Abstract
Objectives
Trefoil factor 1 (TFF1) is a stable secretory protein expressed widely in the gastrointestinal mucosa that is also expressed in pancreatic ductal adenocarcinoma (PDAC). In the current study, we documented the extent and timing of TFF1 expression and investigated the effects of TFF1 on PDAC cells and stellate cells, the primary cells of the PDAC stroma.
Methods
Trefoil factor 1 expression in pancreatic cancer tissues and cell lines was analyzed using microarray, quantitative reverse transcriptase–polymerase chain reaction, and immunohistochemistry. The effects of recombinant TFF1 on cell growth, migration, and invasion of pancreatic cancer cell lines and immortalized human pancreatic stellate cells (HPSCs) were analyzed using MTS and Matrigel-coated invasion chambers. In vivo studies were also conducted in which Mpanc-96 cells stably expressing TFF1 were implanted orthotopically into nude mice.
Results
Trefoil factor 1 was highly increased in preneoplastic lesions. Recombinant TFF1 stimulated motility of both cancer and HPSCs. In contrast, only HPSC cell growth was increased by TFF1. In vivo studies showed that overexpression of TFF1 in PDAC cells did not affect primary tumor growth but greatly increased metastasis.
Conclusions
The present data demonstrate that TFF1 influences both PDAC cells and stellate cells and stimulates metastasis.
Keywords: pancreatic cancer, TFF1, bioluminescence, stellate cells, PanIN
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related deaths in western countries.1 Worldwide, pancreatic cancer causes an estimated 213,000 deaths each year.2 For all stages combined, the 1-year survival rate is around 20%, and the overall 5-year survival rate is less than 5%, despite even the most aggressive therapies available.1 Pancreatic tumors develop a complex microenvironment including cancer, stellate, endothelial, and immune cells.3 The stellate cells are a dominant myofibroblast-like cell that produces abundant extracellular matrix. Cancer cells alter their microenvironment largely by secretion of soluble factors that influence stellate cells.4 Likewise, pancreatic stellate cells (PSCs) modify the phenotype, invasiveness, and metastatic capacity of cancer cells.5 Thus, interaction between cancer and stellate cells plays a major role in pancreatic cancer aggressive behavior. Identification of the key players in this interaction is important to allow understanding of the biology of pancreatic cancer.
In the pancreas, trefoil proteins are expressed in cancerous but not in normal cells.6–8 Trefoil proteins are secreted chiefly from mucus-secreting cells of the gastrointestinal tract and are implicated in the protection of the gastrointestinal tract against mucosal damage and its subsequent repair.9,10 Members of the family each contain 1 or more copies of the trefoil domain comprising 42 to 43 amino acids each with 6 cysteine amino acid residues that form disulfide bonds resulting in the characteristic trefoil structural motif. This motif is formed from the 3 loops that are stacked parallel to each other, with the third loop positioned between the first and second.9,10 Their expression is increased in response to gastrointestinal injury and has been reported to act as motogens to facilitate cell migration into the lesion, thus forming a protective barrier in a process known as restitution.10 In addition, trefoil proteins are potent inhibitors of apoptosis and prevent anoikis (cell death induced by anchorage independence) during the cell migration process.10–12 The distinct signaling pathways that mediate the effects of trefoil proteins have not been fully elucidated, nor have definitive functional receptors for trefoil proteins been identified.13 In addition to the protective and restorative effects of trefoil proteins in the gastrointestinal tract, recent evidence has indicated that members of this family also possess pivotal roles in the development and progression of human cancers including gastric,14 breast,15 colon,16 and prostate.17 Despite the observation that trefoil factor (TFF1) is widely expressed in pancreatic cancer,6–8 its biological functions in this disease are unknown.
In the current study, we investigated the expression and function of TFF1 in pancreatic cancer. We found that TFF1 was expressed early in preneoplastic cells and also in advanced cancer cells but was not expressed by human pancreatic stellate cells (HPSCs). Extracellular addition of TFF1 increased pancreatic cancer cell invasion, but not proliferation. In contrast, TFF1 increased both proliferation and migration HPSCs. Orthotopic tumors that arose from cancer cells expressing high levels of TFF1 showed that the ectopic expression of TFF1 increased metastasis but not primary tumor growth. Thus, TFF1 influenced both cancer and stellate cells and increased the aggressiveness of pancreatic cancer.
MATERIALS AND METHODS
Tissue Specimens and Cell Lines
The primary tumors and normal and pancreatitis tissues analyzed in this study were derived from the University of Texas MD Anderson Cancer Center and conformed to the policies and practices of the MD Anderson Cancer Center Internal Review Board. HPAF II, HPAC, Capan-I, Capan-II, SUR 99, BxPc3, Mpanc-96, CFPAC-1, Panc-1, and SU 86.86 pancreatic cancer cell lines were obtained from the American Type Culture Collection (Manassas, Va), and the nontransformed human ductal epithelial (HPDE) cell line was kindly provided by Dr M. S. Tsao (University of Toronto, Ontario, Canada). MOH cells were a kind gift from Dr R. Mohammad (Wayne State University, Mich). PSN-1 was kindly provided by Dr Jiro Okami (Osaka Medical Center, Osaka, Japan). Cell line identities were verified using DNA fingerprinting (data not shown). Cells were routinely cultured in recommended media. Isolation and immortalization of HPSC have been previously reported.5,18 All cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Polymerase Chain Reaction of TFF1
Standard reverse transcriptase–polymerase chain reaction (RT-PCR) and quantitative RT-PCR (QRT-PCR) used total RNA prepared from normal human pancreas, pancreatic adenocarcinomas, and chronic pancreatitis and different cell lines as described previously. Reverse transcription was for 45 minutes at 45°C from 500 ng of purified total RNA in a 25-µL volume of reverse transcription system reaction mixture by using avian myeloblastosis virus reverse transcriptase (Promega, Madison, Wis). For standard RT-PCR, reverse transcription was followed by 35 cycles of PCR (1-minute denaturation at 94°C, 1-minute annealing at 65°C, and 1-minute extension at 72°C). For the QRT-PCR, the PCR reactions extended through 40 cycles. Primers designed for human TFF1 (GenBank: X52003) were as follows: forward 5′ CCGGA TCCTGGAGCAGAGAGGAGGCA 3′ and reverse, 5′ CCGAATT CGCAGGCAGATCCCTGCAG 3′. Primers designed for Β-actin (GenBank: BC016045), which was used as a loading control for the RT-PCR reactions, were as follows: forward 5′ ATGATATCG CCGCGCTCGTCGTC 3′ and reverse, 5′ CGCTCGGCCGTGGT GGT GAA 3′. Amplified products were separated on 1.5% agarose gels and visualized after staining with ethidium bromide.
Enzyme-Linked Immunosorbent Assay for TFF1
Trefoil factor 1 was quantified in the media collected from TFF1-transfected Mpanc-96 cells. Trefoil factor 1 was captured between an anti–mouse TFF1 monoclonal antibody (cat. no. P3742; Sigma, St Louis, Mo) and the TFF1 polyclonal antibody (cat. no. GTX28762; GeneTex, San Antonio, Tex) using an enzyme-linked immunosorbent assay kit (KPL, Gaithersburg, Md). Recombinant TFF1 was used as a standard. Cells were plated at 5 × 105 cells per well in 6-well plate for 3 days, and medium was collected and concentrated using YM10 Centricon concentrating filters (Centricon, Billerica, Mass). Concentrated media (100 µL) was incubated for 2 hours at room temperature in antibody-coated plates and washed 3 times with wash buffer. Bound TFF1 was detected with horseradish peroxidase–labeled anti–rabbit secondary antibody and TMB (3,3′,5,5′-tetramethylbenzidene) substrate. Color development was blocked with 1Mphosphoric acid and read at 450 nm.
Immunohistochemical Staining
Unstained 4-µm sections of clinical specimens or a tissue microarray were deparaffinized with xylene and rehydrated with ethanol. Immunohistochemistry used RTU Vectastain Elite ABC Universal kit (Vector Laboratories, Burlingame, Calif) according to the manufacturer’s instructions. Primary antibody against TFF1 (cat. no. GTX28762; GeneTex) was diluted 1:250 in 2% bovine serum albumin/0.2% Triton in phosphate-buffered saline (PBS). Finally, slides were developed with 3,3-diaminobenzidine substrate counterstained with hematoxylin, dehydrated with ethanol, fixed with xylene, and mounted.
Cell Growth Studies
Cell growth was analyzed using the MTS reagent (Promega) according to the manufacturer’s directions. Wild-type Mpanc-96, BxPc-3, or HPSCs (5000 cells) were plated on 96-well plates, and TFF1 (0–100 nM) was added to the cells, and cell numbers were estimated at 48 hours by MTS assay, which was added to the wells 1 hour before taking the photometric reading.
Migration and Invasion Assays
For migration and invasion studies, cells were seeded in BioCoat Matrigel migration or invasion chambers, respectively (Becton-Dickinson, Bedford, Mass), according to the manufacturer’s protocol. Briefly, 2.0 × 105 cells suspended in 300 µL of serum-free medium were added to each chamber, and different concentrations of TFF1 were added to the bottom wells, and the cells were allowed to migrate (8 hours) or invade Matrigel (24 hours) at 37°C in a 5% CO2 atmosphere. The noninvaded or nonmigrated cells on the upper surface of the membrane were removed from the chambers with a cotton swab, and the invaded or migrated cells on the lower surface of the membrane were fixed and stained using a Diff-Quick stain kit (Becton-Dickinson). After 2 washes with water, the chambers were allowed to air dry. The number of invaded or migrated cells in 5 adjacent microscope fields per membrane was counted at 20× magnification, and the average number of cells per field was calculated. The assays were performed 3 times.
Tumor Growth and Metastasis Studies In Vivo
MPanc96 cells were stably transfected with a pcDNA 3.1+ TFF1 expression plasmid or control plasmid. Expression of TFF1 was confirmed by enzyme-linked immunosorbent assay. These cells were also transfected stably with luciferase gene by lentivirus transfection.19 The tumorigenic and metastatic capabilities of the TFF1-expressing cells were compared with control cells in 4-week-old male athymic nude mice. Briefly, cells were grown to 80% confluence, harvested by trypsinization, washed twice in PBS, and resuspended to a final concentration of 1 × 106 cells/50 µL and injected into the pancreas. Six mice were inoculated per test group, and tumor growth was assessed every week by bioluminescence imaging for 6 weeks.
Bioluminescence imaging used a cryogenically cooled imaging system coupled to a data acquisition computer running LivingImage software (Xenogen Corp, Alameda, Calif). Before imaging, animals were anesthetized in an acrylic chamber with 1.5% isoflurane-air mixture and injected intraperitoneally with 40 mg/mL of luciferin potassium salt in PBS at a dose of 150 mg/kg body weight. A digital grayscale animal image was acquired followed by acquisition and overlay of a pseudocolor image representing thespatial distribution of detected photons emerging from active luciferase within the animal. Signal intensity was quantified as the sum of all detected photons within the region of interest per second. Tumor volume, peritoneal dissemination, and metastasis were assessed. After the bioluminescence imaging, animals was dissected, and cancer cell dissemination and metastases were visualized and counted. Tissues were also fixed with formaldehyde and examined histologically to verify the accuracy of the bioluminescence data.19
Statistical Analysis
Data are presented as mean ± SEM. Statistically significant differences were determined by unpaired t test. When more than 2 groups were analyzed, analysis of variance was used to analyze the data, and furthermore, Newman-Keuls multiple comparison test was used to check the posttest significance. Statistical significance was defined as P < 0.05. Results were compared using GraphPad Prism 4 software (GraphPad Software, http://www.graphpad.com).
RESULTS
TFF1 Is Expressed in Pancreatic Cancer Cells and in Preneoplastic Lesions
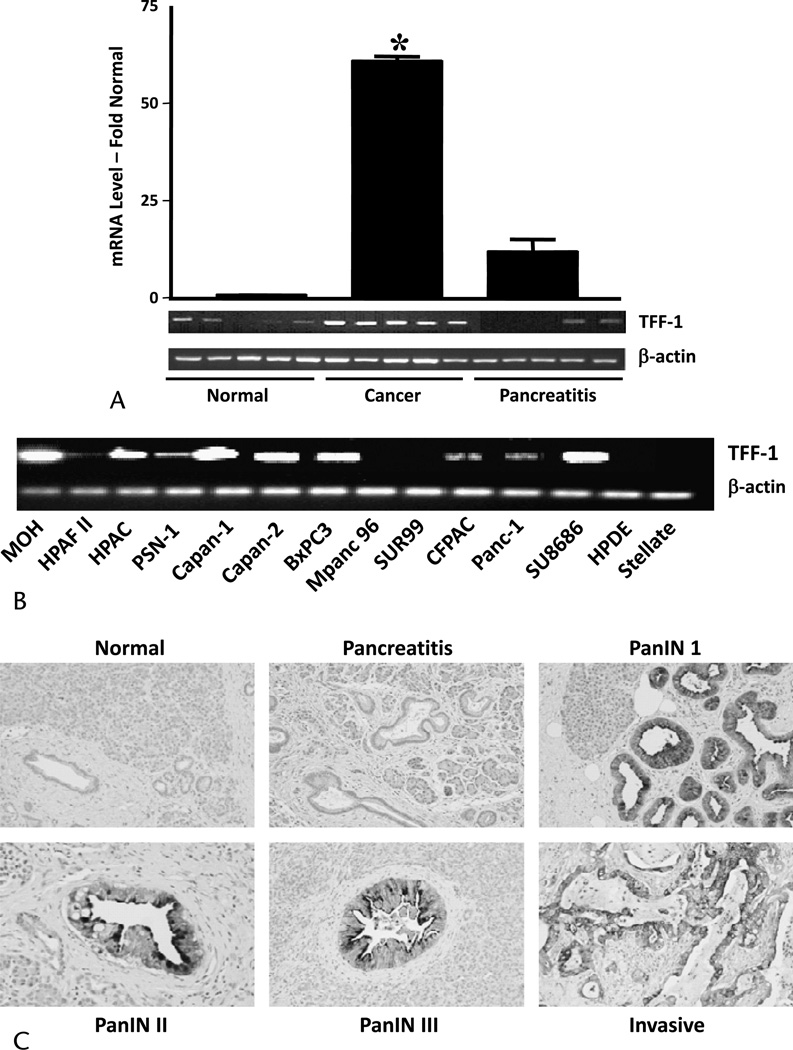
Our previous microarray study indicated that TFF1 mRNA was highly expressed in pancreatic cancer when compared with normal and pancreatitis tissues.6 To verify this result, we quantified TFF1 mRNA in normal, cancer, and pancreatitis tissues by QRT-PCR. Trefoil factor 1 mRNA was expressed at 65-fold higher levels in cancer tissue compared with normal or chronic pancreatitis tissues (Fig. 1A). Trefoil factor 1 was also expressed at detectable levels by most (10/12) of the pancreatic cancer cell lines examined (Fig. 1B). In contrast, TFF1 mRNA was absent in the immortalized HPDE cell line and in HPSCs. Immunohistochemistry of a tissue microarray indicated that TFF1 was expressed in preneoplastic lesions (5/6 pancreatic intraepithelial neoplasia 1 [PanIN1]; 6/6 PanIN2, and 5/6 PanIN3) and in the majority of pancreatic adenocarcinomas (50/70) (Fig. 1C). In contrast, TFF1 was not found either in normal healthy pancreas or in chronic pancreatitis, a nonneoplastic inflammatory condition. Thus, the tissue expression of TFF1 showed promise as a histological marker for pancreatic cancer.
FIGURE 1.
Trefoil factor 1 expression in pancreatic cancer. A, Quantitative RT-PCR showing the expression of TFF1 mRNA in pancreatic cancer tissue using Β-actin as control. Insert shows representative RT-PCR. B, Reverse transcriptase–polymerase chain reaction showing the expression of TFF1 in pancreatic cancer cell lines and HPDE and in HPSC cells using Β-actin as control. C, Immunohistochemistry showing the absence of TFF1 in normal and chronic pancreatitis tissues and early expression of TFF1 in PanINs1–3 and also in pancreatic adenocarcinoma.
TFF1 Stimulates Cancer Cell Invasion But Not Proliferation
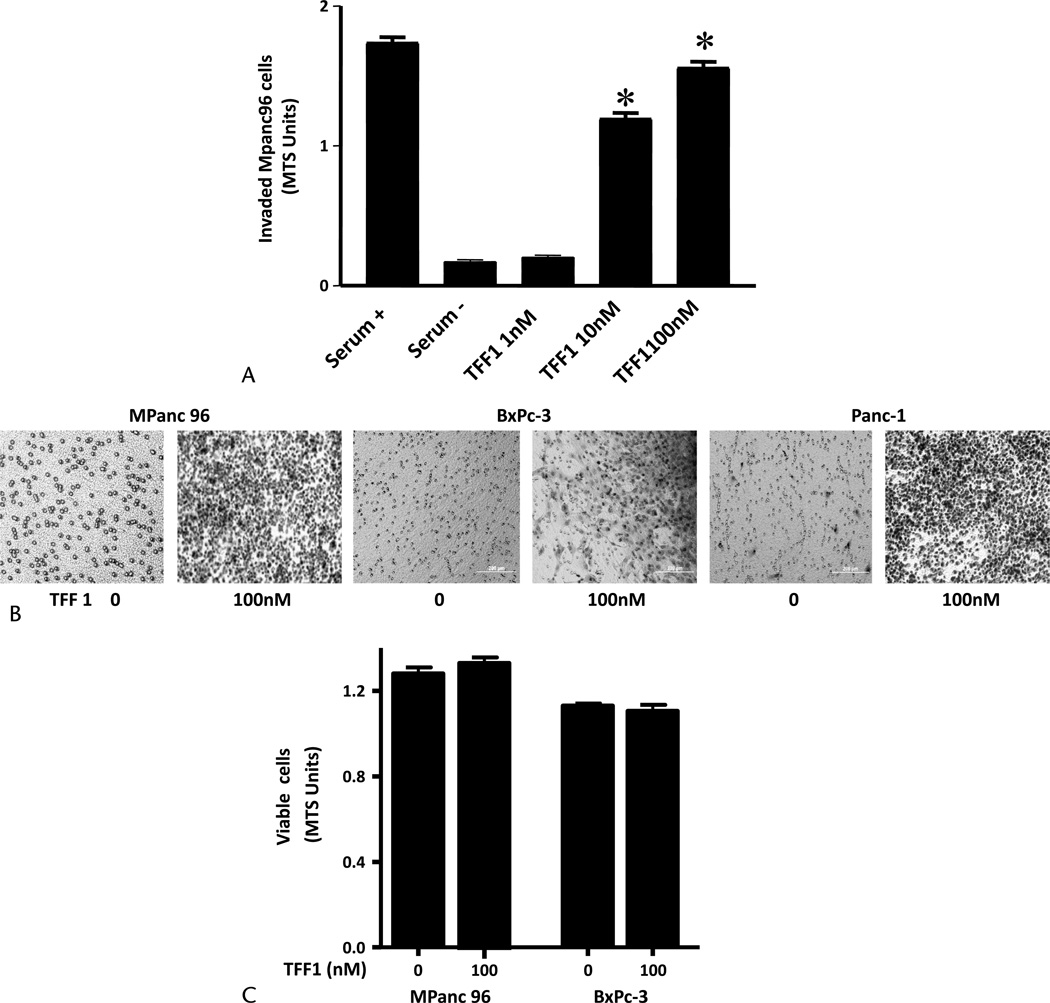
The effects of treatment with recombinant TFF1 were studied on proliferation and invasion of pancreatic cancer cells in vitro. In cultures of Mpanc-96 cells, TFF1 (0–100 nM) was added to the bottom wells of invasion chambers for 24 hours. Trefoil factor 1 increased the number of invading cells in a concentration-dependent manner with significant effects noted with 10 nM of TFF1 (Fig. 2A). Likewise, TFF1 stimulated invasion rates of other pancreatic cancer cell lines BxPC-3 and Panc-1 (Fig. 2B). However, TFF1 had no effect on cell proliferation of Mpanc-96 or BxPC-3 cells (Fig. 2C).
FIGURE 2.
Exogenous TFF1 stimulates pancreatic cancer cell invasion but not proliferation in vitro. A, Trefoil factor 1 stimulated pancreatic cancer cell invasion in a concentration-dependent manner. Wild-type Mpanc-96 cells (20,000 cells) were added into BioCoat Matrigel invasion upper chamber, and different concentrations of TFF1 (0–100 nM) were added into the lower chamber. After 24 hours, noninvaded cells on the upper membrane were removed, and cells invading into the lower membrane were quantified photometrically using MTS reagent added 1 hour before taking the reading. Dulbecco’s modified Eagle medium containing 10% or 0% serum was used as control. B, Photographs of representative membranes of migrated Mpanc-96, BxPc3, and Panc-1 after Diff-Quick staining are shown. C, Trefoil factor 1 did not stimulate pancreatic cancer cell proliferation. Wild-type Mpanc-96 and BxPc-3 cells (5000 cells) were plated on 96-well plates, and TFF1 (0 and 100 nM) was added to the cells for 48 hours. Cell numbers were estimated by MTS assay. Data shown are mean ± SEM for 3 experiments. *P < 0.05 versus control.
TFF1 Stimulates Stellate Cell Proliferation and Migration
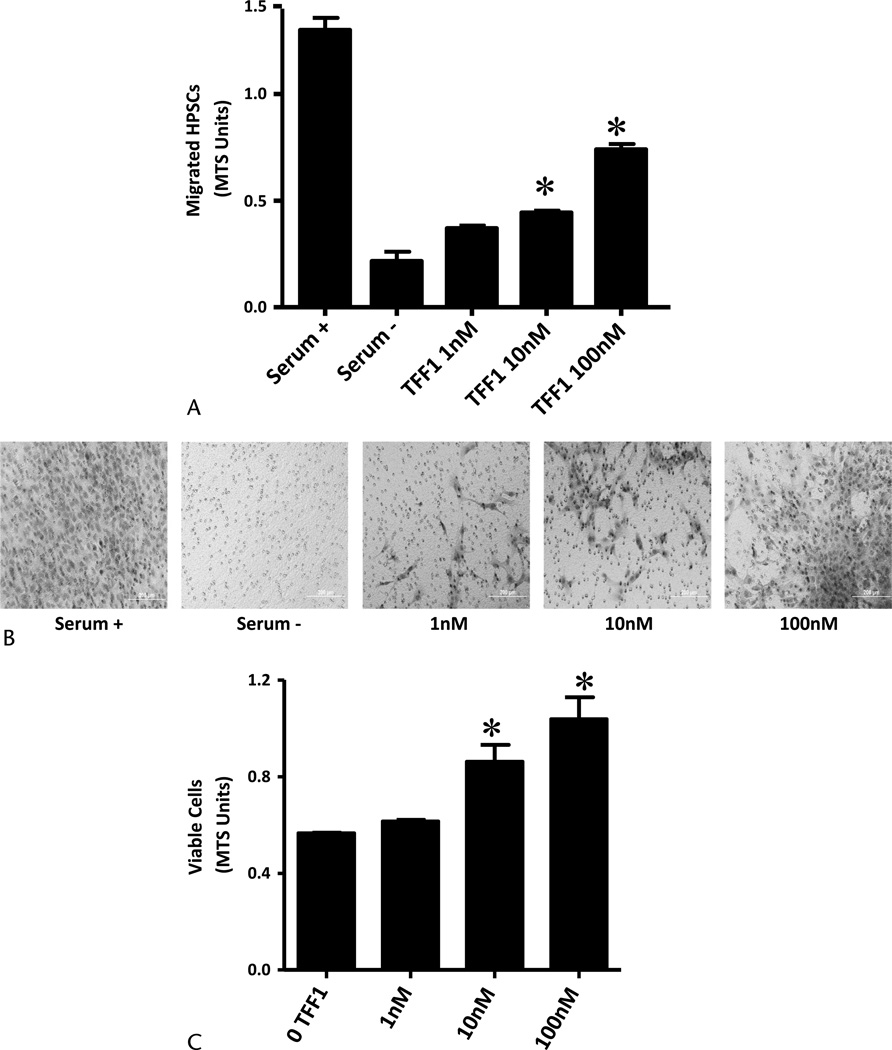
Stellate cells are involved in the prominent desmoplasia associated with pancreatic cancer. To test the hypothesis that TFF1 stimulates this crucial process, we analyzed the effects of TFF1 on proliferation and migration of HPSCs. Trefoil factor 1 was a chemoattractant for HPSCs and significantly increased their migration at concentrations of 1 nM or greater (Figs. 3A, B). In contrast to its effects on proliferation of pancreatic cancer cells, TFF1 stimulated the proliferation of PSCs. After a 48-hour treatment with TFF1, concentrations of 1 nM or greater increased the proliferative activity of HPSCs compared with untreated cells (P < 0.05; Fig. 3C).
FIGURE 3.
Exogenous TFF1 stimulates PSC (HPSC) migration and proliferation in vitro. A, Trefoil factor 1 stimulated PSC migration in a concentration-dependent manner. Stellate cells (20,000 cells) were added into BioCoat migration upper chamber, and different concentrations of TFF1 (0–100 nM) were added into the lower chamber. After 8 hours, nonmigrated cells in the upper membrane were removed, and cells migrated into the lower membrane were quantified photometrically using MTS reagent added 1 hour before taking the reading. Dulbecco’s modified Eagle medium containing 10% or 0% serum was used as control. B, Photographs of representative membranes after Diff-Quick staining are also shown. C, Trefoil factor 1 stimulated PSC proliferation in a concentration-dependent manner. Stellate cells (5000 cells) were plated on 96-well plates, and TFF1 (0–100 nM) was added to the cells for 48 hours. Cell numbers were estimated by MTS assay. Data shown are mean ± SEM for 3 experiments. *P < 0.05 versus control.
TFF1 Increases Metastatic Spread of Pancreatic Cancer In Vivo
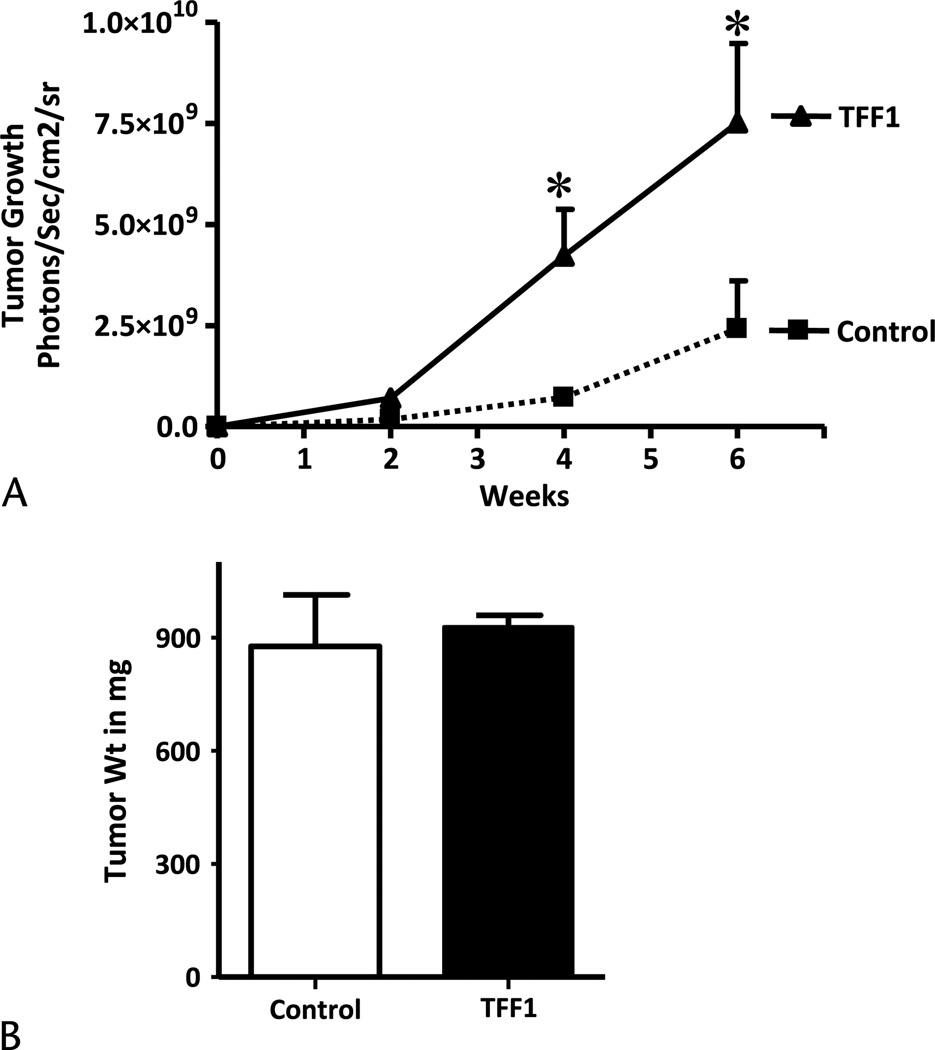
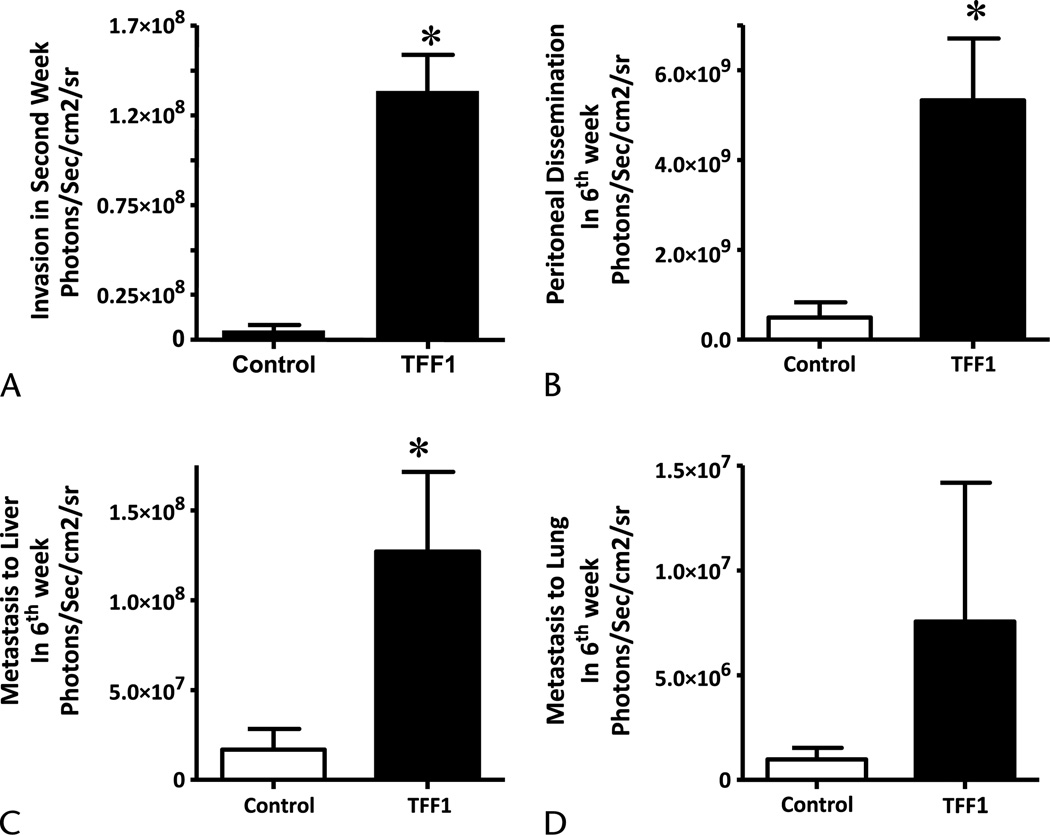
Pancreatic cancer Mpanc-96 cells, which do not express endogenous TFF1, were stably transfected with TFF1 expression vector. Enzyme-linked immunosorbent assay results verified cellular secretion of TFF1 into conditioned media after transfection (37 ± 3 ng/mL). Control and TFF1-expressing Mpanc-96 cells were labeled with a luciferase vector to facilitate the analysis of tumor growth and metastasis by bioluminescence imaging.19 Luciferase-expressing Mpanc-96 cells transfected with the TFF1 expression plasmid or a control vector were injected orthotopically into the pancreas of nude mice, and tumor growth and metastasis were measured every week for 6 weeks. Trefoil factor 1 expression increased the overall cancer burden of the animals 3-fold, as determined by luciferase imaging when compared with control vector-transfected cells (Fig. 4A). However, at the end of the experiment, when primary tumors were excised and weighed carefully, no difference was observed in primary tumor weights between TFF1-expressing and control tumors (Fig. 4B). Rather, a large increase was noted in the nonpancreatic burden of tumor cells in animals injected with TFF1-expressing cells. Cells ectopically expressing TFF1 were observed to begin spreading beyond the pancreas as early as the second week of the experiment as measured by bioluminescence (Fig. 5A). Mpanc-96 cells that express TFF1 invaded the peritoneal cavity while in the same time period no metastatic foci to surrounding areas were observed with cells that did not express TFF1 (Fig. 5A). At the end of the experiment, the volumes of metastatic foci were analyzed by imaging after removal of the primary tumor. We observed a large and statistically significant (P < 0.05) increase in the incidence (data not shown) and growth of invaded cells into the peritoneum (Fig. 5B; 11-fold) and liver (Fig. 5C; 7.5-fold). The extent of lung metastasis increased (Fig. 5D; 7.5-fold), but the increase did not reach statistical significance (P < 0.05) because of high variability.
FIGURE 4.
Trefoil factor 1 increases total tumor burden in vivo. A, The growth of tumors formed from Mpanc-96 cells stably transfected with control or TFF1-expressing vectors was analyzed in 4-week-old male athymic nude mice. Both control and TFF1-expressing cells were stably transfected with luciferase gene, and 1 × 106 cells were injected into the pancreas. Bioluminescent imaging was used to estimate overall tumor burdens from 6 animals in each group for 6 weeks. B, The animals were killed, and tumor weight was measured at the end of the experiment. *P < 0.05 versus control.
FIGURE 5.
Trefoil factor 1 increases metastasis in vivo. The metastasis from Mpanc-96 cells stably transfected with control or TFF1-expressing vectors were analyzed in 4-week-old male athymic nude mice. Both control and TFF1-expressing cells were stably transfected with luciferase gene, and 1 × 106 cells were injected into the pancreas. Bioluminescent imaging was used to estimate metastasis from 6 animals in each group for 6 weeks. A, Trefoil factor 1 expression induces invasion as early as second week. B–D, Trefoil factor 1 expression increased metastasis to peritoneal cavity, liver, and lung, measured at the end of the experiment. Columns, mean for 6 animals (*P < 0.05); bars, SEM.
DISCUSSION
Pancreatic ductal adenocarcinoma is a major clinical challenge with a 5-year survival rate of less than 5%.1,2 The mechanisms responsible for the aggressive nature of this cancer are not well understood. Our previous gene profiling data identified TFF1 mRNA as highly expressed in pancreatic cancer. In the current study, we further investigated the expression of TFF1 in pancreatic cancer tissues and cell lines and in tumor microenvironment cells and also the potential functional role of this molecule in PDAC. We observed that TFF1 was present in the majority of pancreatic tumors and cell lines, and its expression occurred early in the development of the disease. We also observed that exogenous TFF1 stimulated pancreatic cancer cell invasion in vitro and metastasis in vivo in an orthotopic mouse model. Furthermore, ectopic TFF1 also increased the growth and migration of PSCs in vitro. Thus, our observations strongly suggest an involvement of TFF1 in pancreatic cancer through actions on both the cancer cells and the tumor microenvironment and support TFF1 as a potential target for the development of future therapies.
In the current study, we observed that the majority of pancreatic cancer tissues and cell lines expressed TFF1. There is now satisfactory histopathologic and molecular evidence to support the evolution of invasive pancreatic cancer through a series of noninvasive duct lesions called PanIN. Interestingly, we observed that TFF1 expression was less frequently observed in invasive cancer (~70% of patients expressed TFF1) compared with early preneoplastic lesions. In precursor lesions, we observed that almost 100% of the samples expressed TFF1. Prasad et al20 also recently reported expression of TFF1 in early PanIN lesions when compared with normal pancreatic duct. They further demonstrated that transfection of a nontumorigenic human duct cell line, HPDE cells, with Gli1, a downstream mediator of hedgehog signaling, resulted in up-regulation of TFF1. Recent evidence has implicated reactivation of Notch and hedgehog signaling in PanIN development.20,21 This supports the concept that hedgehog signaling is responsible for TFF1 expression in pancreatic cancer.20 Taken together, the evidence indicates strongly that TFF1 expression is an early event in the development of pancreatic tumorigenesis. These results suggest the possibility that TFF1 might be useful as an early cytological marker for PDAC.
Trefoil factor 1 is a secreted protein that is involved in various pathological condition including inflammatory bowel disease,22 prostate cancer,23 breast cancer,24 and lung cancer.25 Because our microarray and immunohistochemistry data indicated that TFF1 expression was specific to pancreatic cancer when compared with normal tissue and pancreatitis, we measured TFF1 in pancreatic cancer cell conditioned media. Pancreatic cancer cell lines released TFF1 into the media bathing the cells. However, we were unable to observe a correlation between TFF1 serum levels with the presence of the disease in patient samples (data not shown). Quantification of TFF1 in pancreatic juice or in fine-needle aspirate samples may be a more useful way in which to examine the utility of TFF1 as a pancreatic cancer biomarker. Future studies will be necessary to fully evaluate the utility of TFF1 as a biomarker for pancreatic cancer.
When we added TFF1 exogenously to cancer cells, we did not observe any significant effect on cell proliferation. In contrast, we observed a robust and concentration-dependent increase in the proliferation of stellate cells. In general, there is a paucity of data on the ability of trefoil proteins to modulate cell cycle progression. Existing published data are complicated by the use of pharmacological concentrations of exogenous trefoil proteins (1–50 µM). At these pharmacological concentrations, TFF1 reduced cell proliferation by delaying G1 to S phase transition in human colon and gastric carcinoma cells.26 Our data indicate that, even at lower concentrations (between 1 nM and 100 nM), TFF1 can stimulate PSC proliferation but not malignant pancreatic epithelial cell proliferation, suggesting that there are important cell-type–dependent responses to TFF1.
Interestingly, both pancreatic cancer and stellate cells responded to treatment with TFF1, demonstrating increased motility. Trefoil factor 1 has been shown previously to stimulate migration of breast cancer and cholangiocarcinoma cells in modified Boyden assays. However, its biological activity has not been investigated previously in pancreatic cancer. Cell migration and invasion are crucial processes in tumor metastasis. In our study, extracellular addition of TFF1 stimulated invasion of cancer cells and migration of stellate cells in vitro. In addition, expression of TFF1 in pancreatic cancer cells induced a more invasive phenotype and a more metastatic phenotype in vivo in an orthotopic model of pancreatic cancer. Pancreatic cancer cells that expressed TFF1 showed significant local invasion 2 weeks after inoculation in the mice and increased invasion to the peritoneal cavity and metastasis to the liver and lungs in the later weeks. This is the first direct demonstration that TFF1 expression drives tumor cell invasion and metastasis in vivo. These data suggest the autocrine and paracrine effects of TFF1 on tumor microenvironment and also support the hypothesis that TFF1 is a metastasis inducer.
The specific receptor of TFF1 has yet to be identified. However, TFF1 has been reported to interact with TFIZ1/GKN2 to protect gastric epithelial cells from damage, and it can also promote invasive properties of gastric tumor cells.27 A recent report demonstrated that TFF2 protein activates signaling through the CXCR4 receptor.28 Interestingly, CXCR4 is overexpressed in PanIN tissues and plays a role in progression from PanIN to invasive cancer.29 A recent study also demonstrated that CXCR4 is expressed in stellate cells.30 Therefore, future studies will be necessary to confirm the possibility that TFF1 mediates its effects through CXCR4 receptor both in cancer and stellate cells.
The significance of the effects of TFF1 on stellate cells is uncertain. The extremely dense desmoplastic infiltration that is characteristic of pancreatic cancer is unique among solid tumors and may impede effective systemic treatments on a molecular level. Importantly, in pancreatic cancer, as in many other tumor types, the local microenvironment is thought to be an active participant in the process of cancer initiation, progression, and metastasis.4 Pancreatic stellate cells are myofibroblast-like cells that were identified originally as the source of the fibrosis in chronic pancreatitis5 and are now thought to be responsible for the dense stroma associated with pancreatic cancer.5 The exact role of PSCs in tumor-stroma interactions in pancreatic cancer has not been well defined, mainly because of lack of knowledge about molecules that play a dual role in the malignant epithelial cells and in the associated stellate cells. Based on the current observations for a potent effect of TFF1 on stellate cells, it is likely that this molecule is one of the important regulators of cancer-stromal interaction.
In summary, this study describes the presence of TFF1 in pancreatic cancer cells and for the first time indicates the role of TFF1 in increasing the aggressive characteristics of pancreatic cancer cells and its effects on stellate cells in vitro. Furthermore, we have demonstrated TFF1 as an important metastatic inducer in vivo. Our observations add a significant new insight into the multifaceted role of TFF1 in pancreatic cancer. The data support the further investigation of TFF1 as a new and potentially important target for pancreatic cancer therapy.
Acknowledgments
This work was supported in part by the MD Anderson Support Core (grant CA16672), the MD Anderson Pancreatic Specialized Programs of Research Excellence (grant P20 CA101936), the Lockton Endowment, and by Public Health Service (grant DK56338), which funds the Texas Medical Center Digestive Diseases Center.
Footnotes
The authors have no any potential conflict of interest including any financial, personal, or other relationships with other people or organizations that could inappropriately influence this work.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37:S4–S66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 3.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- 5.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Can Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Can Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 7.Sagol O, Tuna B, Coker A, et al. Immunohistochemical detection of pS2 protein and heat shock protein-70 in pancreatic adenocarcinomas. Relationship with disease extent and patient survival. Pathol Res Pract. 2002;198:77–84. doi: 10.1078/0344-0338-00190. [DOI] [PubMed] [Google Scholar]
- 8.Terris B, Blaveri E, Crnogorac-Jurcevic T, et al. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002;160:1745–1754. doi: 10.1016/S0002-9440(10)61121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thim L, May FE. Structure of mammalian trefoil factors and functional insights. Cell Mol Life Sci. 2005;62:2956–2973. doi: 10.1007/s00018-005-5484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 11.Perry JK, Kannan N, Grandison PM, et al. Are trefoil factors oncogenic? Trends Endocrinol. Metab. 2008;19:74–81. doi: 10.1016/j.tem.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Buron N, Guery L, Creuzot-Garcher C, et al. The trefoil factor TFF1 protects conjunctival cells from apoptosis at pre and post-mitochondrial levels. Invest Ophthalmol Vis Sci. 2008;49:3790–3798. doi: 10.1167/iovs.07-1270. [DOI] [PubMed] [Google Scholar]
- 13.Emami S, Rodrigues S, Rodrigue CM, et al. Trefoil factor family [TFF] peptides and cancer progression. Peptides. 2005;25:885–898. doi: 10.1016/j.peptides.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Katoh M. Trefoil factors and human gastric cancer. Int J Mol Med. 2003;12:3–9. [PubMed] [Google Scholar]
- 15.Prest SJ, May FE, Westley BR. The estrogen-regulated protein, TFF1, stimulates migration of human breast cancer cells. FASEB J. 2002;16:592–594. doi: 10.1096/fj.01-0498fje. [DOI] [PubMed] [Google Scholar]
- 16.Babyatsky M, Lin J, Yio X, et al. Trefoil factor-3 expression in human colon cancer liver metastasis. Clin Exp Metastasis. 2009;26:143–151. doi: 10.1007/s10585-008-9224-9. [DOI] [PubMed] [Google Scholar]
- 17.Ather MH, Abbas F, Faruqui N, et al. Expression of pS2 in prostate cancer correlates with grade and chromogranin A expression but not with stage. BMC Urol. 2004;4:14. doi: 10.1186/1471-2490-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran V, Arumugam T, Hwang RF, et al. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 2007;67:2666–2675. doi: 10.1158/0008-5472.CAN-06-3362. [DOI] [PubMed] [Google Scholar]
- 19.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–1818. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad NB, Biankin AV, Fukushima N, et al. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of hedgehog signaling on pancreatic ductal epithelial cells. Can Res. 2005;65:1619–1626. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]
- 21.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grønbaek H, Vestergaard EM, Hey H, et al. Serum trefoil factors in patients with inflammatory bowel disease. Digestion. 2006;74:33–39. doi: 10.1159/000096591. [DOI] [PubMed] [Google Scholar]
- 23.Vestergaard EM, Borre M, Poulsen SS, et al. Plasma levels of trefoil factors are increased in patients with advanced prostate cancer. Clin Can Res. 2006;12:807–812. doi: 10.1158/1078-0432.CCR-05-1545. [DOI] [PubMed] [Google Scholar]
- 24.Reshkin SJ, Tedone T, Correale M, et al. Association of pS2 [TFF1] release with breast tumour proliferative rate: in vitro and in vivo studies. Cell Prolif. 1999;32:107–118. doi: 10.1046/j.1365-2184.1999.32230107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashiyama M, Doi O, Kodama K, et al. Estimation of serum level of pS2 protein in patients with lung adenocarcinoma. Anticancer Res. 1996;16:2351–2355. [PubMed] [Google Scholar]
- 26.Bossenmeyer-Pourié C, Kannan R, Ribieras S, et al. The trefoil factor 1 participates in gastrointestinal cell differentiation by delaying G1-S phase transition and reducing apoptosis. J Cell Biol. 2002;157:761–770. doi: 10.1083/jcb200108056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westley BR, Griffin SM, May FE. Interaction between TFF1, a gastric tumor suppressor trefoil protein, and TFIZ1, a BRICHOS domain-containing protein with homology to SP-C. Biochemistry. 2005;44:7967–7975. doi: 10.1021/bi047287n. [DOI] [PubMed] [Google Scholar]
- 28.Dubeykovskaya ZA, Dubeykovskiy AN, Solal-Cohen J, et al. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem. 2009;284:3650–3662. doi: 10.1074/jbc.M804935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas RM, Kim J, Revelo-Penafiel MP. The chemokine receptor CXCR4 is expressed in pancreatic intraepithelial neoplasia. Gut. 2008;57:1555–1560. doi: 10.1136/gut.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Z, Wang X, Wu K, et al. Pancreatic stellate cells increase the invasion of human pancreatic cancer cells through the stromal cell–derived factor-1/CXCR4 axis. Pancreatology. 2010;10:186–193. doi: 10.1159/000236012. [DOI] [PubMed] [Google Scholar]