Abstract
Several lines of evidence suggest that β-cyclodextrin (β-CD) derivatives initiate the efflux of accumulated, unesterified cholesterol from the late endosomal/lysosomal compartment in Niemann Pick C (NPC) disease models. Unfortunately, repeated injections or continuous infusions of current β-CD therapies are required to sustain suppression of symptoms and prolong life. In an effort to make CD treatment a more viable option by boosting efficacy and improving pharmacokinetics, a library of Pluronic surfactant-based β-CD polyrotaxanes has been developed using biocompatible poly(ethylene glycol) (PEG)–polypropylene glycol (PPG)–PEG triblock copolymers. These compounds carry multiple copies of β-CD as shown by 1H NMR, 2D nuclear Overhouser effect spectroscopy, gel permeation chromatography/multiangle light scattering, analytical ultracentrifugation analysis, matrix assisted laser desorption/ionization mass spectrometry, and diffusion-ordered spectroscopy. Analyses of free β-cyclodextrin contamination in the compounds were made by reverse phase high pressure liquid chromatography and hydrophilic interaction liquid chromatography. Dethreading kinetics were studied by reverse phase high pressure liquid chromatography, UV/vis, and 1H NMR analysis. Filipin staining studies using npc2−/− fibroblasts show significant reversal of cholesterol accumulation after treatment with polyrotaxane compounds. The rate and efficacy of reversal is similar to that achieved by equivalent amounts of monomeric β-CD alone.
1. INTRODUCTION
Niemann-Pick Type C (NPC) disease is one of a large set of lysosomal storage disorders that arise as a consequence of abnormal function within the late endosome/lysosome (LE/LY) compartment. In this rare disease, the accumulation of cholesterol and other lipids within the LE/LY compartment gives rise to various disease states in the patient.1–3 Often, the defect manifests itself in the form of neurological, hepatic, and/or pulmonary symptoms that increase in intensity over time.4 These can involve enlargement of organs, dysarthria, dysphagia, ataxia, and other neurological symptoms including epilepsy and dementia.2 NPC typically manifests in children and is ultimately fatal, even with current treatment regimens.5
The underlying causes of NPC are genetic mutations that disrupt the function or localization of either the NPC1 (95% of cases) or NPC2 (5% of cases) proteins. These proteins are ubiquitously expressed and are localized to the LE/LY compartment.1,2 In a normally functioning cell, NPC1 and NPC2 are responsible for the efflux of unesterified cholesterol (UC) from the LE/LY compartment into the cytosol and are generally thought to function in a common UC efflux pathway.6 The dysfunction of either NPC protein causes aberrant sequestration of UC in the LE/LY compartment.5 The accumulation of UC, in turn, causes lysosomal swelling, upregulation of the genes controlling cholesterol synthesis and LDL uptake, elevation in intrinsic total cholesterol synthesis and lipoprotein uptake, and neurological effects including demyelination of brain cell axons and the death of Purkinje cells.3,7,8 Additionally, the onset of macrophage activity in affected tissues results in the release of pro-inflammatory cytokines in most organs.7,9–11 The cumulative impact of these factors is cell death and presentation of the NPC disease state.
Since its demonstration by Camargo et al., an increasing body of evidence shows that derivatives of β-cyclodextrin (β-CD), a macrocycle consisting of 7 glucose subunits, are able to initiate the efflux of accumulated UC from the LE/LY compartment of npc1 and npc2 deficient cells.3,4,7–13 Dietschy and co-workers have recently reported several studies that demonstrate the ability of β-CD derivatives (e.g., HP-β-CD) to mobilize UC in the murine model of NPC1 disease.3–5,8,9,14 Upon treatment with HP-β-CD, a normalization of intracellular UC levels, suppression of intrinsic cholesterol synthesis, activation of the target genes for the LXR nuclear receptors, and suppression of target genes for SREBP were observed in several organs. All of these factors contribute to rapid egress of sequestered UC from sites of abnormal storage.8,10,11 When injected directly into the CNS, similar effects were seen in the brains of npc1−/− mice,4,7 and continuous infusion with β-CD derivatives was shown to prevent expected neurodegeneration.7 β-CD has also been shown to have similar positive effects in npc2−/− mice.13 At the cellular level, addition of β-CD to either npc1−/− or npc2−/− patient fibroblasts has been shown to rescue the cholesterol accumulation phenotype of these cells.12,13
While reversal of UC storage in mice was seen after administration of β-CD, evidence indicated that the animals began reaccumulating cholesterol shortly after dosing was terminated.8,9,11 β-CD derivatives, therefore, overcome cholesterol transport defects in NPC1 disease, but the alleviation is temporary such that repeated or continuous infusions are needed for prolonged effects.7,9 Increasing the efficiency of β-CD delivery, by improving the pharmacokinetics and biodistribution of the dose, would make β-CD much more viable as a possible NPC therapeutic. One potential method for achieving these goals would be to deliver β-CD via a high molecular weight, long circulating vehicle. A β-CD formulation offering improved circulation time and lower clearance rates could potentially deliver a more efficacious dose and be beneficial in NPC treatment.
Polyrotaxanes (PRTx) are supramolecular assemblies that have been of considerable interest in biomaterials applications such as drug and gene delivery and hydrogel formation.15–19 These complexes are typically prepared by threading macro-cycles noncovalently onto a polymer core to produce a pseudopolyrotaxane with subsequent addition of bulky end-caps onto the central polymer termini to yield a polyrotaxane. The end-caps prevent macrocycle dethreading and can be tuned to be bioresponsive toward changes in pH20 or toward enzyme activity,21,22 allowing for control over when and how the PRTx degrades to release its macrocyclic cargo. Cyclo-dextrin-based PRTx derivatives have been synthesized using many polymer cores including polyesters,23 polyamides,24 poly(ethylene glycol) (PEG),25–27 polypropylene glycol (PPG),28 and many di- and triblock copolymers.29
In this study, a library of PRTx derivatives has been synthesized using Pluronic block copolymers and β-cyclo-dextrin as building blocks. Pluronic polymers have a PEG–PPG–PEG triblock copolymer architecture that differs in the relative lengths of the PEG and PPG blocks. Molecular weights of the Pluronics used in this study range from 1900 to 12 600 g·mol−1. These polymers were chosen because of their previous uses in pharmaceutical applications and their known bio-compatibility.30 Our aim was to increase the efficiency of β-CD delivery into NPC cells using biocompatible Pluronic-derived β-CD PRTx.
The PRTx library was subsequently characterized by solubility tests, nuclear magnetic resonance (NMR), 2D nuclear overhouser effect spectroscopy (NOESY), gel permeation chromatography (GPC), analytical ultracentrifugation (AUC), atomic force microscopy (AFM), matrix assisted laser desorption/ionization mass spectrometry (MALDI), and diffusion-ordered spectroscopy (DOSY). Analyses of free β-CD contamination levels in the compounds were made by reverse phase high-pressure liquid chromatography (RPLC) and hydrophilic interaction liquid chromatography (HILIC). Dethreading kinetics were studied by RPLC, 1H NMR, and UV/vis analysis. Finally, the capacity of these compounds to mobilize UC in npc2−/− fibroblasts was evaluated by filipin staining, while PRTx localization to LE/LY compartments was studied by fluorescent PRTx colocalization with Lysotracker Blue.
2. MATERIALS AND METHODS
2.1. Materials
(1,1)-Carbonyldiimidazole (CDI) and 2,4,6-trinitrobenzenesulfonic acid (TNBS) were purchased from Research Organics. Tris(2-aminoethyl)amine (TREN), β-cyclodextrin, Pluronic polymers, and 2,2′-(ethylenedioxy)bis-(ethylamine) were purchased from Sigma-Aldrich. The four Pluronics used in this study were F127, F68, L64, and L35. All solvents were dried and distilled from an appropriate desiccant prior to use unless otherwise noted. Human fibroblasts cells from an apparently healthy donor (GM03652) and from an NPC2 patient (GM18455) were from Coriell Institute of Medical Research (Camden, NJ). Filipin and fluorescein were purchased from Sigma and Lysotracker Blue from Molecular Probes.
2.2. Synthesis of TREN-Terminated Pluronics
The synthesis of F127 Pluronic-based β-CD polyrotaxane will be described in detail below. All other β-CD:Pluronic PRTx were synthesized and purified in the same manner, except where noted.
The Pluronic precursor was added to a dry round-bottom flask under a dry N2 atmosphere and then dissolved in benzene before solvent evaporation. This azeotropic drying procedure was repeated three times before finally drying the polymer under a vacuum at 80 °C overnight.
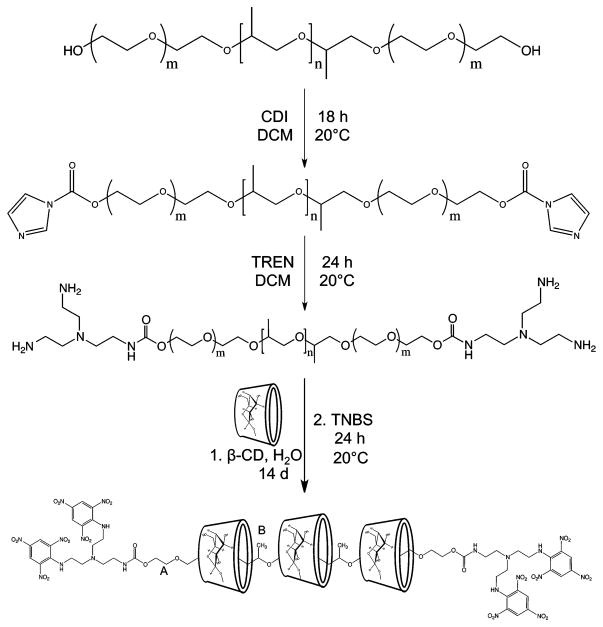
Pluronic F127 (4.0 g) was dissolved in 20 mL of dichloromethane (DCM) and added to CDI (9.5 mmol, 30 equiv, dissolved in 20 mL of DCM) by syringe over the course of 6 h. The solution was stirred for 18 h at 20 °C before concentration by evaporation and addition to TREN (6.4 mmol, 20 equiv, dissolved in 20 mL of DCM) over 3 h. After being stirred at 20 °C for 24 h, the solution was concentrated by rotary evaporation, and the residue was precipitated in ether (3 × 350 mL), before collection by centrifugation and drying overnight under a vacuum. The TREN derivatives of L35 and L64 were isolated as viscous yellow liquids, whereas the F127 and F68 derivatives were isolated as white solids. TREN-polymers proved difficult to purify completely and as such were used without further purification. Extensive washing and dialysis of the final PXR compounds would serve to remove any impurity retained after the earlier steps.
2.3. Threading, End-Capping, and Purification of TREN-Terminated β-CD:Pluronic Polyrotaxanes
TREN-F127 (2.5 g) was added to a saturated aqueous solution of β-CD (5.9 mmol β-CD in 361 mL H2O). After dissolution of the polymer, the solution was bath sonicated for 10 min at 65 °C to ensure complete mixing. Finally, the solution was probe sonicated using a 1/2 in. tipped transducer for 3 min, followed by agitation on a rocker plate for 14 days to promote threading. For the liquid polymers L35 and L64, a white precipitate formed in the flask almost immediately and increased over time. The threading progressed much more slowly for the solid polymers (F127 and F68), with precipitate appearing usually after 24 h and increasing thereafter.
After completion of the threading reaction, TNBS (4 equiv, 1.5 mmol, 5.1 mL in a 10% solution) was added to the reaction mixture and left on the rocker plate overnight to effect the end-capping reaction.
The L35-, L64-, and F68-based PRTx were first collected by centrifugation from the reaction mixture for 3 min at 7000 rpm. They were then diluted with 30 mL of H2O, vortexed, and recentrifuged under identical conditions. Water washes were continued in this manner until the washes ran nearly clear; usually 3–5 repetitions. The F127-based PRTx was not collected by centrifugation due to the formation of viscous suspensions; therefore, this product was purified by extensive dialysis as described below.
The products were again suspended in 30 mL of H2O and dialyzed against H2O using dialysis tubing correlating to the estimated MW of the final product. L35- and L64-based PRTx were dialyzed with MWCO 3500 and 6–8000 kDa tubing, respectively. F68 and F127-based PRTx were dialyzed against water in MWCO 12–15 kDa tubing. The products were dialyzed for 3 days with an average of six water changes, or until water remained clear for two consecutive water changes.
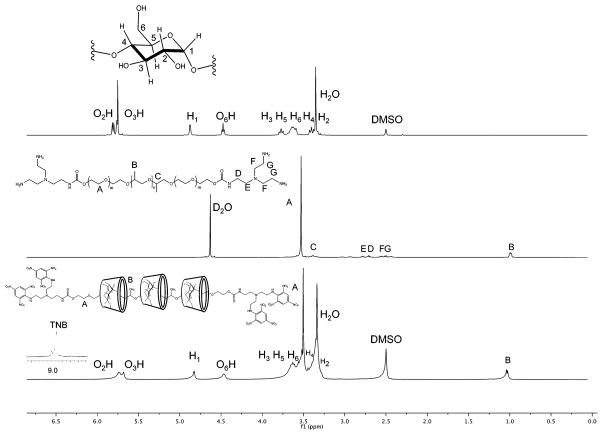
Upon completion of dialysis, the purified products were frozen and lyophilized to yield yellow-orange powders. β-CD:F127 PRTx was obtained in 32.9% yield (1.883 g). β-CD:F68 PRTx, β-CD:L64 PRTx, and β-CD:L35 PRTx gave 13.29% (0.502 g), 29.1% (4.773 g), and 19.1% (1.12 g) of solid, respectively. F127 1H NMR: (400 MHz, DMSO-d6, 22 °C) δ 8.94–8.76 (br s., 8H, TNB), 5.73–5.65 (m, 266H, O2H, O3H of CD), 4.8 (s, 133H, H1 of CD), 4.45 (t, 133H, O6H of CD), 3.67–3.59 (m, 532H, H3, H6, H5, H4 of CD), 3.51–3.48 (m, 995H, PPO and PPG –CH2–), 1.04–1.01 (d, 195H, PPG –CH3).
After the initial purification processes, the PRTx were analyzed by both RPLC and HILIC, with the two methods consistently agreeing within 8%. Initial free CD measurements for β-CD:F127 PRTx were 19.9% by HILIC and 26.9% by RPLC. Samples containing >5% free β-CD by RPLC were suspended in 15 mL of H2O, vortexed for 2 min, and gathered by centrifugation as previously described. After three repetitions, the samples were lyophilized and reanalyzed for free β-CD content. This process was repeated until the HILIC and/or RPLC analyses indicated that the free β-CD content was ≤5% free β-CD.
The inability to wash and collect β-CD:F127 PRTx by centrifugation made them more difficult to purify of free and/or entrained β-CD contamination. Samples containing >5% free β-CD contamination after initial purification were resuspended in H2O, vortexed for 3 min, and again left to dialyze against water in either MWCO 12–14 kDa dialysis tubing or a 20 kDa dialysis cassette for 2 days, lyophilized, and retested. This process was repeated until the HILIC and/or RPLC analyses indicated that the free β-CD content was ≤5% free β-CD.
Fluorescein modified β-CD:F127 PRTx was synthesized by dissolution of 400 mg β-CD:F127 PRTx in dry DMSO before activation with CDI (6.1 mmol) and coupling to ethylene diamine (7.4 mmol). A second activation with CDI (6.1 mmol), followed by fluorescein coupling (6.1 mmol), and precipitation twice in MeOH yielded the fluorescent PRTx derivative. Fluorescent PRTxs were used without further purification.
2.4. Determination of Threading Efficiency by 1H NMR
1H NMR spectra were acquired on a 400 MHz Bruker ARX400 spectrometer, using the solvent peak as the reference standard and the chemical shift given in parts per million. NOESY spectra were taken on a 500 MHz Bruker DRX500 spectrometer with a 5 mm TBI probe. DMSO-d6 was used as NMR solvent unless otherwise noted. Determinations of PRTx threading efficiencies were initially performed by 1H NMR spectral integration. The characteristic β-CD H1 proton at ~4.8 ppm was directly compared to the PPG methyl protons of the polymer at 1.0 ppm. The PPG resonance was normalized to the average number of hydrogens expected for that specific Pluronic’s methyl group. Given that each β-CD monomer has seven acetal protons, the corresponding integration for the number of β-CD H1 protons present in the sample was divided by seven. This yielded an average number of β-CDs per PRTx. This estimate of threading efficiency was then recalculated once the amount of free, unthreaded β-CD in the sample was determined by chromatographic analysis.
2.5. Determination of Free β-CD in β-CD/Pluronic PRTx
Determinations of free β-CD in PRTx samples were done in two ways. First, PRTx solutions of ~1 mg/mL were prepared in H2O for analysis on a custom-made HILIC silica packed column with 700 nm nonporous silica nanoparticles that were surface-modified with amide groups.31 Before analysis, PRTx were subjected to two rounds of vortexing for 2 min and bath sonication at 50 °C for 10 min. The temperature of the column oven was maintained at 25 °C. All runs were performed under the gradient conditions of 90–70% acetonitrile (ACN) with 0.1% formic acid. Determinations were made on a Thermo Accela UPLC system (Thermo Fischer Scientific, MA, USA) and LTQ Velos LC-MS. Mobile phases A and B were ACN with 0.5% (v/v) formic acid and water with 0.5% (v/v) formic acid. Chromatographic separation started at an initial mobile phase of 95% A, held for 1 min, then followed by a linear gradient from 80 to 60% mobile phase A in 15 min. The solvent composition then changed to 50% A over 0.1 min and was maintained for 5 min before being re-equilibrated to 95% A. The flow rate was 150 μL/min.
Mass spectrometric data were acquired in the positive ion mode. The ESI conditions were as follows: ESI spray voltage, 4 kV; temperature setting, 300 °C; sheath gas flow, 30 U (arbitrary units); auxiliary gas flow, 8 U.
Additionally, PRTx solutions in H2O (~1 mg/mL) were analyzed by RPLC using an H2O:ACN gradient elution as described by Agueros, Campanero and Irache.32 Analyses were performed using an Agilent Technologies 1200 Series HPLC equipped with an ESA Corona Detector and an Agilent Zorbax Eclipse XDB-Phenyl column (2.1 × 1250 mm, 5 μm particles). Known concentrations of β-CD were used for both HILIC and RPLC measurements to construct standard curves for fitting the PRTx sample data.
2.6. Gel Permeation Chromatography
Corroboration of NMR molecular weight measurements and threading efficiencies were made by gel permeation chromatography (GPC) analysis. PRTx samples (~30 mg/mL in DMSO) were prepared and analyzed as described by Beirne, Truchan, and Rao.33 GPC measurements were made using an Agilent Technologies GPC system equipped with an OHpak SB-803HQ column (8 mm × 300 mm), Dawn Helios II multiangle light scattering detector (Wyatt Technology Corporation), and Optirex RI detector (Wyatt Technology Corporation) using DMSO as the mobile phase. The detectors were normalized using 25 kDa pullulan and calibrated using 11.6 kDa and 48.6 kDa dextran standards. The flow rate was 0.16 mg/mL and a 100 μL injection volume was used.33
2.7. Analytical Ultracentrifugation
Analytical ultracentrifugation was done in the Purdue Bindley Bioscience Center on a Beckman Coulter XLI (Beckman-Coulter, CA) ultracentrifuge using absorbance optics at 346 and 422 nm and interference optics at 675 nm. PRTx were characterized in PBS buffer at pH 7.4 at concentrations of 0.1, 0.2, and 0.4 OD. Sedimentation equilibrium (SE) experiments were done at speeds of 25 000, 35 000 and 40 000 rpm for a total of 67.8 h and analyzed by the species analysis model in SEDPHAT (v. 9.4).34 The system was standardized by running unmodified F127 Pluronic (found to be 12 500 g/mol as expected). Sedimentation velocity experiments were conducted at 50 000 rpm using a 2 channel charcoal centerpiece for 15 h and analyzed using SEDFIT (v. 12.43). 35 All experiments were carried out at 20 °C using an Anton 60 Ti rotor. Buffer density and viscosity were calculated using SEDNTERP.36 The partial specific volume of polyrotaxane without a PEG derivative was calculated to be 0.62 mL/g.37 The partial specific volume of the polyrotaxanes with PEG derivatives was 0.85 mL/g, which agrees well with published values.38
2.8. Atomic Force Microscopy
AFM images were acquired by depositing and drying 1 μL of probe sonicated PRTx-DMSO solution onto a mica plate. All samples were fixed ~5 min after probe sonication. Images were obtained using a Dimension 3100 AFM from Veeco Instruments (USA). Tapping mode images were collected using an NSC15/AIBS 325 kHz cantilever with a 46 N/m force constant and an amplitude set point of 1 V.
2.9. Mass Spectrometry Analysis
An ionic liquid matrix (ILM) was made for analyzing the PRTx by MALDI. The ILM was made as follows: 2′,4′,6′-trihydroxyacetophenone monohydrate (THAP) and 1,1,3,3-tetramethylguanidine (TMG) were mixed at a molar ratio of 1:2 in methanol. The solution was then sonicated for 15 min at 40 °C. After removal of methanol by centrifugal evaporation in a SpeedVac for 3 h at 20 °C, ILMs were left under a vacuum overnight. Final solutions were then prepared at a concentration of 90 mg/mL in DMF for use as a matrix. The polyrotaxane samples were prepared at 3 mg/mL in DMF. The samples for MALDI-MS analysis were prepared by mixing the PRTx sample and ILM in a 1:80 ratio. Then, 0.6 μL of the mixture was deposited on a mirror polished stainless steel MALDI target and allowed to dry at 20 °C and atmospheric pressure overnight. The spectra were acquired at a laser power of 6500 using 600 laser shots in linear mode on an AB4800 MALDI TOF/TOF analyzer.
ESI mass spectrometry of the TNB terminated 2,2′-(ethylenedioxy)bis(ethylamine) was done on a Waters ZQ electrospray mass spectrometer in negative ion mode.
2.10. Dethreading Analysis
Bulk dethreading experiments of β-CD:Pluronic PRTx were done by suspending each PRTx in H2O, adjusted to pH 7.4 or 5.5, at concentrations of 1 mg/mL. Samples were placed in a constant temperature bath at 37 °C and sampled at 1, 2, 4, 8, 16, and 24 h. Aliquots were analyzed by RPLC as described above to monitor the increase in free β-CD in each sample. 1H NMR analysis of similarly incubated PRTx was also conducted to reveal any changes in TNB peaks characteristic of successful end-capping.
A short, unthreaded TNB end-capped PEG-bis-amine (MW 570.4 g/mol) was synthesized to better visualize any possible changes in the encap 1H NMR spectrum in the presence of glutathione (structure, synthesis, and NMR given in SI). This compound was subjected to a D2O solution of 10 mM glutathione at pH 7.4, incubated at 37 °C, and monitored at intervals for 48 h. The PEG-TNB concentration was 1 mg/mL.
UV/vis analyses of TNB end-caps were made using an Agilent HP 8453 UV/vis spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA, USA). PBS solutions of an unthreaded F68-TNB polymer were dissolved at 1 mg/mL with 10 mM glutathione at either pH 7.4 or 5.5. Emission spectra were taken at 1, 2, 4, 8, 16, 24, and 48 h, with monitoring at 346 and 420 nm wavelengths.
2.11. Diffusion-Ordered Spectroscopy
Experiments were performed on a Bruker AVIII300WB spectrometer using a diff50 diffusion probe with a maximum gradient strength of 30 T/m. The pulse sequence used was a stimulated echo diffusion sequence (PGSTE)39 with an additional spoiler gradient in the diffusion period. Signal attenuation was achieved by varying the gradient strength while all the timings in the pulse sequence were kept constant. The main experimental parameters were as follows. The effective gradient pulse duration (δ) was 1 ms using a sinusoidal gradient shape, the diffusion time (Δ) was 20 ms, and the repetition time 2 s. The gradient amplitude was varied in 64 steps linearly between 9 and 572 G/cm (90–5720 mT/m). For each gradient step, 64 scans were performed. The samples were dissolved in DMSO at 1 mg/mL and the temperature was kept constant at 25 °C. Data was processed using the Bruker Dynamics Center program.
2.12. Cell Studies
Human fibroblasts from npc2−/− or unaffected patients were seeded on eight-well tissue culture slides at a density of 6 × 103 cells, in Eagle’s minimum essential medium with Earle’s salts and nonessential amino acids +15% FBS at 37 °C with 5% CO2. Sterile β-CD:Pluronic PRTx samples were solubilized in DMSO and diluted in culture media to a concentration that would yield 25 μM β-CD. The final DMSO concentration in all preparations was ≤0.001% (v/v). Treatments were administered to cells 36–48 h after plating. Following varying incubation periods, as indicated, cells were rinsed with PBS and fixed in situ with 10% buffered formalin. Cell density and viability, determined by trypan blue exclusion, were determined using cells collected immediately prior to fixation. No changes in cell numbers or viability (>95%) were found, indicating an absence of toxicity under the range of conditions used in the present studies. Fixed cells were washed with PBS and stained with 50 μg/mL filipin in PBS for 1 h. Images were acquired using a DAPI filter set on a Nikon Eclipse E800 epifluorescence microscope (Nikon Inc.), equipped with NIS-Elements BR 3.2 software (Nikon Inc.). Antifade mounting agents and microscope light settings ensured the absence of photobleaching. Filipin accumulation in cells was determined using NIS-Elements selection tools and calculated as a ratio of filipin area to cell area. The results in β-CD:Pluronic PRTx and β-CD treated cells were normalized to filipin accumulation in untreated npc2−/− fibroblasts and are expressed as mean ± SE.
Fluorescein labeled β-CD:F127 PRTx was prepared as described above and administered to npc2−/− fibroblasts approximately 24 h after plating. Cells were rinsed with PBS 0.5 or 1 h after addition of the compound and incubated with 50 nM Lysotracker Blue (Life Technologies) in PBS for 10 min. Cells were rinsed again and fixed in situ with buffered formalin. Images were obtained using DAPI and B-2A filter sets for the fluorescent PRTx and Lysotracker, respectively.
3. RESULTS AND DISCUSSION
3.1. β-CD/Pluronic PRTx Characterization
Multiple techniques were used to characterize the β-CD:Pluronic PRTx products, including verification of successful end-capping reactions, characterization of β-CD threading efficiencies, determination of PRTx product molecular weights, and analysis of their purity with respect to free β-CD content.
The β-CD:Pluronic PRTx products were poorly soluble in all solvents tested, except DMSO. Poor solubility is a well-known property of PEG-based PRTx compounds.40 Poor solvent access to the threaded polymer core, combined with their high molecular weights, produce limited solubility of these derivatives.19 Pluronic-based β-CD PRTx were expected to have similar properties.
This characteristic was exploited to purify the PRTx from the β-CD and Pluronic precursors that are both quite water-soluble. Free Pluronic polymers, TREN-terminated Pluronic intermediates, and free β-CD were removed by repeated aqueous washing of the relatively water-insoluble PRTx products. Unsuccessfully and partially end-capped PRTx are also expected to dethread upon repeated suspension in water since there is nothing obstructing diffusion of β-CD off the end of the unreacted polymer core, eventually rendering these byproducts water-soluble and removable by the washing and dialysis processes.
Multiple lines of 1H NMR evidence suggest that PRTx formation was successful. End-cap peaks were observed indicating successful reaction of TNBS with the terminal amines of the TREN-pseudorotaxane intermediate. These peaks were present in NMR spectra of each β-CD:Pluronic PRTx species. There was also evidence of broadening in the β-CD O2H, O3H, H1, and O6H protons as well as in the signal corresponding to the Pluronic PPG methyl group. These characteristic changes, previously reported for β-CD PRTx, are a result of restricted β-CD mobility upon threading onto the Pluronic core.40
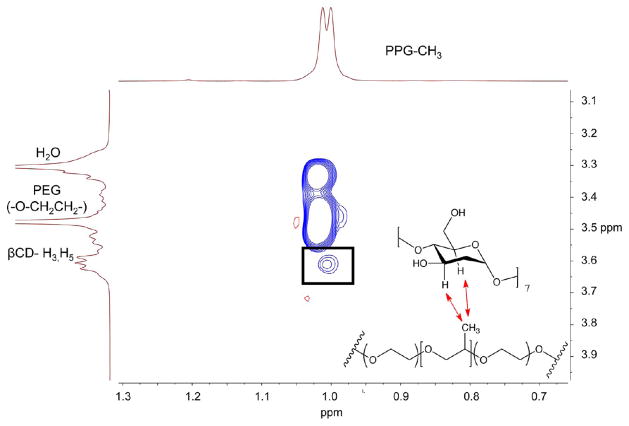
2D NOESY NMR was also performed as an additional confirmation of PRTx formation. Signals corresponding to the inward facing protons (H-3 and H-5) of the β-CD cavity (Figure 2) and the proton resonances of the Pluronic PPG methyl groups are found to be cross-correlated. The close proximity of these substituents indicates that the cyclodextrin molecules are threaded onto the Pluronic core.41
Figure 2.
Partial 2D-NOESY spectra of PRTx-F27. The characteristic cross-peak between β-CD hydrogens H3/H5 and the PPG methyl group is shown.
Initial characterization of the threading efficiency was done using 1H NMR integration with subsequent correction for the free β-CD contamination levels present in the sample as determined by chromatographic analysis. Since β-CD has seven identical H1 protons at the acetal position of each glucose subunit that appear at ~4.8 ppm, the intensity of this signal can be directly related to the signal intensity of the Pluronic PPG block methyl group. This method indicated that the PRTx threading efficiencies typically ranged between 62 and 100%.
Initial attempts to produce highly threaded Pluronic PRTx were less than satisfactory, with threading efficiencies often in the range of 20–30%. We attributed these failed attempts to aggregation of the Pluronic precursors. Since the critical aggregation concentration for the Pluronic precursors used is quite low (0.004–1 wt %), we attempted to disperse the micelles into Pluronic unimers in the threading mixture.31 Decreasing the Pluronic feed concentration and addition of a probe sonication step into the threading procedure increased the threading efficiencies for each of the Pluronic PRTx cores (Table 1). It is hypothesized that probe sonication of solutions at the initiation of the threading reaction disrupts micelle formation, thus allowing for the initial threading of β-CD molecules onto the Pluronic unimer which, in turn, prevents the immediate reformation of micelles and allows for further threading (Figure 3).
Table 1.
Average Number of CDs per PRTx, Threading Efficiencies, Yields, and Free CD Content
| β-CD PRTx | base MW (Da) | PEG:PPG | # CDs | % threading | % yield | % free CD |
|---|---|---|---|---|---|---|
| F127 | 12,500 | 200:65 | 15 | 71.4 | 32.9 | 4.4 |
| F68 | 8,350 | 151:29 | 14 | 100 | 13.3 | 3.6 |
| L64 | 2,900 | 26:30 | 12 | 92 | 29.1 | 2.4 |
| L35 | 1,900 | 22:16 | 5 | 63 | 19.1 | 1.2 |
Figure 3.
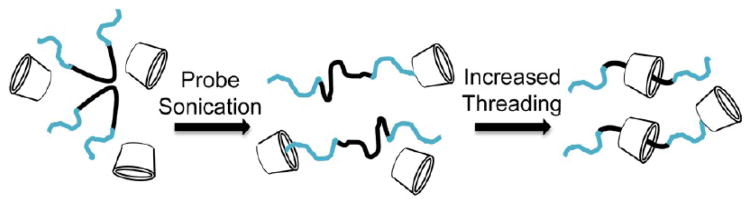
Hypothesized mechanism of increased β-CD threading upon probe sonication.
Quantification of the free β-CD content in a given PRTx sample is an important parameter to measure so that the performance of these compounds can be properly evaluated during in vitro and in vivo testing of cholesterol mobilization in NPC models. By having PRTx compounds essentially free of unthreaded β-CD, it can be safely assumed that any observed increases in cholesterol efflux or decreases in cholesterol synthesis are a product of β-CD initially entering the cell as a PRTx complex where it is then dethreaded into the active monomeric form. A goal of ≤5% free CD was selected as an acceptable target of purity. PRTx that did not meet this criterion were further purified as described. One round of further purification was usually enough to bring the PRTx samples below the 5% free β-CD threshold as determined by RPLC and/or HILIC.
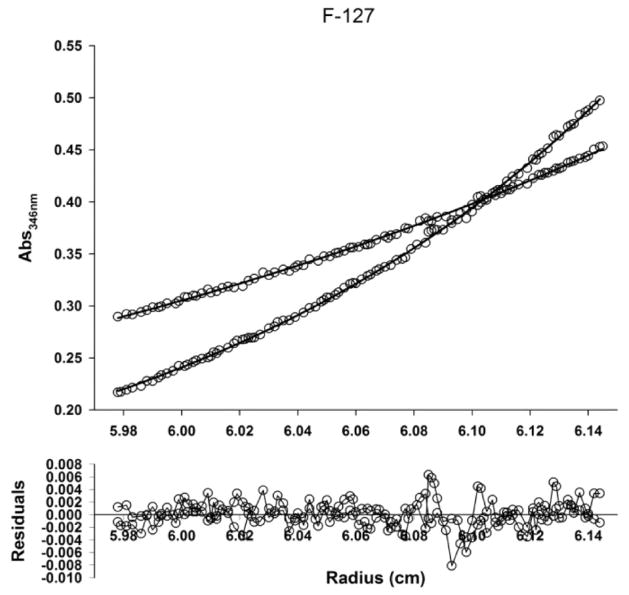
GPC analysis of PRTx compounds produced molecular weight estimates that were in agreement with the masses determined by NMR (after correction for free β-CD). The β-CD:F127 PRTx molecular weight was 30 800 g/mol. GPC analysis indicated a mass of 33 300 g/mol, with evidence of low levels of free β-CD noted in all samples analyzed. A mass of 30 000 g/mol was determined by both sedimentation velocity and sedimentation equilibrium experiments (Figure 4). Taken together, these data suggest that the average molecular weight of β-CD:F127 PRTx is ~31 kDa. Molecular weights for each member of the β-CD:Pluronic PRTx library corresponded well with respect to GPC, AUC, and NMR analysis (Table 2).
Figure 4.
Sedimentation equilibrium (SE) profile of β-CD:F127 PRTx. The data were fitted using SEDPHAT using a species analysis model. The molecular weight obtained from the SE experiment agrees well with the apparent molecular weights calculated from the sedimentation velocity.
Table 2.
Molecular Weight Measurements by NMR, GPC, and AUC
| CD:Pluronic PRTx | NMR (g/mol) | GPC (g/mol) | AUC (g/mol) |
|---|---|---|---|
| F127 | 30,800 | 33,300 | 30,000 |
| F68 | 25,500 | 28,500 | 27,000 |
| L35 | 11,000 | 13,200 | 12,000 |
| L64 | 17,700 | 17,100 | 21,000 |
MALDI-MS analysis gave additional confirmation of the β-CD:F127 PRTx molecular weights as well as information about the heterogeneity of the material. The spectrum contains a mass peak at ~30 kDa, representing a PRTx with a threading of 15 β-CDs as found by other measures. Other peaks in the spectrum correspond to members of the β-CD:F127 PRTx population with greater and lesser degrees of threading. Specifically, additional m/z peaks corresponding to 18, 8, 7, 6, and 1 threaded CD also appear (Figure 5). As expected, there is some heterogeneity in the amount of threading on any given PRTx. Additionally, MALDI spectra of lower molecular weight PRTx showed evidence of dimer formation. This fits well with the aggregation behavior noted in AFM analysis (see below). Full MALDI spectra for each PRTx are available in Supporting Information.
Figure 5.
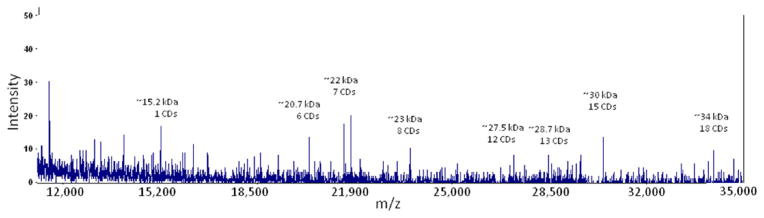
MALDI-MS spectrum of β-CD:F127 PRTx at laser power 6,500. Peak assignments were made by addition of modified polymer MW and 1135n where n is the potential number of bound CDs. Lesser peaks flanking assigned masses can be attributed to variations in MW inherent in the base polymer.
AFM analysis of the β-CD:F127 PRTx material produced evidence of PRTx aggregation after DMSO evaporation. To try and image nonaggregated particles, PRTx samples were probe sonicated before fixation onto mica plates. Sonication did not prevent aggregation, but did decrease the diameter and height of imaged particles. It is hypothesized that β-CD:Pluronic PRTx are forming hollow micelles in solution as previously reported in a related β-CD:Pluronic PRTx system.41 Consistent with this expectation, we found that higher PRTx concentrations produced larger aggregates. Samples at a concentration of 10−5 mg/mL produced an average diameter of 45 nm after probe sonication. Samples at 10−7 mg/mL averaged 41 nm in diameter, while concentrations of 10−9 mg/mL gave particles with an average diameter of 32 nm (Figure 6A–C). Thus, higher β-CD:F127 PRTx concentrations promote aggregation and micellization into particles whose diameter depends on the PRTx concentration in solution.
Figure 6.
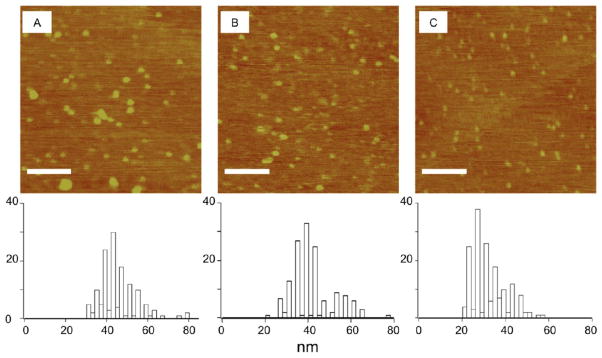
AFM images of β-CD:F127 PRTx at 10−5 mg/mL (A), 10−7 mg/mL (B) and 10−9 mg/mL (C) with probe sonication. Scale bar represents 500 nm. Size distributions are shown below images.
DOSY measurements were also able to confirm rotaxanation and provide further insight into how the β-CD:Pluronic PRTx constructs behave in solution. The diffusion constants (D) for β-CD:L35, β-CD:L64, and β-CD:F68 PRTx were 5.84 × 10−11 m2/s, 5.30 × 10−11 m2/s, and 3.38 × 10−11 m2/s, respectively. These diffusion constants scale with molecular weight and are in reasonably good agreement with the diffusion behavior of similar systems.42,43 Additionally, the least-squares fit of a log(D) vs log(MW) plot gave a scaling constant of −0.63 (plots, spectra, and diffusion constants available in Supporting Information S.10). Scaling constants < −0.6 are indicative of semiflexible wormlike systems,44 showing that the PRTx system has more rigidity in DMSO solution than would a well-solvated unbranched random coil polymer.45
3.2. Dethreading Behavior
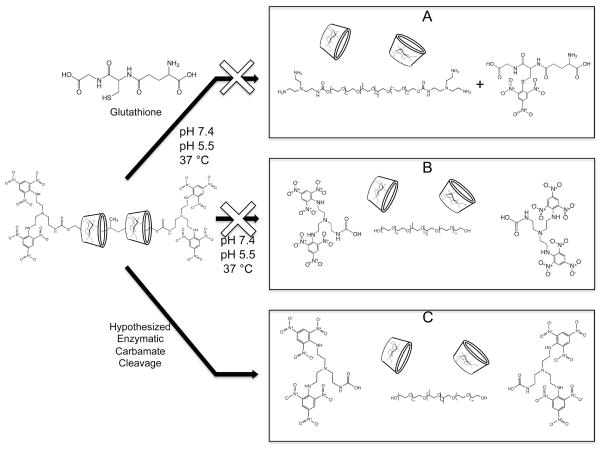
Experiments were performed to characterize the dethreading behavior of the β-CD:Pluronic PRTx under acidic and reducing conditions in an attempt to mimic the LE/LY environment (Figure 7). Experiments in which β-CD:Pluronic PRTx were suspended in solutions at either pH 7.4 or 5.5 and then sampled at various time intervals showed no increase in free β-CD levels by RPLC analysis up to 24 h after dissolution. This was in agreement with the design of the PRTx, which were intended to be stable under acidic conditions.
Figure 7.
Hypothesized and experimentally tested mechanisms for PRTx dethreading. (A) Glutathione incubation with PRTx at acidic and neutral pH and (B) acid catalyzed dethreading at 37 °C showed no signs of dethreading. Hypothesized dethreading (C) by enzymatic cleavage of carbamate linkages yields free β-CD in vitro.
Additionally, 1H NMR and UV/vis studies were conducted to monitor changes in end-cap status upon exposure to acidic pH and 10 mM glutathione. 1H NMR analysis of a short bis-amine PEG-NH-TNB (SI Section S.8) analogue showed no changes in the characteristic TNB peaks even after 48 h incubation with glutathione at 37 °C, indicating that the TNB nitro groups had not been reduced. Similarly, the UV/vis spectra of the F68-TNB end-caps remained unchanged even after 48 h of incubation in glutathione at both acidic and neutral pH. We infer from these findings that dethreading of β-CD:Pluronic PRTx is not triggered by acid-mediated end-cap cleavage or glutathione reduction of the TNB end-cap.
Finally, the possibility that β-CD:Pluronic PRTx were dethreaded upon solubilization in DMSO and addition to the cell culture media was investigated. 1H NMR analysis of PRTx samples showed that neither incubation with DMSO/MEM nor DMSO/PBS buffer resulted in any changes in the threaded extent of the samples when compared to PRTx controls (Supporting Information, Figure S.9). β-CD:Pluronic PRTx should, therefore, be stable in culture before cellular encounter and internalization.
3.3. Cell Studies
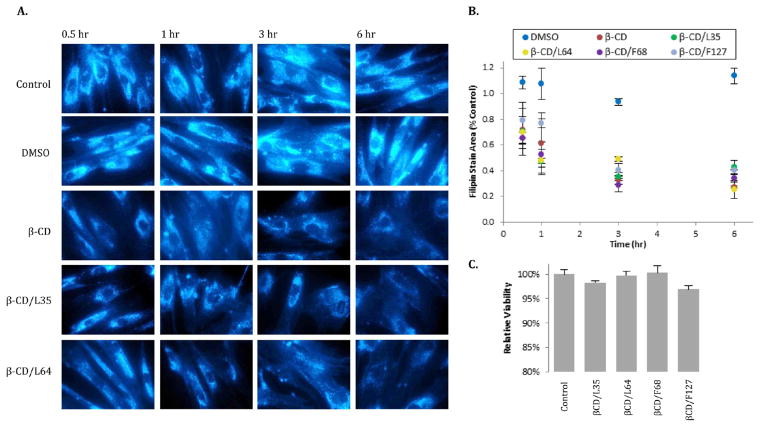
Npc2−/− fibroblasts were incubated with each β-CD:Pluronic PRTx derivative, and reductions in cellular cholesterol accumulation were monitored over time to evaluate their impact on a cellular model of NPC disease. Initially, it was hypothesized that this library of PRTx constructs, which was not designed to be either acid sensitive or reductively labile, would stimulate little or no reduction in npc2−/− cholesterol accumulation. Figure 8A and B, however, shows that a single dose of any β-CD:Pluronic PRTx species, yielding 25 μM β-CD, results in a reduction of approximately 60–75% in accumulated cholesterol levels by 6 h, relative to untreated controls. Results were similar to monomeric β-CD, which decreased cholesterol levels to ~30% of the level of untreated cells over the same time period.
Figure 8.
Filipin accumulation in NPC2-defficient fibroblasts treated with β-CD:Pluronic PRTx. Human NPC2 deficient fibroblasts were incubated with 25 μM monomeric β-CD or PRTx yielding 25 μM β-CD and were fixed and stained with 0.05 mg/mL filipin at varying time points. (A) Representative images reveal stimulation of cholesterol efflux from npc2−/− fibroblasts by β-CD and two PRTx compounds over time. (B) Filipin accumulation was determined as the ratio of filipin stain area to total cell area. Results are expressed relative to control untreated cells, and are represented as mean ± SE (n = 3). (C) Viability of npc2−/− fibroblasts was determined by Trypan Blue staining 6 h after administration of each β-CD:Pluronic PRTx. Results are expressed relative to control untreated cells.
It is worth noting that the levels of β-CD monomers used in the present studies are within the range of effective concentrations shown by Peake and Vance to clear cholesterol from murine npc1−/− neurons and glial cells,46 as well as by Storch and co-workers, to clear cholesterol from human npc2−/− fibroblasts.12 Levels that were demonstrated to cause neuronal cell toxicity, e.g., 10 mM, were not used in the present studies.46 In addition, although the fibroblasts were exposed to potentially cytotoxic TNB upon end-cap release, no alterations in cell density or viability were observed in any of the cell samples (Figure 8C).
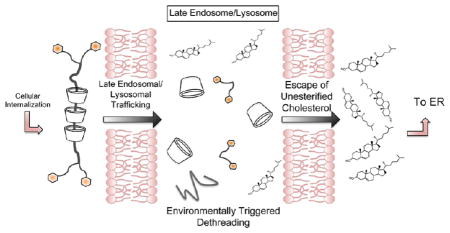
While each of the compounds induces rapid efflux of cholesterol in a manner similar to monomeric β-CD, differences in the efficacy of the PRTx species are apparent. For example, L35, carrying 5 β-CDs, stimulates the lowest degree of cholesterol efflux from npc2−/− cells while L64, with 12 β-CD monomers, is the most effective (p < 0.05). These differences in relative cholesterol clearance by the PRTx species suggest that end-cap cleavage and dethreading of CD is occurring within the LE/LY, following bulk phase endocytosis of the intact compounds (Figure 8B). Furthermore, if dethreading were occurring in the media prior to cellular uptake, approximately equal degrees of cholesterol clearance would instead be expected for all the β-CD:Pluronic PRTx compounds, since each treatment would yield 25 μM monomeric β-CD.
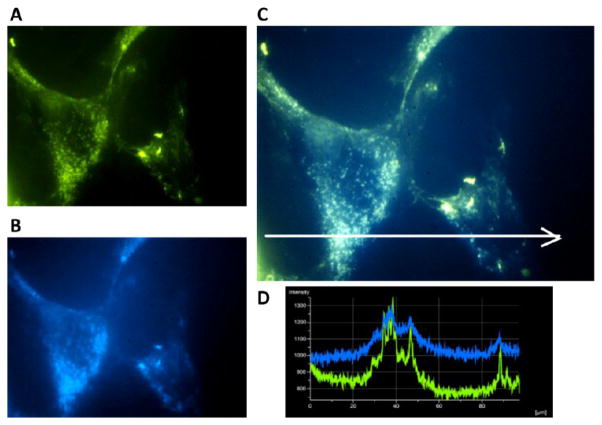
In order to determine whether the β-CD:Pluronic PRTx compounds were in fact entering the LE/LY compartment, a fluorescein labeled β-CD:F127 PRTx was synthesized. Thirty minutes after addition of the compound to npc2−/− fibroblasts, fluorescein was observed to overlap entirely with Lysotracker Blue staining, indicating localization of the compound to the LE/LY (Figure 9). Therefore, it is likely that the carbamate linkages used to attach the PRTx end-caps are being cleaved by enzymatic activation within the LE/LY. Hydrolysis of carbamate linkages has been previously reported and, indeed, has been used as a method for designing prodrugs.47–49 Thus, carbamate cleavage would allow for dethreading of β-CD monomers at high concentration within the LE/LY and subsequent efflux of cholesterol from this compartment.
Figure 9.
Fluorescein-labeled β-CD/F127 PRTx localizes to the LE/LY in npc2−/− fibroblasts. (A) Representative image of fluorescein localization in NPC2 deficient fibroblasts following a 1 h incubation period with labeled β-CD:F127 PRTx. (B) Lysotracker Blue staining identifies LE/LY compartments. (C) Fluorescein fluorescence overlays with Lysotracker Blue, indicating presence of labeled β-CD:F127 PRTx in the LE/LY compartment. (D) Intensity plot for each stain throughout an image section as indicated by the white arrow in panel C. Overlap in the localization of peak intensities is shown.
4. CONCLUSIONS
We have synthesized a family of β-CD:Pluronic PRTx compounds as potential therapeutics for treating Niemann-Pick Type C disease. The threading efficiency, molecular weight, and free β-CD contamination in these analogues have been characterized. Each of the compounds has shown the npc2−/− capacity to trigger the efflux of cholesterol from fibroblasts in vitro via filipin staining studies. The rate and efficacy of β-CD:Pluronic PRTx mediated cholesterol removal has been shown to be comparable to monomeric β-CD treatment alone. On the basis of these findings, β-CD:Pluronic polyrotaxanes warrant further investigation with regard to their safety, pharmacokinetics, biodistribution, and durability of response toward cholesterol efflux and/or synthesis suppression in an in vivo model of Niemann-Pick C disease.
Supplementary Material
Figure 1.
1H NMR spectra of free β-CD and PRTx-F127 product in DMSO-d6. Peak broadening shows inclusions of polymer inside β-CD core.
Scheme 1. Synthesis of β-CD/Pluronic PRTxa.
aFor Pluronic base F127, m = 100 and n = 32.
Acknowledgments
Funding
National Niemann-Pick Disease Foundation-Breakthrough Fund, Ara Parseghian Medical Research Foundation, American Heart Association. 1H NMR and MS were acquired in the Purdue Interdepartmental NMR Facility and the Campus Wide Mass Spectrometry Center, respectively, both of which is supported by NCI CCSG CA23168 to the Purdue University Center for Cancer Research.
We would like to thank Peter Pentchev for many helpful discussions.
ABBREVIATIONS USED
- NPC
Niemann-Pick Type C
- CD
cyclodextrin
- β-CD
β-cyclodextrin
- PRTx
polyrotaxane
- UC
unesterified cholesterol
- PEG
poly(ethylene glycol)
- LE/LY
late endosome/lysosome
- PPG
poly(propylene glycol)
- NMR
nuclear magnetic resonance
- NOESY
2D nuclear Overhouser effect spectroscopy
- GPC
gel permeation chromatography
- AUC
analytical ultracentrifugation
- AFM
atomic force microscopy
- RPLC
reverse phase high-pressure liquid chromatography
- HILIC
hydrophilic interaction liquid chromatography
- DOSY
diffusion-ordered spectroscopy
- CDI
(1,1)-carbonyldiimdazole
- TNBS
2,4,6-trinitrobenzenesulfonic acid
- TNB
2,4,6-trinitrobenzene
- TREN
tris(2-aminoethyl)amine
- DCM
dichloromethane
- ILM
ionic liquid matrix
- MALDI
matrix assisted laser desorption/ionization mass spectrometry
- THAP
2′,4′,6′-trihydroxyacetophenone monohydrate
- TMG
1,1,3,3-tetramethylguanidine
- ACN
acetonitrile
- DCM
dichloromethane
Footnotes
The authors declare no competing financial interest.
1H NMR spectra, 2D NOESY spectra, GPC chromatograms, AFM images, MALDI mass spectrometry data, DOSY data, and filipin staining images are reported for each compound. The complete syntheses and 1H NMR spectra for the unthreaded F68-TNB polymer and short PEG-TNB compound are also provided. Additionally, procedures and spectra for DMSO and media stability tests are given. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Carstea ED, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 2.Naureckiene S, et al. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- 3.Camargo F, Erickson RP, Garver WS, Hossain GS, Carbone PN, Heidenreich RA, Blanchard J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001;70:131–142. doi: 10.1016/s0024-3205(01)01384-4. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez C, Liu B, Aqui A, Taylor A, Repa J, Turley SD, Dietschy JM. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J Lipid Res. 2011;54:688–698. doi: 10.1194/jlr.M013789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie C, Turley SD, Pentchev PG, Dietschy JM. Cholesterol balance and metabolism in mice with loss of function of Niemann-Pick C protein. Am J Physiol. 1999;276:E336–E344. doi: 10.1152/ajpendo.1999.276.2.E336. [DOI] [PubMed] [Google Scholar]
- 6.Storch J, Xu Z. Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim Biophys Acta. 2009;1791:671–678. doi: 10.1016/j.bbalip.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aqul A, Liu B, Ramirez C, Pieper AA, Estill SJ, Burns DK, Liu B, Repa JJ, Turley SD, Dietschy JM. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J Neurosci. 2011;31:9404–9413. doi: 10.1523/JNEUROSCI.1317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc−/− mouse. Proc Natl Acad Sci U S A. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez C, Liu B, Taylor AM, Repa JJ, Burns DK, Weinberg AG, Turley SD, Dietschy JM. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr Res. 2010;68:309–315. doi: 10.1203/PDR.0b013e3181ee4dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Xie C, Richardson JA, Turley SD, Dietschy JM. Receptor-mediated and bulk-phase endocytosis cause macrophage and cholesterol accumulation in Niemann-Pick C disease. J Lipid Res. 2007;48:1710–1723. doi: 10.1194/jlr.M700125-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Liu B, Ramirez C, Miller AM, Repa JJ, Turley SD, Dietschy JM. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res. 2010;51:933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCauliff LA, Xu Z, Storch J. Sterol transfer between cyclodextrin and membranes: similar but not identical mechanism to NPC2-mediated cholesterol transfer. Biochemistry. 2011;50:7341–7349. doi: 10.1021/bi200574f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum AI, Zhang G, Warren DJ, Maxfield FR. Endocytosis of beta-cyclodextrin is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc Natl Acad Sci U S A. 2010;107:5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Ni X, Leong KW. Injectable drug-delivery systems based on supramolecular hydrogels formed by poly(ethylene oxide)s and α-cyclodextrin. J Biomed Mater Res A. 2003;65:196–202. doi: 10.1002/jbm.a.10444. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Li X, Ni X, Wang X, Li H, Leong KW. Self-assembled supramolecular hydrogels formed by biodegradable PEO-PHB-PEO triblock copolymers and α-cyclodextrin for controlled drug delivery. Biomaterials. 2006;27:4132–4140. doi: 10.1016/j.biomaterials.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Yui N, Ooya T, Kumeno T. Effect of biodegradable polyrotaxanes on platelet activation. Bioconjugate Chem. 1998;9:118–125. doi: 10.1021/bc970189c. [DOI] [PubMed] [Google Scholar]
- 18.Ooya T, Eguchi M, Yui N. Supramolecular design for multivalent interaction: maltose mobility along polyrotaxane enhanced binding to concanavalin A. J Am Chem Soc. 2003;125:13016–13017. doi: 10.1021/ja034583z. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wang H, Chen Y, Ke CF, Liu M. Supramolecular aggregates constructed from gold nanoparticles and l-try-CD polypseudorotaxanes as captors for fullerenes. J Am Chem Soc. 2005;127:657–666. doi: 10.1021/ja046294w. [DOI] [PubMed] [Google Scholar]
- 20.Loethen S, Ooya T, Choi HS, Yui N, Thompson DH. Synthesis, characterization and pH-triggered dethreading of α-cyclodextrin-poly(ethylene glycol) polyrotaxanes bearing cleavable endcaps. Biomacromolecules. 2006;7:2501–2506. doi: 10.1021/bm0602076. [DOI] [PubMed] [Google Scholar]
- 21.Ooya T, Mori H, Terano M, Yui N. Synthesis of a biodegradable polymeric supramolecular assembly for drug delivery. Macromol Rapid Commun. 1995;16:259–263. [Google Scholar]
- 22.Ooya T, Yui N. Synthesis and characterization of biodegradable polyrotaxanes as a novel supramolecular structured drug carrier. J Biomater Sci, Polym Ed. 1997;8:437–455. doi: 10.1163/156856297x00371. [DOI] [PubMed] [Google Scholar]
- 23.Harada A, Nishiyama T, Kawaguchi Y, Kamachi M. Preparation and characterization of inclusion complexes of aliphatic polyesters with cyclodextrins. Macromolecules. 1997;30:7115–7118. [Google Scholar]
- 24.Ogata N, Sanui K, Wada J. Novel synthesis of inclusion polyamides. J Polym Sci, Polym Lett Ed. 1976;14:459–462. [Google Scholar]
- 25.Harada A, Kamachi M. Complex formation between cyclodextrin and poly(propylene glycol) J Chem Soc, Chem Commun. 1990;19:1322–1323. [Google Scholar]
- 26.Harada A, Li J, Kamachi M. The molecular necklace: A rotaxane containing many threaded α-cyclodextrins. Nature. 1992;356:325–327. [Google Scholar]
- 27.Harada A, Li J, Kamachi M. Double-stranded inclusion complexes of cyclodextrin threaded onto poly(ethylene glycol) Nature. 1994;370:126–128. [Google Scholar]
- 28.Harada A, Okada M, Li J, Kamachi M. Preparations and characterization of inclusion complexes of poly-(propylene glycol) with cyclodextrins. Macromolecules. 1995;28:8406–8411. [Google Scholar]
- 29.Panova IG, Gerasimov VI, Grokhovskaya TE, Topchieva IN. New nanostructures based on block-copolymers, inclusion compounds of proxanols with cyclodextrins. Dokl Akad Nauk. 1996;347:61–65. [Google Scholar]
- 30.Kabanov AV, Lemieux P, Viogradov S, Alakhov V. Pluronic block copolymers: novel functional molecules for gene therapy. Adv Drug Delivery Rev. 2002;54:223–233. doi: 10.1016/s0169-409x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- 31.Hemstrom P, Irgum K. Hydrophilic interaction chromatography. J Sep Sci. 2006;29:1784–1821. doi: 10.1002/jssc.200600199. [DOI] [PubMed] [Google Scholar]
- 32.Agueros M, et al. Simultaneous quantification of different cyclodextrins and Gantrez by HPLC with evaporative light scattering detection. J Pharm Biomed Anal. 2005;39:495–502. doi: 10.1016/j.jpba.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 33.Beirne J, Truchan H, Rao L. Development and qualification of a size exclusion chromatography coupled with multiangle light scattering method for molecular weight determination of unfractionated heparin. Anal Bioanal Chem. 2011;399:717–715. doi: 10.1007/s00216-010-4187-5. [DOI] [PubMed] [Google Scholar]
- 34.Vistica J, Dam J, Balbo A, Yikilmaz E, Mariuzza RA, Rouault TA, Schuck P. Sedimentation equilibrium analysis of protein interactions with global implicit mass conservation constraints and systematic noise decomposition. Anal Biochem. 2004;326:234–256. doi: 10.1016/j.ab.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laue TM, Shah BD, Ridgeway TM, Pelletier SL. In: Analytical Ultracentrifugation in Biochemistry and Polymer Science. Harding S, Rowe A, editors. Royal Society of Chemistry; 1992. pp. 90–125. [Google Scholar]
- 37.Michels J, O’Connell MJ, Taylor PN, Wilson JS, Cacialli F, Anderson HL. Synthesis of conjugated polyrotaxanes. Chem—Eur J. 2003;9:6167–6176. doi: 10.1002/chem.200305245. [DOI] [PubMed] [Google Scholar]
- 38.Tziatzios C, Precup AA, Weidl CH, Schubert US, Schuck P, Durchschlag H, Machtle W, Van den Broek JA, Schubert D. Studies on the partial specific volume of a poly(ethylene glycol) derivative in different solvent systems. Prog Colloid Polym Sci. 2002;119:24–30. [Google Scholar]
- 39.Tanner JE. Use of the Stimulated Echo in NMR Diffusion Studies. J Chem Phys. 1970;52:2523–2526. [Google Scholar]
- 40.Zhao T, Beckham HW. Direct synthesis of cyclodextrin-rotaxanated poly(ethylene glycol)s and their self-diffusion behavior in dilute solution. Macromolecules. 2003;36:9859–9865. [Google Scholar]
- 41.Qin J, Meng X, Li B, Wei H, Xiaoqu Y, Zhang S. Self-assembly of β-cyclodextrin and Pluronic into hollow nanospheres in aqueous solution. J Colloid Interface Sci. 2010;350:447–452. doi: 10.1016/j.jcis.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Teuchert C, Michel C, Hausen F, Park D, Beckham HW, Wenz G. Cylindrical polymer brushes by atom transfer radical polymerization from cyclodextrin-PEG polyrotaxanes: Synthesis and Mechanical Stability. Macromolecules. 2013;46:2–7. [Google Scholar]
- 43.Zhao T, Beckham HW. direct synthesis of cyclodextrin-rotaxanated poly(ethylene glycol)s and their self-diffusion behavior in dilute solution. Macromolecules. 2003;36:9859–9865. [Google Scholar]
- 44.Yin Y, Zhao C, Kuroki S, Ando I. Diffusion of rodlike polypeptides with different main-chain lengths in the thermotropic liquid crystalline state as studied by the field-gradent 1H NMR method. Macromolecules. 2002;35:2335–2338. [Google Scholar]
- 45.Callaghan PT, Pinder DN. Self-diffusion of random-coil polystyrene determined by pulsed field gradient nuclear magnetic resonance: dependence on concentration and molar mass. Macromolecules. 1981;14:1334–1340. [Google Scholar]
- 46.Peake KB, Vance JE. Normalization of Cholesterol Homeostasis by 2-Hydroxypropyl-β-cyclodextrin in Neurons and Glia from Niemann-Pick C1 (NPC1)-deficient Mice. J Bio Chem. 2012;12:9290–9298. doi: 10.1074/jbc.M111.326405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe AL, Duncan KK, Parelkar NK, Weir SJ, Vielhauer GA, Boger DL. A novel, unusually efficacious duocarmycin carbamate prodrug that releases no residual byproduct. J Med Chem. 2012;55:5878–5886. doi: 10.1021/jm300330b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolkenberg SE, Boger DL. Mechanisms of in situ activation for DNA targeting antitumor agents. Chem Rev. 2002;102:2477–2495. doi: 10.1021/cr010046q. [DOI] [PubMed] [Google Scholar]
- 49.Boger DL, Boyce CW, Garbaccio RM, Searcey M, Jin Q. CBI prodrug analogs of CC-1065 and the duocarmycins. Synthesis. 1999:1505–1509. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.