Abstract
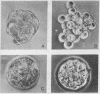
Addition of Fab fragments from rabbit antiserum to surface antigen F9 to 2-cell stage mouse embryos in culture does not alter cleavage; however, the addition prevents culture does not alter cleavage; however, the addition prevents the formation of compact morulae and blastocysts. A similar effect is observed when Fab fragments are added to already compact 8-cell stage or even older morulae, but disappears at the beginning of blastocoel formation. This effect is reversible: uncompact 30-cell embryos washed free of Fab become compact in a few hours, produce blastocysts, and upon reimplantation into pseudopregnant mothers can produce mice. Development is not altered by divalent anti-F9 antibodies, by Fab fragments from sera directed against other embryo surface antigens, or by succinyl concanavalin A.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K., Dubois P., Bennett D., Condamine H., Babinet C., Jacob F. Surface antigens common to mouse cleavage embryos and primitive teratocarcinoma cells in culture. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2988–2992. doi: 10.1073/pnas.70.10.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinet C., Kemler R., Dubois P., Jacob F. Les fragments monovalents d'immunoglobulines de lapin anti-F9 empêchent la formation de blastocystes chez la Souris. C R Acad Sci Hebd Seances Acad Sci D. 1977 May 16;284(19):1919–1922. [PubMed] [Google Scholar]
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J., Colombani M., Shreffler D. C., David C. S. A lymphocyte and platelet complement fixation microtechnique for the study of H-2 region- and I region-associated (Ia) antigens. Transplantation. 1975 Jul;20(1):84–88. doi: 10.1097/00007890-197507000-00018. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Pflumm M. N., Edelman G. M. Isolation and proteolytic cleavage of the intact subunit of concanavalin A. Biochemistry. 1972 Aug 15;11(17):3233–3239. doi: 10.1021/bi00767a016. [DOI] [PubMed] [Google Scholar]
- Ducibella T., Anderson E. Cell shape and membrane changes in the eight-cell mouse embryo: prerequisites for morphogenesis of the blastocyst. Dev Biol. 1975 Nov;47(1):45–58. doi: 10.1016/0012-1606(75)90262-6. [DOI] [PubMed] [Google Scholar]
- Granholm N. H., Brenner G. M. Effects of cytochalasin B (CB) on the morula-to-blastocyst transformation and trophoblast outgrowth in the early mouse embryo. Exp Cell Res. 1976 Aug;101(1):143–153. doi: 10.1016/0014-4827(76)90423-7. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Jacob F. Mouse teratocarcinoma and embryonic antigens. Immunol Rev. 1977 Jan;33:3–32. doi: 10.1111/j.1600-065x.1977.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Kemler R., Babinet C., Condamine H., Gachelin G., Guenet J. L., Jacob F. Embryonal carcinoma antigen and the T/t locus of the mouse. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4080–4084. doi: 10.1073/pnas.73.11.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- McLaren A., Smith R. Functional test of tight junctions in the mouse blastocyst. Nature. 1977 May 26;267(5609):351–353. doi: 10.1038/267351a0. [DOI] [PubMed] [Google Scholar]
- Mintz B. Experimental Study of the Developing Mammalian Egg: Removal of the Zona Pellucida. Science. 1962 Nov 2;138(3540):594–595. doi: 10.1126/science.138.3540.594. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]