyme. Neither crossreacting protein nor the 60,000 molecular weight subunit was adsorbed onto concanavalin A, which adsorbed the intact enzyme as well as the larger subunit. It would appear that crossreacting protein is identical to one of the subunits of prolyl hydroxylase or metabolically related to it.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Chichester C. O., 3rd, Fuller G. C., Cardinale G. J. In vivo labeling and turnover of prolyl hydroxylase and a related immunoreactive protein. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1056–1062. doi: 10.1016/0006-291x(76)90230-8. [DOI] [PubMed] [Google Scholar]
- Guzman N. A., Berg R. A., Prockop D. J. Concanavalin A binds of purified prolyl hydroxylase and partially inhibits its enzymic activity. Biochem Biophys Res Commun. 1976 Nov 22;73(2):279–285. doi: 10.1016/0006-291x(76)90704-x. [DOI] [PubMed] [Google Scholar]
- Hartman B. K., Udenfriend S. A method for immediate visualization of proteins in acrylamide gels and its use for preparation of antibodies to enzymes. Anal Biochem. 1969 Sep;30(3):391–394. doi: 10.1016/0003-2697(69)90132-8. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Ascorbate increases the synthesis of procollagen hydroxyproline by cultured fibroblasts from chick embryo tendons without activation of prolyl hydroxyla. Biochim Biophys Acta. 1975 Dec 5;411(2):202–215. doi: 10.1016/0304-4165(75)90300-1. [DOI] [PubMed] [Google Scholar]
- Kuttan R., Cardinale G. J., Udenfriend S. An activatable form of prolyl hydroxylase in fibrobalast extracts. Biochem Biophys Res Commun. 1975 Jan 2;64(3):947–954. doi: 10.1016/0006-291x(75)90139-4. [DOI] [PubMed] [Google Scholar]
- Kuutti E. R., Tuderman L., Kivirikko K. I. Human prolyl hydroxylase. Purification, partial characterization and preparation of antiserum to the enzyme. Eur J Biochem. 1975 Sep 1;57(1):181–188. doi: 10.1111/j.1432-1033.1975.tb02289.x. [DOI] [PubMed] [Google Scholar]
- McGee J. O., Langness U., Udenfriend S. Immunological evidence for an inactive precursor of collagen proline hydroxylase in cultured fibroblasts. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1585–1589. doi: 10.1073/pnas.68.7.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee J. O., Udenfriend S. Partial purification and characterization of peptidyl proline hydroxylase precursor from mouse fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):216–221. doi: 10.1016/0003-9861(72)90209-3. [DOI] [PubMed] [Google Scholar]
- Miller R. L. Chromatographic separation of the enzymes required for hydroxylation of lysine and proline residues of protocollagen. Arch Biochem Biophys. 1971 Nov;147(1):339–342. doi: 10.1016/0003-9861(71)90342-0. [DOI] [PubMed] [Google Scholar]
- Ooshima A., Fuller G. C., Cardinale G. J., Spector S., Udenfriend S. Increased collagen synthesis in blood vessels of hypertensive rats and its reversal by antihypertensive agents. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3019–3023. doi: 10.1073/pnas.71.8.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus F. J., Newman E. S., Hansen H. J. Affinity in radioimmunoassay of antibody cross-reactive with carcinoembryonic antigen (CEA) and colon carcinoma antigen-III (CCA-III). J Immunol. 1977 Jan;118(1):55–61. [PubMed] [Google Scholar]
- Rhoads R. E., Udenfriend S. Purification and properties of collagen proline hydroxylase from newborn rat skin. Arch Biochem Biophys. 1970 Aug;139(2):329–339. doi: 10.1016/0003-9861(70)90485-6. [DOI] [PubMed] [Google Scholar]
- Risteli J., Tuderman L., Kivirikko K. I. Intracellular enzymes of collagen biosynthesis in rat liver as a function of age and in hepatic injury induced by dimethylnitrosamine. Purification of rat prolyl hydroxylase and comparison of changes in prolyl hydroxylase activity with changes in immunoreactive prolyl hydroxylase. Biochem J. 1976 Aug 15;158(2):369–376. doi: 10.1042/bj1580369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. E., McGee J. O., Udenfriend S. Antibody to prolyl hydroxylase from rat skin and its cross-reactivity with enzyme from other species. Connect Tissue Res. 1973;2(1):65–69. doi: 10.3109/03008207309152601. [DOI] [PubMed] [Google Scholar]
- Rubinstein M., Stein S., Gerber L. D., Udenfriend S. Isolation and characterization of the opioid peptides from rat pituitary: beta-lipotropin. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3052–3055. doi: 10.1073/pnas.74.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryhänen L. Lysyl hydroxylase. Further purification and characterization of the enzyme from chick embryos and chick embryo cartilage. Biochim Biophys Acta. 1976 Jun 7;438(1):71–89. doi: 10.1016/0005-2744(76)90224-2. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., McGee J. O., Udenfriend S. Prolyl hydroxylase and an immunologically related protein in mammalian tissues. Arch Biochem Biophys. 1974 Jan;160(1):340–345. doi: 10.1016/s0003-9861(74)80042-1. [DOI] [PubMed] [Google Scholar]
- Stein S., Chang C. H., Böhlen P., Imai K., Udenfriend S. Amino acid analysis with fluorescamine of stained protein bands from polyacrylamide gels. Anal Biochem. 1974 Jul;60(1):272–277. doi: 10.1016/0003-2697(74)90154-7. [DOI] [PubMed] [Google Scholar]
- Tuderman L. Developmental changes in prolyl hydroxylase activity and protein in chick embryo. Eur J Biochem. 1976 Jul 15;66(3):615–621. doi: 10.1111/j.1432-1033.1976.tb10589.x. [DOI] [PubMed] [Google Scholar]
- Tuderman L., Kuutti E. R., Kivirikko K. I. An affinity-column procedure using poly(L-proline) for the purification of prolyl hydroxylase. Purification of the enzyme from chick embryos. Eur J Biochem. 1975 Mar 3;52(1):9–16. doi: 10.1111/j.1432-1033.1975.tb03967.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]