Abstract
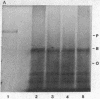
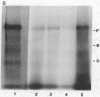
Purified oat phytochrome was labeled with 125I without altering the photoreversibility or absorbance properties of the pigment. The radiolabeled phytochrome was used in experiments in vitro to quantitate the binding of the pigment to both crude and purified membrane preparations from oat tissue. After the membranes were allowed to react with 125I-labeled phytochrome, washed free of unbound material, and pelleted, they were found to have significant levels of radioactivity bound to them. Qualitative identification of phytochrome as the bound radioactive species was confirmed by autoradiography of sodium dodecyl sulfate gels after electrophoresis of the proteins contained in the washed membranes. Data supporting the specificity of the binding are that the binding shows saturation kinetics and that unlabeled phytochrome, but not bovine serum albumin, will competitively inhibit the binding of labeled phytochrome. This technique permits the detection of less than a nanogram of phytochrome and provides a new method for quantifying bound phytochrome that is independent of the spectral detectability of the pigment. It should be useful in elucidating the nature of phytochrome attachment to cellular membranes.
Keywords: purified mitochondria, membrane receptors
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Boisard J., Marmé D., Briggs W. R. In Vivo Properties of Membrane-bound Phytochrome. Plant Physiol. 1974 Sep;54(3):272–276. doi: 10.1104/pp.54.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Christensen E. L., Bonner W. D., Jr Preparation of intaintact plant mitochondria. Biochim Biophys Acta. 1972 Aug 17;275(2):148–160. doi: 10.1016/0005-2728(72)90035-7. [DOI] [PubMed] [Google Scholar]
- Douce R., Mannella C. A., Bonner W. D., Jr The external NADH dehydrogenases of intact plant mitochondria. Biochim Biophys Acta. 1973 Jan 18;292(1):105–116. doi: 10.1016/0005-2728(73)90255-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackenzie J. M., Jr, Coleman R. A., Briggs W. R., Pratt L. H. Reversible redistribution of phytochrome within the cell upon conversion to its physiologically active form. Proc Natl Acad Sci U S A. 1975 Mar;72(3):799–803. doi: 10.1073/pnas.72.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman I. A., Briggs W. R. Phytochrome-mediated Electric Potential Changes in Oat Seedlings. Plant Physiol. 1972 Dec;50(6):687–693. doi: 10.1104/pp.50.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Marmé D. Red Light-enhanced Phytochrome Pelletability: Re-examination and Further Characterization. Plant Physiol. 1976 Nov;58(5):686–692. doi: 10.1104/pp.58.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail P. Interaction of phytochrome with other cellular components. Photochem Photobiol. 1975 Dec;22(6):299–301. doi: 10.1111/j.1751-1097.1975.tb06755.x. [DOI] [PubMed] [Google Scholar]
- Roux S. J. Chemical evidence for conformational differences between the red- and far-red-absorbing forms of oat phytochrome. Biochemistry. 1972 May 9;11(10):1930–1936. doi: 10.1021/bi00760a030. [DOI] [PubMed] [Google Scholar]
- Roux S. J., Hillman W. S. The effect of glutaraldehyde and two monoaldehydes on phytochrome. Arch Biochem Biophys. 1969 May;131(2):423–429. doi: 10.1016/0003-9861(69)90414-7. [DOI] [PubMed] [Google Scholar]
- Satter R. L., Galston A. W. Potassium flux: a common feature of albizzia leaflet movement controlled by phytochrome or endogenous rhythm. Science. 1971 Oct 29;174(4008):518–520. doi: 10.1126/science.174.4008.518. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]