Abstract
AIM
To assess plasma myeloperoxidase (MPO) levels in autistic children and to test the hypothesis that there is an association between decreased MPO concentration and probiotic therapy.
SUBJECTS AND METHODS
Plasma from 49 autistic children (39 males; mean age 11.4 years) (17 with diagnosed gastrointestinal (GI) disease – chronic diarrhea and/or constipation (10 of these GI patients were taking probiotics) and 26 receiving probiotic therapy) and 36 neurotypical controls (29 males; mean age 10.2 years; controls were not assessed for GI disease) were tested for MPO plasma concentration using Enzyme Linked Immunosorbent Assays (ELISAs). Plasma concentration of MPO in autistic individuals was compared to plasma concentration of copper and zinc.
RESULTS
We found that individuals with autism, receiving no therapy, did not have significantly lower plasma MPO levels when compared to controls. In the autistic group, MPO levels were significantly lower in individuals taking probiotic therapy. In addition, plasma copper levels were significantly lower in autistic individuals taking probiotics compared to those not taking probiotics, but plasma zinc levels were not different in the probiotic group.
DISCUSSION
These results suggest a relationship between low MPO levels found in a group of autistic individuals and probiotic therapy. By possibly changing gut bacterial flora and thereby changing absorption properties in the gut, probiotic therapy was also associated with lower copper levels.
Keywords: ASD, autism, myeloperoxidase, MPO, copper, probiotics, oxidative stress
Introduction
Myeloperoxidase (MPO) is an enzyme found in the azurophilic granules of neutrophilic polymorphonuclear leukocytes (PMNs) and in the lysosomes of monocytes in humans. Its major role is to aid in microbial killing. MPO is a dimeric molecule, consisting of a pair of heavy- and light-chain protomers and two iron atoms. MPO has only one gene; thus, the mature enzyme is synthesized from a single polypeptide product. The synthesis of MPO primarily occurs during the promyelocytic stage of myeloid development, concurrent with the development of the azurophilic granules. The MPO gene, located on chromosome 17 (17q23.1), encodes a single-stranded primary translational product, which is glycosylated to yield an enzymatically inactive precursor, apopro-MPO. Heme is then inserted, yielding the enzymatically active precursor pro-MPO.1,2
When neutrophils become activated, in conjunction with phagocytosis, they undergo respiratory burst. This causes production of superoxide, hydrogen peroxide, and other reactive oxygen derivatives, which are all toxic to microbes. During respiratory bursts, granule contents are released into the phagolysosomes and outside the cell, allowing released contents to come into contact with any microbes present. MPO catalyzes the conversion of hydrogen peroxide and chloride ions (Cl) into hypochlorous acid. Hypochlorous acid is 50 times more potent in microbial killing than hydrogen peroxide.2
MPO may have a role in atherosclerosis, carcinogenesis, and degenerative neurological diseases. The role of MPO in atherosclerosis is supported by the fact that MPO deficiency may protect against cardiovascular disease.3,4
In addition to killing bacteria, the products of the MPO–hydrogen peroxide–Cl system are believed to play a role in killing fungi, parasites, protozoa, viruses, tumor cells, natural killer (NK) cells, red cells, and platelets, and they may be involved in terminating the respiratory burst, because individuals with MPO deficiency have a prolonged reaction.3–8
Other functions of MPO include tyrosyl radical production, generation of tyrosine peroxide, mediation of the adhesion of myeloid cells via b2-integrins, and oxidation of serum lipoproteins.3,5 Because of this, MPO is considered a marker for inflammation and oxidation.
Autistic spectrum disorder (ASD) is a neurodevelopmental syndrome with onset prior to age of 36 months. Diagnostic criteria consist of impairments in socialization and communication plus repetitive and stereotypic behaviors.9 Traits strongly associated with autism include movement disorders and sensory dysfunctions.10 Although autism may be apparent soon after birth, many autistic children experience at least several months, up to a year or more in some cases, of normal development, followed by regression, defined as loss of function or failure to progress.10–12
Children with ASDs frequently have accompanying gastrointestinal (GI) symptoms13–15 and pathology, which includes inflammation of the GI tract.16,17 Many autistic children, particularly those with GI disease, may have increased incidence of fungal infection,18,19 but one study found no difference in levels of intestinal yeast between children with autism and controls using both culture methods and microscopic evaluation.20
We recently reported that there was a relationship between low MPO levels and GI disease seen in autism spectrum disorder individuals.21 In this study, we measured MPO levels in a general population of autistic children and analyzed the relationship between these levels and probiotic therapy.
Materials and Methods
ELISAs were used to measure plasma MPO (ELISA Kits, R&D Systems, Minneapolis, MN). The ELISA and plasma protocols have been previously reported and are explained below.22
All reagents and specimens were equilibrated at room temperature before each assay was performed. A 1:51 dilution of the patient samples was prepared by mixing 10 μL of the patient’s plasma with 0.5 mL of plasma diluent. In all, 100 μL of calibrators (20–200 EU/mL antibodies), positive and negative control plasma, plasma diluent alone, and diluted patient samples were added to the appropriate microwells of a microculture plate (each well contained affinity purified polyclonal IgG to the appropriate marker). Wells were incubated for 60 minutes (±5 minutes) at room temperature, and then were washed four times with wash buffer. In all, 100 μL of pre-diluter anti-human IgG conjugated with Horse Radish Peroxidase (HRP) was added to all microwells, incubated for 30 minutes (±5 minutes) at room temperature, and then washed for four times with wash buffer. A total of 100 μL of enzyme substrate was added to each microwell. After approximately 30 minutes at room temperature, the reaction was stopped by adding 50 μL of 1 M sulfuric acid, and then the wells were read at 405 nm with an ELISA reader (BioRad Laboratories, Inc.).
Copper and zinc measurements
Copper and zinc plasma concentrations were measured by LabCorp, Inc. using inductively coupled plasma-mass spectrometry, as previously described.23
Plasma
All plasma, experimental and control, were treated in an identical fashion – frozen at −70°C immediately after collection and cell/serum separation, and then stored at −70°C until thawed for use in ELISAs. Plasmas were collected on a non-fasting basis, under conditions which were the same for both individuals with autism and controls.
Subjects
Plasma and diagnostic criteria (below) has been previously reported.22 Plasma from 49 autistic children (39 males; mean age 11.4 years) (17 with diagnosed GI disease – chronic diarrhea and/or constipation (10 of these GI patients were taking probiotics) and 26 receiving probiotic therapy) and 36 neurotypical controls (29 males; mean age 10.2 years; controls were not assessed for GI disease) were tested for MPO plasma concentration using ELISAs. Plasma concentration of MPO in autistic individuals was compared to plasma concentration of copper and zinc.
The diagnostic criteria used in this study were defined by the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria. In 2012, the separate diagnostic labels of autistic disorder, Asperger’s disorder, and pervasive developmental disorder – not otherwise specified (PDD-NOS) were replaced by one umbrella termed autism spectrum disorder.
Plasma was obtained from patients presenting to the Health Research Institute (HRI) (The HRI is a comprehensive treatment and research center, specializing in the care of neurological disorders, including autism.) over a two-year period. All autistic individuals who presented to HRI were asked to participate. Plasmas from individuals who participated in this study were randomly chosen from all patients who volunteered. Neurotypical control plasma was obtained from HRI (wellness patients) and the Autism Genetic Resource Exchange (AGRE) (The AGRE is a repository of biomaterials and phenotypic and genotypic data to aid research on autism spectrum disorders.), and randomly chosen from a selection of about 200 samples. The autistic individuals in this study met the DSM-IV criteria, and many were diagnosed using the autism diagnostic interview – revised (ADI-R) before presenting to the HRI.
Patient consent was obtained from all patients involved in this study, and this study was approved by the institutional review board (IRB) of the HRI, and this research complied with the principles of the Declaration of Helsinki.
Statistics
Inferential statistics were derived from unpaired t-test and odds ratios with 95% confidence intervals. Pearson moment correlation test was used to establish degree of correlation between groups.
Results
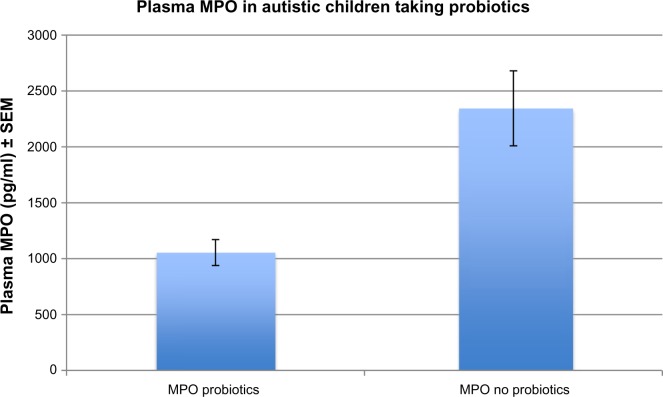
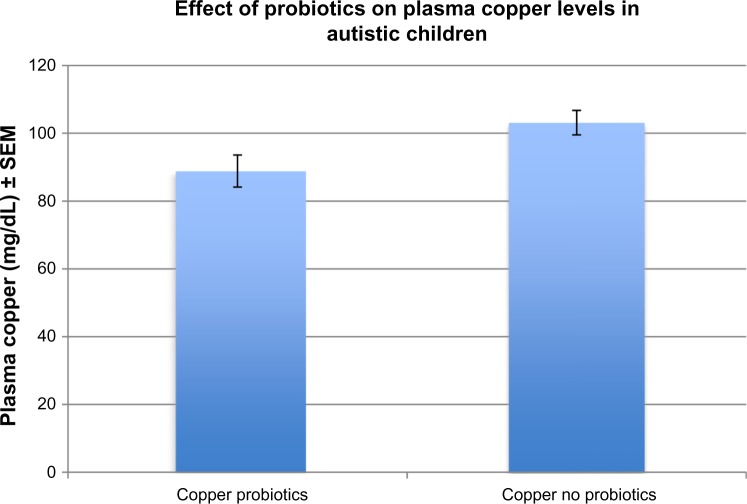
We found that individuals with autism, receiving no probiotic therapy, had lower, but did not have significantly lower plasma MPO levels (2463 pg/mL ± 420) when compared to controls (3161 pg/mL ± 150) (P = 0.06). In the autistic group, however, MPO levels were significantly lower (1054 pg/mL ± 116) in individuals taking probiotic therapy, compared to those not taking probiotics (3161 pg/mL ± 336) (P = 0.0009) (Fig. 1). In addition, plasma copper levels were significantly lower in autistic individuals taking probiotics (88.8 mg/dL ± 4.7) compared to those not taking probiotics (103.1 mg/dL ± 3.6) (P = 0.02) (Fig. 2), but plasma zinc levels were not different in the probiotic group (89.30 mg/dL ± 25.9) compared to the non-probiotic group (86.40 ± 24.7) (P = 0.7). MPO levels did not correlate by gender (P = 0.4) or age (P = 0.7). GI disease (N = 17) had no effect on MPO levels (P = 0.46). Autistic patients receiving zinc therapy (N = 25) did not have different plasma concentrations of MPO when compared to individuals with autism who had not received zinc therapy (P = 0.46). Table 1 summarizes the above data.
Figure 1.
MPO levels were significantly lower (1054 pg/ml ± 116) in individuals taking probiotic therapy, compared to those not taking probiotics (3161 pg/ml ± 336) (P = 0.0009).
Figure 2.
Plasma copper levels were significantly lower in autistic individuals taking probiotics (88.8 mg/dL ± 4.7) compared to those not taking probiotics (103.1 mg/dL ± 3.6) (P = 0.02).
Table 1.
Effects of probiotics on MPO concentration (pg/ml ± SEM).
| 1. Plasma MPO concentration significantly lower in autistic children compared to controls | |
| MPO autistic | MPO controls |
| Mean = 1692.03 ± 181.1 | Mean = 3161.28 ± 150.5 |
| 2. Plasma MPO concentration significantly lower in Autistic individuals taking probiotics compared to autistic individuals not taking probiotics | |
| MPO probiotics | MPO no probiotics |
| Mean = 1054.21 ± 116.8 | Mean = 2344.56 ± 336.8 |
| 3. Plasma MPO levels are not significantly lowered by zinc therapy | |
| MPO pre zinc therapy | MPO post zinc therapy |
| Mean = 2100.54 ± 411.3 | Mean = 1557.36 ± 228.7 |
| 4. Plasma MPO is not affected by GI disease. | |
| MPO GI disease | MPO without GI disease |
| Mean = 1929.87 ± 442.4 | Mean = 1607.18 ± 212.9 |
| 5. Plasma copper levels (mg/dl ± SEM) are significantly lower on autistic children taking probiotics compared to those not taking probiotics | |
| Copper probiotics | Copper no probiotics |
| Mean = 88.84 ± 4.7 | Mean = 103.14 ± 6.3 |
| 6. Plasma zinc levels (mg/dl ± SEM) are not affected by probiotics | |
| Zinc probiotics | Zinc no probiotics |
| Mean = 89.30 ± 7.1 | Mean = 86.40 ± 6.3 |
Discussion
Our results show that probiotic therapy may serve to significantly decrease MPO concentration in individuals with autism. We also found that probiotic therapy is associated with significantly lower copper levels.
A large population of autistic children suffers from GI disorders, implying a link between autism and abnormalities in gut microbial functions. Increasing evidence associated with sequencing analyses indicates disturbances in composition and diversity of gut microbiome, and that these changes may be associated with various disease conditions.20,24,25
Recent research indicates that autism and accompanying GI symptoms are characterized by distinct and less diverse gut microbial compositions than controls, with lower levels of Prevotella, Coprococcus, and unclassified Veillonellaceae.25
Probiotics are live microorganisms, such as Lactobacilli sp., Bifidobacteria sp., and Streptococcus thermophilus, that provide various health benefits upon ingestion and serve to often normalize gut flora populations.26 These probiotics are commercially available as spores in lyophilized forms or in the form of probiotic-fortified fermented dairy products. Probiotics have shown potential as a component of treatment for eczema,27 pediatric antibiotic-associated diarrhea,28 acute upper respiratory tract infections,29 chronic and acute enteric infections, and their associated diarrheal complexes.30
Probiotics have been shown to modulate the intestinal immune system by production of secreted factors and metabolites that affect the growth and function of intestinal epithelial and immune cells.31
Probiotics may manipulate intestinal microbial communities and suppress growth of pathogens by inducing the host’s production of β-defensin and IgA. Probiotics may be able to fortify the intestinal barrier by maintaining tight junctions and inducing mucin production. Probiotic-mediated immunomodulation may occur through mediation of cytokine secretion through signaling pathways such as NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) and MAPK (mitogen activated protein kinase), which can also affect proliferation and differentiation of immune cells (such as T cells) or epithelial cells. Gut motility may be modulated through regulation of pain receptor expression and secretion of neurotransmitters.32
The gut microbiota comes into contact with metals and other contaminants as they are ingested through diet.33 It is likely that microbes are presented with metals in water and food and may play a role in protecting the host from metal adsorption. Microbial sequestering of heavy metals by the intestinal microbiota is strongly supported by studies that show that when these contaminants are consumed at much higher concentrations, there is a lower detection in clinical samples, excluding absorption and dilution factors.34,35 Mechanisms for the binding of metals to bacterial cell walls include ion exchange reactions with peptidoglycan and teichoic acid, precipitation through nucleation reactions, and complexation with nitrogen and oxygen ligands.36–38 Since we found that copper levels, but not zinc levels, are lowered when associated with probiotic therapy, changes in microbiota may selectively affect the absorption of copper. The dynamics of this will need to be elucidated with future studies.
There were limitations to this study. We were unable to differentiate effect of different types of probiotics. This was based on varied therapy and the number of individuals in our study. Also, we did not follow individual patients, but the data represent a comparison of individual MPO levels, patients who had not taken probiotics with those who received the therapy. We did not find a relationship between length of probiotic use and MPO levels (unpublished data).
These data strongly suggest that autistic children taking probiotics have significantly lower levels of MPO and copper. It is possible that altered gut flora, because of probiotic therapy, results in less inflammation, which, in turn, results in lower MPO levels. Lower copper levels may also be the result of changes in gut flora, resulting in bacteria that more readily bind heavy metals and prevent high absorption rates.
Acknowledgments
The author would like to thank the Autism research Institute for financial support for this project. The author would like to acknowledge the resources provided by the Autism Genetic Resource Exchange (AGRE) Consortium and the participating AGRE families. The AGRE is a program of Autism Speaks and is supported, in part, by grant 1U24MH081810 from the National Institute of Mental Health to Clara M. Lajonc.
Footnotes
Author Contributions
Conceived and designed the experiments: AJR. Analyzed the data: AJR. Wrote the first draft of the manuscript: AJR. Contributed to the writing of the manuscript: AJR. Agree with manuscript results and conclusions: AJR. Jointly developed the structure and arguments for the paper: AJR. Made critical revisions and approved final version: AJR. The author reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Praveen Kumar, Associate Editor
FUNDING: This project was funded by the Autism Research Institute. The author confirms that the funding agency had no influence on study design, content of the article or selection of this journal.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–45. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 2.Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 3.Heinecke JW, Li W, Francis GA, Goldstein JA. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91(6):2866–72. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nauseef WM. Insights into myeloperoxidase biosynthesis from its inherited deficiency. J Mol Med. 1998;76(10):661–8. doi: 10.1007/s001090050265. [DOI] [PubMed] [Google Scholar]
- 5.Kutter D, Devaquet P, Vanderstocken G, Paulus JM, Marchal V, Gothot A. Consequences of total and subtotal myeloperoxidase deficiency: risk or benefit. Acta Haematol. 2000;104(1):10–5. doi: 10.1159/000041062. [DOI] [PubMed] [Google Scholar]
- 6.Cramer R, Soranzo MR, Dri P, et al. Incidence of myeloperoxidase deficiency in an area of northern Italy: histochemical, biochemical and functional studies. Br J Haematol. 1982;51:81–7. doi: 10.1111/j.1365-2141.1982.tb07292.x. [DOI] [PubMed] [Google Scholar]
- 7.Nauseef WM. Lessons from MPO deficiency about functionally important structural features. Jpn J Infect Dis. 2004;57(5):S4–5. [PubMed] [Google Scholar]
- 8.Lanza F. Clinical manifestation of myeloperoxidase deficiency. J Mol Med. 1998;76(10):676–81. doi: 10.1007/s001090050267. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington D.C: American Psychiatric Association; 1994. [Google Scholar]
- 10.Gillberg C, Coleman M. The Biology of the Autistic Syndromes. 2nd. London: Mac Keith Press; 1992. [Google Scholar]
- 11.Filipek PA, Accardo PJ, Baranek GT, et al. The screening and diagnosis of autistic spectrum disorders. J Autism Dev Disord. 1999;29(6):439–84. doi: 10.1023/a:1021943802493. [DOI] [PubMed] [Google Scholar]
- 12.Bailey A, Phillips W, Rutter M. Autism: towards an integration of clinical, genetic, neuro-psychological, and neurobiological perspectives. J Child Psychol Psychiatry. 1996;37(1):89–126. doi: 10.1111/j.1469-7610.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 13.Horvath K, Perman J. Autistic disorder and gastrointestinal disease. Curr Opin Pediatr. 2002;14(5):583–7. doi: 10.1097/00008480-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Molloy C, Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003;7(2):165–71. doi: 10.1177/1362361303007002004. [DOI] [PubMed] [Google Scholar]
- 15.Valicenti-McDermott M, McVicar K, Rapin I. Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. J Dev Behav Pediatr. 2006;27(suppl 2):S128–36. doi: 10.1097/00004703-200604002-00011. [DOI] [PubMed] [Google Scholar]
- 16.Ashwood P, Anthony A, Pellicer AA, Torrente F, Walker-Smith JA, Wakefield AJ. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. J Clin Immunol. 2003;23(6):504–17. doi: 10.1023/b:joci.0000010427.05143.bb. [DOI] [PubMed] [Google Scholar]
- 17.Balzola F, Daniela C, Repici A, et al. Autistic enterocolitis: confirmation of a new inflammatory bowel disease in an Italian cohort of patients. Gastroenterology. 2005;128(suppl 2):A299–303. [Google Scholar]
- 18.Shaw W, Kassen E, Chaves E. Increased urinary excretion of analogs of Krebs cycle metabolites and arabinose in two brothers with autistic features. Clin Chem. 1995;41:1094–104. [PubMed] [Google Scholar]
- 19.Shaw W, Kassen E, Chaves E. Assessment of antifungal drug therapy in autism by measurement of suspected microbial metabolites in urine with gas chromatography-mass spectrometry. Clin Pract Alternat Med. 2000;1:15–26. [Google Scholar]
- 20.Adams JB. Gastrointestinal flora and gastrointestinal status in children with autism – comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11:22. doi: 10.1186/1471-230X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo AJ, Krigsman A, Jepson B, Wakefield A. Low serum myeloperoxidase in autistic children with gastrointestinal disease. Clin Exp Gastroenterol. 2009;2:85–94. doi: 10.2147/ceg.s6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo AJ. Correlation between hepatocyte growth factor (HGF) and gamma-aminobutyric acid (GABA) plasma levels in autistic children. Biomark Insights. 2013;8:69–75. doi: 10.4137/BMI.S11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanner S, Baranov V, Bandura D. Reaction cells and collision cells for ICP–MS: a tutorial review. Spectrochimica Acta. 2002;57:1361–452. [Google Scholar]
- 24.Finegold SM. State of the art; microbiology in health and disease. Intestinal bacterial flora in autism. Anaerobe. 2011;17(6):367–8. doi: 10.1016/j.anaerobe.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Kang D-W, Park JG, Ilhan ZE, et al. Reduced Incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8(7):e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rijkers GT, de Vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P. Health benefits and health claims of probiotics: bridging science and marketing. Br J Nutr. 2011;106(9):1291–6. doi: 10.1017/S000711451100287X. [DOI] [PubMed] [Google Scholar]
- 27.Gore C, Custovic A, Tannock GW, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy. 2011;42(1):112–22. doi: 10.1111/j.1365-2222.2011.03885.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2011;9:CD004827. doi: 10.1002/14651858.CD004827.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Hao Q, Lu Z, Dong BR, Huang CQ, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2011;9:CD006895. doi: 10.1002/14651858.CD006895.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Sleator RD. Probiotics – a viable therapeutic alternative for enteric infections especially in the developing world. Discov Med. 2010;10(51):119–24. [PubMed] [Google Scholar]
- 31.Preidis G, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–31. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. 2013;6(1):39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turroni F, Foroni E, Pizzetti P, et al. Exploring the diversity of the bifido-bacterial population in the human intestinal tract. Appl Environ Microbiol. 2009;75:1534–45. doi: 10.1128/AEM.02216-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fierens S, Mairesse H, Heilier JF, et al. Impact of iron and steel industry and waste incinerators on human exposure to dioxins, PCBs, and heavy metals: results of a cross-sectional study in Belgium. J Toxicol Environ Health A. 2007;70:222–6. doi: 10.1080/15287390600884628. [DOI] [PubMed] [Google Scholar]
- 35.Zhitkovich A. Chromium in drinking water: sources, metabolism, and cancer risks. Chem Res Toxicol. 2011;24:1617–29. doi: 10.1021/tx200251t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beveridge TJ, Murray RG. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141:876–87. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beveridge TJ, Fyfe WS. Metal fixation by bacterial cell walls. Can J Earth Sci. 1985;22:1893–8. [Google Scholar]
- 38.Mueller JG, Chapman PJ, Pritchard PH. Creosote-contaminated sites. Their potential for bioremediation. Environ Sci Technol. 1989;23:1197–201. [Google Scholar]