Abstract
Background
Atrial fibrillation (AF) is associated with left atrial (LA) structural and functional changes. Cardiac magnetic resonance (CMR) late gadolinium enhancement (LGE) and feature-tracking are capable of noninvasive quantification of LA fibrosis and myocardial motion, respectively. We sought to examine the association of phasic LA function with LA enhancement in patients with AF.
Methods and Results
LA structure and function was measured in 90 AF patients (age 61 ± 10 years, 76% male) referred for ablation and 14 healthy volunteers. Peak global longitudinal LA strain (PLAS), LA systolic strain rate (SR-s), and early (SR-ed) and late diastolic (SR-ld) strain rates were measured using cine-CMR images acquired during sinus rhythm. The degree of LGE was quantified. Compared to patients with paroxysmal AF (60% of cohort), those with persistent AF had larger maximum LA volume index (LAVImax, 56 ± 17ml/m2 versus 49 ± 13ml/m2 p=0.036), and increased LGE (27.1± 11.7% versus 36.8 ± 14.8% p<0.001). Aside from LA active emptying fraction, all LA parameters (passive emptying fraction, PLAS, SR-s, SR-ed and SR-ld) were lower in patients with persistent AF (p< 0.05 for all). Healthy volunteers had less LGE and higher LA functional parameters compared to AF patients (p<0.05 for all). In multivariable analysis, increased LGE was associated with lower LA passive emptying fraction, PLAS, SR-s, SR-ed, and SR-ld (p<0.05 for all).
Conclusions
Increased LA enhancement is associated with decreased LA reservoir, conduit, and booster pump functions. Phasic measurement of LA function using feature-tracking CMR may add important information regarding the physiological importance of LA fibrosis.
Keywords: atrial fibrillation, left atrial function, feature-tracking CMR, late gadolinium enhancement
Atrial fibrillation (AF) is associated with extensive abnormalities in atrial structure and function1-3. It is well-established that structural atrial changes precede the development of AF and progress with increased duration of sustained AF4. The changes in atrial function impair not only the booster pump function but also the atrial reservoir and conduit functions during ventricular systole and early diastole 5, 6. Progressive atrial remodeling includes fibrotic changes that promote AF maintenance7. This idea is supported by observations of increased left atrial (LA) fibrosis in patients with long-standing persistent AF 4. LA structural and functional remodeling is associated with increased incidence of AF, as well as AF recurrence after cardioversion or ablation8-11.
Late gadolinium enhanced (LGE) cardiac magnetic resonance (CMR) can noninvasively quantify the extent of LA fibrosis12, 13. Atrial function is commonly evaluated by speckle-tracking echocardiography; however, the technique is limited for resolution of the thin and asymmetric LA myocardium and for the analysis of the posterior LA where most of the fibrosis is located7. In contrast, myocardial motion can be accurately tracked with CMR due to its ability to accurately define endocardial and epicardial borders14. CMR-feature tracking, a novel post–processing technique which tracks myocardial motion using cine CMR images, has recently been developed15-19. In this study, we sought to examine the association of LA fibrosis measured with LGE-CMR with phasic LA remodeling measured with feature-tracking CMR in patients with AF. We hypothesized that increased atrial LGE is associated with reduced LA function as assessed by feature tracking CMR.
Methods
The Johns Hopkins Institutional Review Board approved the study and all patients provided written informed consent. Between December 2011 and May 2013, 147 consecutive patients with drug refractory AF were referred for CMR prior to catheter ablation of AF. Out of the total 142 patients, 49 were in AF at the time of CMR and were excluded. In 3 patients the cine images were not analyzable due to poor quality, leaving a final study cohort of 90 patients. Left atrial structure and function was also assessed in 14 volunteers free of cardiovascular disease.
CMR protocol
Images were acquired using 1.5 Tesla scanners (Avanto and Aera, Siemens, Erlangen, Germany) and a 6-channel–phased array body coil in combination with a 6-channel spine matrix coil. Cine-CMR images were acquired using a steady-state free precession sequence with the following parameters: minimal TR/TE, slice thickness 8 mm, spacing 2 mm, flip angle 78°, and field of view36-40 cm, typical in-plane resolution 1.5 × 1.5 mm LGE-CMR images were acquired within a range of 15–25 minutes (mean 18.8 ± 2.4 minutes) after a gadolinium injection (0.2 mmol/kg; gadopentetate dimeglumine; Bayer Healthcare Pharmaceuticals, Montville, NJ) using a fat-saturated 3D IR-prepared fast spoiled gradient recalled echo sequence with respiratory navigation and electrocardiogram gating, echo time of 1.52 ms, repetition time of 3.8 ms, in-plane resolution of 1.3 × 1.3 mm, slice thickness of 2.0 mm, and flip angle of 10°. Trigger time for 3D LGE-CMR images was optimized to acquire imaging data during diastole of LA as observed from the cine images. The optimal inversion time was identified with an inversion time scout scan (median 270 ms; range 240–290 ms) to maximize nulling of the LA myocardium.
Quantification of LA Enhancement
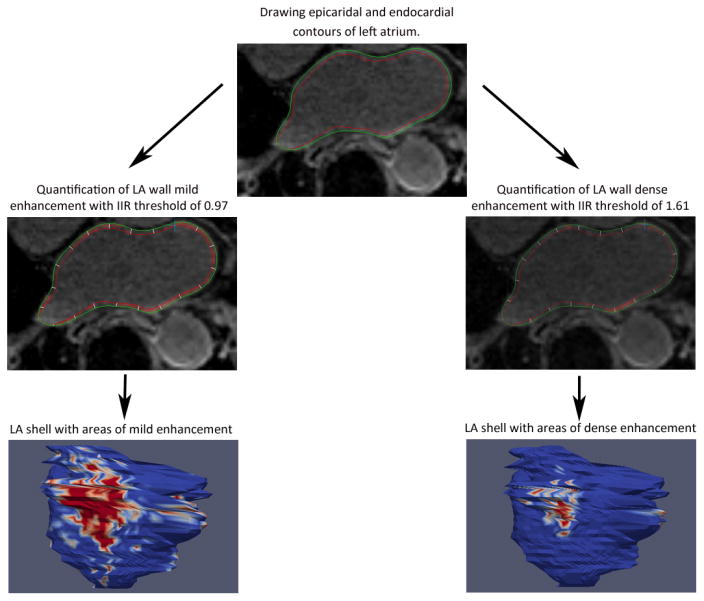
The method we used for the measurement of LA enhancement has been previously described in detail20. Briefly, an operator blinded to the measured LA functional parameters processed images off-line by using QMass MR software (version 7.2, Leiden University Medical Center, Leiden, The Netherlands). Multiplanar reformatted axial images with 3.5-mm slice thickness were reconstructed from 3D axial image data. Epicardial and endocardial contours were manually drawn around the LA myocardium (Figure 1). LA wall thickness was measured by averaging the distance between LA endocardium and epicardium in all segments. For measurement of LA enhancement we used the image intensity ratio (IIR), defined as the mean pixel intensity of each sector divided by the mean pixel intensity of the entire LA blood pool. A threshold of 0.97 and 1.61 for IIR, which previously was shown to be to a bipolar voltage of < 0.5mV and <0.1mV respectively, was used for mild LA enhancement and dense enhancement determination respectively20. Inter- and intra-observer variability of IIR has been previously assessed in the same study with inter- and intra-class correlation coefficients of 0.97 and 0.98 respectively20.
Figure 1. Assessment of LA structure using LGE-MRI.
LA Functional Analysis
Multimodality Tissue Tracking software (MTT; version 6.0, Toshiba, Japan) was used to measure phasic LA volumes, strain and strain rate from 4-chamber and 2-chamber cine CMR images 18, 19. An experienced operator contoured endocardial and epicardial LA borders in 2- and 4- chamber cine CMR images, taking care to exclude the pulmonary veins and the LA appendage. Once contouring is complete in one phase, the software automatically tracks on screen pixels during the cardiac cycle (Video 1). The operated then reviewed all contours generated by the software for quality control. Based on the biplane area-length method, the software generates LA volume curves during the cardiac cycle (Figure 2). Using the volume/time curves measurements for maximum LA volume (LAVmax), LA volume before LA contraction (LAVpre-a), and minimum LA volume (LAVmin) were extracted. All volume measurements were indexed to body surface area. LA passive and active emptying fractions were then calculated as follows:
Figure 2. LA volume and function during the cardiac cycle.
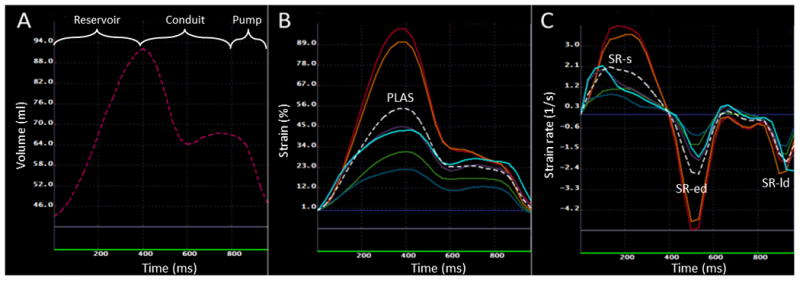
A: Changes in LA volume during reservoir, conduit and booster pump phases. The volume curve is a composite of measured volumes using 2 and 4 chamber views. B: LA longitudinal strain in different segments of LA, the dotted white line shows the average of LA longitudinal strain in all segments. PLAS: peak global longitudinal LA strain; C: LA longitudinal strain rate in different LA segments. The white dotted line shows the average of the strain rate in all segments. The points for systolic strain rate (SR-s), early diastolic strain rate (SR-ed) and late diastolic strain rate (SR-ld) have been shown.
LA passive emptying fraction (LAPEF): 100×(LAVmax − LAVpre-a)/LAVmax
LA active emptying fraction (LAAEF): 100 ×(LAVpre-a − LAVmin)/LAVpre-a
Strain measurement
Global longitudinal strain and strain rate curves were generated by averaging longitudinal strain and strain rate measurements in all LA segments, as shown in Figure 2. Global peak LA longitudinal strain (PLAS) was measured from the global longitudinal strain curve. Using longitudinal strain rate curves, peak systolic LA strain rate (SR-s), absolute values of peak early and late diastolic strain rates (SR-ed and SR-ld respectively) were measured (Figure 2).
To assess the inter- and intra-observer reproducibility of measured LA functional parameters, two readers blinded to other measurements re-measured LA parameters using the same technique in 20 patients randomly selected from the whole cohort. Additionally, LA functional parameters were re-measured by the original reader blinded to the first measurement.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are presented as frequencies and percentages. Differences between group means were evaluated with t tests (continuous variables) or chi-square analysis (categorical variables) as appropriate. The association of fibrosis with measured LA parameters was assessed using Pearson correlation in all patients and also in patients with paroxysmal and persistent AF separately. Multivariable linear regression was performed with LA fibrosis as the dependent variable and each LA functional and structural parameters as an independent variable. For each LA functional and structural parameter a separate model was analyzed. To avoid co-linearity, correlations between continuous variables were tested using the Spearman Correlation Coefficient and variables with r>0.50 were not included in the same multivariable model. Model 1 was adjusted for age and gender. Model 2 was additionally adjusted for history of hypertension, heart failure, left ventricular ejection fraction, type of AF (paroxysmal or persistent), LA volume index and LA wall thickness. Intra-class correlation coefficient analysis was performed to evaluate inter- and intra-observer agreement. Statistical analyses were performed using Stata software (Version 11.2, Stata Corp, TX).
Results
Of 90 patients in the cohort 68 were male (76%), 36 (40%) had persistent AF, and 20 (22%) had prior AF ablation. The average age was 61 ± 10 years (range 36-83 years). Heart failure was more prevalent among patients with persistent AF (22 % vs. 6%, P=0.018, respectively). None of the patients had significant valvular disease. Healthy volunteers included 4 women and 10 men with an average age of 43 ± 9 years (range 25-57 years). The baseline demographic and clinical characteristics of patients have been summarized in Table 1.
Table 1. Baseline characteristics of patients.
| Total (n=90) | Paroxysmal (n=54) | Persistent (n=36) | P Value | |
|---|---|---|---|---|
|
|
|
|
||
| Age (years) | 61 ± 10 | 61 ± 10 | 60 ± 9 | 0.416 |
| Sex (male) | 76% | 72 % | 81 % | 0.367 |
| Body mass index (kg/m2) | 29 ± 5 | 28 ± 5 | 29 ± 5 | 0.256 |
| Hypertension | 48 % | 48% | 47% | 0.931 |
| History of heart failure | 12 % | 6 % | 22% | 0.018 |
| Coronary artery disease | 12 % | 13% | 11% | 0.768 |
| Diabetes mellitus | 7 % | 6% | 8% | 0.605 |
| Obstructive sleep apnea | 24% | 24 % | 25 % | 0.920 |
| Left ventricular ejection fraction | 58 ± 6 % | 59 ± 5 % | 56 ± 8 % | 0.104 |
LA Structure and Function in Persistent and Paroxysmal AF
Structural and functional LA parameters in patients with paroxysmal and persistent AF have been summarized in Table 2. Persistent AF patients had more extensive LGE using both thresholds (36.8 ± 14.8% versus 27.1 ± 11.7%, for mild LA enhancement and 5.8 ± 4.4 versus 9.2 ± 7.3% for dense LA enhancement, P<0.01). At the same time persistent AF patients had larger LA (LAVImax: 56 ± 17 ml/m2 versus 49 ± 13, p= 0.036 and LAVImin: 36 ± 14 ml/m2 versus 28 ± 11 ml/m2, p=0.008). LA conduit phase function determined by LAPEF was significantly lower in persistent AF patients (17 ± 6% versus 20 ± 6%, p=0.019). Patients with persistent AF also had lower PLAS representing decreased LA reservoir function. The absolute values of LA systolic, early diastolic, and late diastolic strain rates, representing reservoir, conduit, and LA booster pump functions, respectively, were significantly lower in patients with persistent AF. However, LAAEF was not significantly different among patients with persistent versus paroxysmal AF (22 ± 8 % versus 23 ± 8 %, P=0.294).
Table 2. LA structure and function in patients stratified by type of AF.
| LA parameters | Total AF Patients (n=90) | Paroxysmal (n=54) | Persistent (n=36) | P Value |
|---|---|---|---|---|
|
|
|
|
||
| LA Mild Enhancement (%) | 31 ± 13.8 | 27.1 ± 11.7 | 36.8 ± 14.8 | <0.001 |
| LA Dense Enhancement (%) | 7.2 ± 5.9 | 5.8 ± 4.4 | 9.1 ± 7.3 | 0.009 |
| LA Wall Thickness | 2.06 ± 0.31 | 2.09 ± 0.31 | 2.00 ± 0.28 | 0.150 |
| LAVImax (ml/m2) | 52 ± 15 | 49 ± 13 | 56 ± 17 | 0.036 |
| LAVImin (ml/m2) | 31 ± 13 | 28 ± 11 | 36 ± 14 | 0.008 |
| LAPEF (%) | 19 ± 7 | 20 ± 6 | 17 ± 6 | 0.019 |
| LAAEF (%) | 23 ± 8 | 23 ± 8 | 22 ± 8 | 0.294 |
| PALS (%) | 22 ± 8 | 24 ± 8 | 20 ± 7 | 0.012 |
| SR-s (1/s) | 0.93 ± 0.36 | 1 ± 0.39 | 0.83 ± 0.28 | 0.024 |
| SR-ed (1/s) | 0.82 ± 0.39 | 0.89 ± 0.42 | 0.72 ± 0.33 | 0.049 |
| SR-ld (1/s) | 1.07 ± 0.57 | 1.17 ± 0.62 | 0.91 ± 0.47 | 0.030 |
LAVImax (maximum LA volume index), LAVImin (minimum LA volume index), LAPEF (LA passive emptying fraction), LAAEF (LA active emptying fraction), PALS (peak global LA longitudinal strain), SR-s (systolic strain rate), SR-ed (early diastolic strain rate), SR-ld (late diastolic strain rate)
LA Structure and Function in Patients with Prior Ablation
Our study cohort consists of 20 patients with prior ablation who were undergoing a repeat ablation due to the failure of the first procedure. While the amount of mild enhancement was comparable in these patients with those with no history of ablation (34.6 ± 12.1% vs. 30 ± 14.1% p=0.193) they had lower measurements of LA function (LAPEF: 16 ± 5% vs. 19 ± 7%, p=0.028; PLAS: 17 ± 5% vs. 24 ± 8%, p<0.001). All measures of the phasic LA strain rate were also lower in patients with prior ablation (p<0.05 for all).
LA Structure and Function in Healthy Volunteers
Structural and functional LA parameters in AF patients and healthy volunteers have been summarized in Table 3. Healthy volunteers had smaller LA size (LAVImax: 36 ± 10 ml/m2 versus 52 ± 15, and LAVImin: 18 ± 6 ml/m2 versus 31 ± 13 ml/m2, p=<0.001 for both). All patients with AF had at least mild LA enhancement (IIR >0.97, range 7-69%), however, in the healthy volunteer group, 12 (85%) had mild LA enhancement (range 2-18%). Only one healthy volunteer had 2% of dense LA enhancement, however, in patients with AF, 89 (99%) had dense LA enhancement (IIR> 1.61, range 1-29%). Mean LA wall thickness was comparable among two groups (2.06 ± 0.32mm versus 2.15 ± 0.30mm in AF patients and healthy volunteers respectively, p=0.308). All measures of LA function were significantly higher in healthy volunteers compared with AF patients (P<0.01 for all).
Table 3. LA structure and function in healthy volunteers and AF patients.
| LA parameters | Total AF Patients (n=90) | Healthy Volunteers (n=14) | P Value |
|---|---|---|---|
|
|
|
|
|
| LA mild enhancement (%) | 31 ± 13.8 | 8.9 ± 6.0 * | <0.001 |
| LA dense enhancement (%) | 7.1 ± 5.9 | 0.1 ± 0.5¶ | <0.001 |
| LA Wall Thickness | 2.06 ± 0.32 | 2.15 ± 0.30 | 0.308 |
| LAVImax (ml/m2) | 52 ± 15 | 36 ± 10 | <0.001 |
| LAVImin (ml/m2) | 31 ± 13 | 18 ± 6 | <0.001 |
| LAPEF (%) | 19 ± 7 | 24 ± 6 | 0.002 |
| LAAEF (%) | 23 ± 8 | 34 ± 10 | <0.001 |
| PALS (%) | 22 ± 8 | 33 ± 9 | <0.001 |
| SR-s (1/s) | 0.93 ± 0.36 | 1.39 ± 0.48 | <0.001 |
| SR-ed (1/s) | 0.82 ± 0.39 | 1.1 ± 0.40 | 0.01 |
| SR-ld (1/s) | 1.07 ± 0.57 | 1.75 ± 0.89 | <0.001 |
Abbreviations as in Table 2.
LA fibrosis was present in 12 patients.
Only one patient had 2% of scar
Association of LA function and LA Enhancement
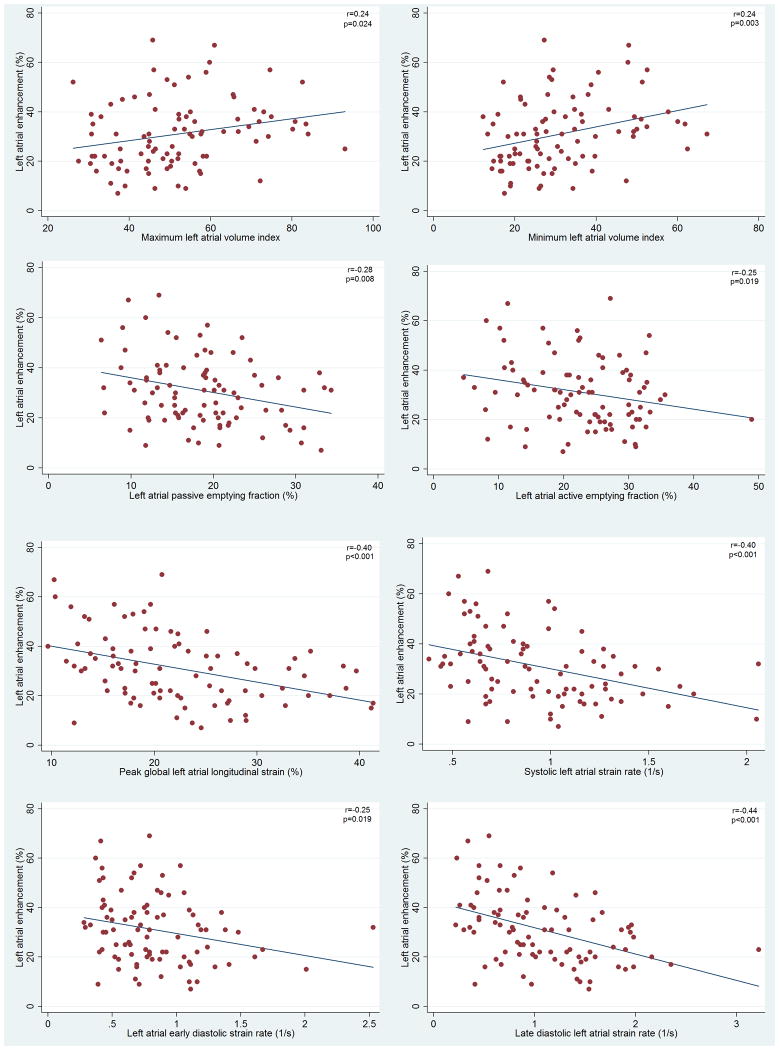
Figure 3 illustrates the association of LA functional and structural measurements with LA enhancement. More extensive LA enhancement was associated with larger LA maximum and minimum volume indexes, and lower LAPEF and LAAEF. More extensive LA enhancement was also inversely associated with lower PLAS and lower absolute values for LA strain rates in all phases of LA function. Figure 4 compares LA function in two AF patients with mild and extensive LA enhacement. Multivariable regression models to examine the association of LA fibrosis extent with LA structural and functional parameters have been summarized in Table 4. In model 1 (adjusted for age and gender), all LA functional and structural parameters were associated with LA enhancement. In model 2 (adjusted for age, gender, hypertension, heart failure, left ventricular ejection fraction, type of AF, maximum LAVI, and LA wall thickness) LAPEF, peak longitudinal strain and absolute values of strain rate in all phases of LA function were inversely associated with LA enhancement (p value <0.05 for all). Because of high correlation between LAVImax and LAVImin (r=0.88) only LAVImax was included the multivariable models.
Figure 3.
Correlation of LA enhancement and A: maximum LA volume index, B: minimum LA volume index, C: LA passive emptying fraction, D: LA active emptying fraction, E: LA peak global longitudinal strain, F: systolic LA strain rate, G: early diastolic atrial strain rate (absolute value), and H: late diastolic LA strain rate (absolute values)
Figure 4.
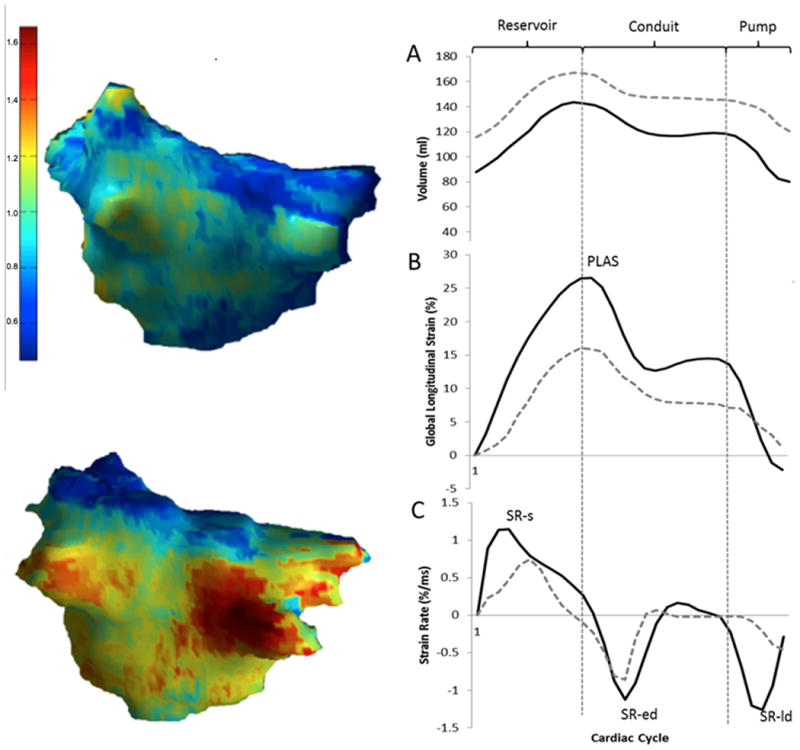
Left: Examples of image intensity ratio map of two LA with mild (top) and extensive (bottom) enhancement. Right: Comparing LA function in two patients with mild (solid black line) and extensive (dotted gray line) LA enhancement A: changes in LA volume during systole (reservoir), early diastole (conduit), and late diastole (pump). B: changes in global longitudinal LA strain during cardiac cycle. More extensive LA enhancement was associated with smaller peak global longitudinal atrial strain (PLAS). C: LA longitudinal strain rate during cardiac cycle. In this example the patient with more extensive LA enhancement has smaller systolic strain rate (SR-s), early diastolic strain rate (SR-ed), and late diastolic strain rate (SR-ld)
Table 4. Association of LA functional and structural parameters with LA fibrosis.
| LA Parameters | Model 1 (N=90) | Model 2 (n=90) | ||
|---|---|---|---|---|
|
|
|
|
||
| β* | P Value | β | P Value | |
| LAVImax(ml/m2) | 3.6 | 0.021 | ||
| LAVImin(ml/m2) | 4.7 | 0.002 | 9.0 | 0.013 |
| LAPEF (%) | -5.3 | 0.002 | -4.1 | 0.032 |
| LAAEF (%) | -3.25 | 0.030 | -3.2 | 0.052 |
| PALS (%) | -5.9 | <0.001 | -4.9 | 0.002 |
| SR-s (%/ms) | -6.1 | <0.001 | -5.2 | 0.002 |
| SR-ed (%/ms) | -4.7 | 0.007 | -3.9 | 0.030 |
| SR-ld (%/ms) | -6.4 | <0.001 | -5.3 | 0.001 |
The table illustrates multivariable regression analyses of the association of LA parameters with LA fibrosis. Six different regression models were analyzed. Each row represents a separate multivariable model with LA fibrosis as the dependent variable and the LA parameter as the independent variable. Model 1 was adjusted for age and gender. Model 2 was additionally adjusted for history of hypertension, heart failure, left ventricular ejection fraction, type of AF (persistent or paroxysmal), maximum LA volume index, and LA wall thickness.
All β coefficient values are per SD.
Reproducibility of LA Functional Parameters
Reproducibility results of feature-tracking CMR measured LA parameters in 20 randomly selected subjects have been summarized in Table 5. Intra-class correlation coefficients for inter- and intra-observer reproducibility for all LA parameters ranged between 0.88-0.99. The inter- and intra-observer reproducibility of IIR measurements by our investigators has been previously reported20.
Table 5. Reproducibility results of feature-tracking CMR measured LA parameter in 20 randomly selected subjects.
| LA Parameter | Intra-Observer | Inter-Observer | ||
|---|---|---|---|---|
|
|
|
|||
| ICC* | CI | ICC | CI | |
| LAVmax | 0.99 | 0.96-0.99 | 0.96 | 0.92-0.99 |
| LAVmin | 0.97 | 0.95-0.99 | 0.93 | 0.89-0.97 |
| LAPEF | 0.96 | 0.93-0.99 | 0.95 | 0.90-0.98 |
| LAAEF | 0.92 | 0.87-0.97 | 0.90 | 0.82-0.96 |
| PLAS | 0.94 | 0.89-0.98 | 0.92 | 0.85-0.97 |
| SR-s | 0.91 | 0.82-0.96 | 0.90 | 0.81-0.95 |
| SR-ed | 0.90 | 0.82-0.96 | 0.88 | 0.80-0.95 |
| SR-ld | 0.91 | 0.83-0.96 | 0.88 | 0.78-0.93 |
|
|
|
|||
ICC: Intra-Class Correlation. CI: Confidence Interval, other abbreviations as in Table 2.
Discussion
To the best of our knowledge, this is the first study to examine the association of phasic LA function with LA enhancement using feature-tracking CMR. In this study we note an inverse association between the extent of LA enhancement and passive LA emptying fraction, peak global LA strain and LA strain rate during systole, early diastole and late diastole as surrogates of reservoir, conduit, and booster pump functions of LA, respectively. This association was independent of age, gender, hypertension, heart failure, left ventricular ejection fraction, type of AF, LA size and wall thickness. The study also shows that AF patients have more structural and functional changes in LA compared to healthy volunteers. Moreover, persistent AF is associated with greater reduction in all phases of LA function and more advanced LA structural changes.
Challenges in the Assessment of LA Structure and Function
Left atrial anatomical, contractile, and electrical remodeling promotes the maintenance of AF and further structural and functional deterioration. Therefore assessment of LA structure and function has both predictive and prognostic values8, 21-23. Left atrial velocity measured with Tissue Doppler Imaging has been traditionally used in the assessment of LA function. However, because of the translation and tethering effect of mitral valve and left ventricle on LA velocity and angle dependency, TDI is not able to differentiate between true atrial contraction and mitral annular and ventricular motion 24. More recently, speckle-tracking echocardiography has been used to assess LA function and to measure strain and strain rate 25-27. However, given the posterior location and thin wall of the LA, and the presence of pulmonary veins and appendage, assessment of LA wall motion might be challenging despite having a higher temporal resolution compared with CMR. On the other hand, CMR is not only the gold standard imaging modality in the assessment of cardiac motion, but also the only modality capable of quantification of LA fibrosis as the hallmark of structural changes in AF patients using both LGE or T1 mapping 12, 13, 28. Feature-tracking MRI has been recently proposed as a novel method in the assessment and quantification of LA function using cine CMR images18, 19. One limiting factor in the assessment of LA function is the quality of images. Given the high image quality and spatial resolution of CMR images compared to echocardiography, LA function can be assessed using the volumetric method (by measuring LA volumes in different phases of cardiac cycle and calculating the emptying fractions) and also by strain and strain rate analyses in all segments of the LA. In our study out of 107 participants in sinus rhythm the images were analyzable in 104 subjects (97.2%). However the feasibility of speckle tracking echocardiography in measurement of LA strain and strain rate ranges between 76- 94% 25, 29. Qualitative factors that influenced the reliability of feature-tracking observations included significant arrhythmia and inability to breath hold.
LA Structure and Function in patient with AF
In our study, the IIR was utilized to detect differences in LA wall enhancement between healthy volunteers and patients with AF. All AF patients had at least mild LA enhancement, however, healthy subjects had much less mild LA enhancement and almost no dense enhancement. These findings are comparable to the study of Oakes et al in which 6 healthy volunteers had only minimal LA wall enhancement (1.7 ± 0.3%) compared to the average LA enhancement of 8.0, 21 and 50% in AF patients with mild, moderate and extensive enhancement, respectively 30. As it has been shown previously patients with persistent AF have more extensive atrial structural changes and deterioration of atrial function. In a study of 74 paroxysmal and 44 persistent AF patients, those with persistent AF had lower absolute values for both strain and strain rate measurements and also lower total LA emptying fraction21. Kuppahally et al. also reported lower LA midseptal strain and systolic strain rate and lower midlateral strain but not systolic strain rate in 31 patients with persistent AF compared to 24 paroxysmal AF patients7. In the same study, persistent AF patients also had more fibrosis, larger LA size, and lower LA emptying fraction. Similar results were presented in another study of 971 individuals with AF, where LA size increased across AF types (paroxysmal, persistent and permanent respectively) while LA function measured by total and passive emptying fraction deteriorated31. In this study, LAAEF was not significantly different among AF subtypes. Our study is in agreement with these findings, as we also found that patients with persistent AF had larger LA size and lower LAPEF but not LAAEF. Additionally, we found that patients with persistent AF had a higher percentage of LA fibrosis and lower global strain and strain rate measurements in all phases of LA function. These findings support the hypothesis of progressive atrial remodeling with the maintenance of AF.
Association of LA fibrosis and LA function
Deposition of extracellular matrix and fibrosis are hallmarks of LA structural changes in AF. Several experimental models have demonstrated that LA fibrosis is a substrate for promotion and maintenance of AF. Despite several studies investigating the progressive nature of LA remodeling and fibrosis in the setting of AF, the association between LA LGE as a measure of LA fibrosis and phasic LA dysfunction has not been extensively studied. To our knowledge, the only previous study examining this association has been performed by Kuppahally et al. on 55 patients with AF 7. The authors reported an inverse association between LA midseptal and midlateral strain and strain rate measured with speckle-tracking echocardiography and the percentage of LA fibrosis detected with LGE-CMR. This association was more prominent in patients with persistent AF than in patients with paroxysmal AF. Our findings complement the results of this study as we found an inverse association between LAPEF (representing conduit function), peak global LA strain (representing reservoir function) and systolic, early diastolic and late diastolic strain rates (representing reservoir, conduit and booster pump functions) with the extent of LA enhancement.
Compared to the study of Kuppahally et al., we examined this association with global strain as well as phasic strain rate measurements of the entire LA including measurements from the posterior segments where most of the fibrosis is located. Importantly, all of our study population was in sinus rhythm at the time of CMR enabling measurement of both passive and active emptying fractions. Additionally, both LA enhancement and strain/strain rate measurements were performed using the same CMR images, thus minimizing bias due to temporal changes in LA function/fibrosis between different modalities.
The extent of fibrosis, and LA function in patients with prior ablation should be interpreted cautiously because some LA remodeling is likely due to the prior procedure13, 32, whereas some differences in these parameters are likely due to the selection of patients with more advanced structural disease in the subset with repeat ablation 11, 21-23.
Limitations
This is a relatively small study with 90 participants; however, based on another study using feature-tracking CMR, a sample size of 29 has 90% power and α error of 0.05 to detect a change of 5% in strain measurements33. The LGE-MRI sequence used in this study provided a 1.3 × 1.3 mm in-plane resolution and was obtained during ventricular diastole/atrial systole, thus maximizing atrial myocardial thickness for measurement. Nevertheless, atrial myocardial thickness may be near the limit of image resolution in some cases. Because of volume averaging, the analyzed LA myocardial sector may have included blood pool or epicardial fat in some cases. The cohort included patients with paroxysmal and persistent AF as well as patients with and without prior ablations. The resulting increased variability in the extent of fibrosis and LA myocardial function, likely increased the standard error for the magnitudes of associations. On the other hand, the heterogeneity of the cohort improves the generalizability of our results. Additionally, adjusted and stratified analyses were performed to minimize the possibility of confounding. The objective of the current study was to examine the association of LA enhancement and function using LGE-MRI and feature-tracking MRI. The association of clinical endpoints with LA enhancement or atrial transport function was not examined and will be the subject of future manuscripts once appropriate clinical follow-up has been performed. Due to time constraints, and in order to obtain functional image data, MOLLI sequences were not obtained in this cohort of patients. Therefore the analysis of fibrosis has been performed using LGE sequences. Future studies to examine the association of LA T1 mapping with LA function are warranted.
Conclusions
Left atrial LGE was inversely correlated with passive LA emptying fraction, peak global LA strain and strain rate in all phases of LA function. Patients with persistent AF had worse LA function through the entire cardiac cycle compared to those with paroxysmal AF. Feature-tracking CMR, a novel tool in the analysis of LA function, enables detailed assessment of phasic LA volume, strain and strain rate. Quantification of LA function using feature-tracking CMR may provide additional insight for procedural outcomes and stroke risk stratification in AF patients.
Acknowledgments
Sources of Funding: The study was funded by a Biosense-Webster grant and NIH grants K23HL089333 and R01HL116280 to Dr. Nazarian, the Dr. Francis P. Chiaramonte Foundation, and The Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: Dr. Nazarian is on the MRI advisory panel for Medtronic, and is a scientific advisor to and principal investigator for research funding to Johns Hopkins University from Biosense-Webster Inc.
References
- 1.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 2.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 3.Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. 2002;54:361–379. doi: 10.1016/s0008-6363(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, Odim J, Laks H, Sen L. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–368. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 5.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 6.Inaba Y, Yuda S, Kobayashi N, Hashimoto A, Uno K, Nakata T, Tsuchihashi K, Miura T, Ura N, Shimamoto K. Strain rate imaging for noninvasive functional quantification of the left atrium: Comparative studies in controls and patients with atrial fibrillation. J Am Soc Echocardiogr. 2005;18:729–736. doi: 10.1016/j.echo.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, Macleod RS, McGann C, Litwin SE, Marrouche NF. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: Relationship to left atrial structural remodeling detected by delayed-enhancement mri. Circ Cardiovasc Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 8.Di Salvo G, Caso P, Lo Piccolo R, Fusco A, Martiniello AR, Russo MG, D'Onofrio A, Severino S, Calabro P, Pacileo G, Mininni N, Calabro R. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: A color doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005;112:387–395. doi: 10.1161/CIRCULATIONAHA.104.463125. [DOI] [PubMed] [Google Scholar]
- 9.Shaikh AY, Maan A, Khan UA, Aurigemma GP, Hill JC, Kane JL, Tighe DA, Mick E, McManus DD. Speckle echocardiographic left atrial strain and stiffness index as predictors of maintenance of sinus rhythm after cardioversion for atrial fibrillation: A prospective study. Cardiovasc Ultrasound. 2012;10:48. doi: 10.1186/1476-7120-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rondano E, Dell'Era G, De Luca G, Piccinino C, Bellomo G, Marino PN. Left atrial asynchrony is a major predictor of 1-year recurrence of atrial fibrillation after electrical cardioversion. J Cardiovasc Med (Hagerstown) 2010;11:499–506. doi: 10.2459/JCM.0b013e32833757b5. [DOI] [PubMed] [Google Scholar]
- 11.Akoum N, Daccarett M, McGann C, Segerson N, Vergara G, Kuppahally S, Badger T, Burgon N, Haslam T, Kholmovski E, Macleod R, Marrouche N. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: A DE-MRI guided approach. J Cardiovasc Electrophysiol. 2011;22:16–22. doi: 10.1111/j.1540-8167.2010.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGann CJ, Kholmovski EG, Oakes RS, Blauer JJ, Daccarett M, Segerson N, Airey KJ, Akoum N, Fish E, Badger TJ, DiBella EV, Parker D, MacLeod RS, Marrouche NF. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol. 2008;52:1263–1271. doi: 10.1016/j.jacc.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 13.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement mr imaging: Initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 14.Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. doi: 10.1186/1532-429X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M, Benson W, Mazur W. Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp. 2011;48:2356–2361. doi: 10.3791/2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuster A, Morton G, Hussain ST, Jogiya R, Kutty S, Asrress KN, Makowski MR, Bigalke B, Perera D, Beerbaum P, Nagel E. The intra-observer reproducibility of cardiovascular magnetic resonance myocardial feature tracking strain assessment is independent of field strength. Eur J Radiol. 2013;82:296–301. doi: 10.1016/j.ejrad.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:43. doi: 10.1186/1532-429X-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G, Bluemke DA, Lima JA. Association of cmr-measured la function with heart failure development: Results from the mesa study. JACC Cardiovasc Imaging. 2014;7:570–579. doi: 10.1016/j.jcmg.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai M, Venkatesh BA, Samiei S, Donekal S, Habibi M, Armstrong AC, Heckbert SR, Wu CO, Bluemke DA, Lima JA. Multi-ethnic study of atherosclerosis: Association between left atrial function using tissue tracking from cine mr imaging and myocardial fibrosis. Radiology. 2014;131971 doi: 10.1148/radiol.14131971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khurram IM, Beinart R, Zipunnikov V, Dewire J, Yarmohammadi H, Sasaki T, Spragg DD, Marine JE, Berger RD, Halperin HR, Calkins H, Zimmerman SL, Nazarian S. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart rhythm. 2014;11:85–92. doi: 10.1016/j.hrthm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider C, Malisius R, Krause K, Lampe F, Bahlmann E, Boczor S, Antz M, Ernst S, Kuck KH. Strain rate imaging for functional quantification of the left atrium: Atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J. 2008;29:1397–1409. doi: 10.1093/eurheartj/ehn168. [DOI] [PubMed] [Google Scholar]
- 22.Hammerstingl C, Schwekendiek M, Momcilovic D, Schueler R, Sinning JM, Schrickel JW, Mittmann-Braun E, Nickenig G, Lickfett L. Left atrial deformation imaging with ultrasound based two-dimensional speckle-tracking predicts the rate of recurrence of paroxysmal and persistent atrial fibrillation after successful ablation procedures. J Cardiovasc Electrophysiol. 2012;23:247–255. doi: 10.1111/j.1540-8167.2011.02177.x. [DOI] [PubMed] [Google Scholar]
- 23.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P, Brachmann J. Association of atrial tissue fibrosis identified by delayed enhancement mri and atrial fibrillation catheter ablation: The decaaf study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 24.Rosca M, Lancellotti P, Popescu BA, Pierard LA. Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart. 2011;97:1982–1989. doi: 10.1136/heartjnl-2011-300069. [DOI] [PubMed] [Google Scholar]
- 25.Saraiva RM, Demirkol S, Buakhamsri A, Greenberg N, Popovic ZB, Thomas JD, Klein AL. Left atrial strain measured by two-dimensional speckle tracking represents a new tool to evaluate left atrial function. J Am Soc Echocardiogr. 2010;23:172–180. doi: 10.1016/j.echo.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Azemi T, Rabdiya VM, Ayirala SR, McCullough LD, Silverman DI. Left atrial strain is reduced in patients with atrial fibrillation, stroke or tia, and low risk chads(2) scores. J Am Soc Echocardiogr. 2012;25:1327–1332. doi: 10.1016/j.echo.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. 2010;8:14. doi: 10.1186/1476-7120-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beinart R, Khurram IM, Liu S, Yarmohammadi H, Halperin HR, Bluemke DA, Gai N, van der Geest RJ, Lima JA, Calkins H, Zimmerman SL, Nazarian S. Cardiac magnetic resonance t1 mapping of left atrial myocardium. Heart rhythm. 2013;10:1325–1331. doi: 10.1016/j.hrthm.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurt M, Wang J, Torre-Amione G, Nagueh SF. Left atrial function in diastolic heart failure. Circ Cardiovasc Imaging. 2009;2:10–15. doi: 10.1161/CIRCIMAGING.108.813071. [DOI] [PubMed] [Google Scholar]
- 30.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, Deenadayalu N, Hoffman E, Patel I, Shi M, Mercuri M, Mitrovic V, Braunwald E, Solomon SD. Effective aNticoaGulation with factor × AnGiAFTIMIESI. Left atrial structure and function in atrial fibrillation: Engage AF-TIMI 48. Eur Heart J. 2014;35:1457–1465. doi: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wylie JV, Jr, Peters DC, Essebag V, Manning WJ, Josephson ME, Hauser TH. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart rhythm. 2008;5:656–662. doi: 10.1016/j.hrthm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E. Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson. 2012;14:43. doi: 10.1186/1532-429X-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]