Abstract
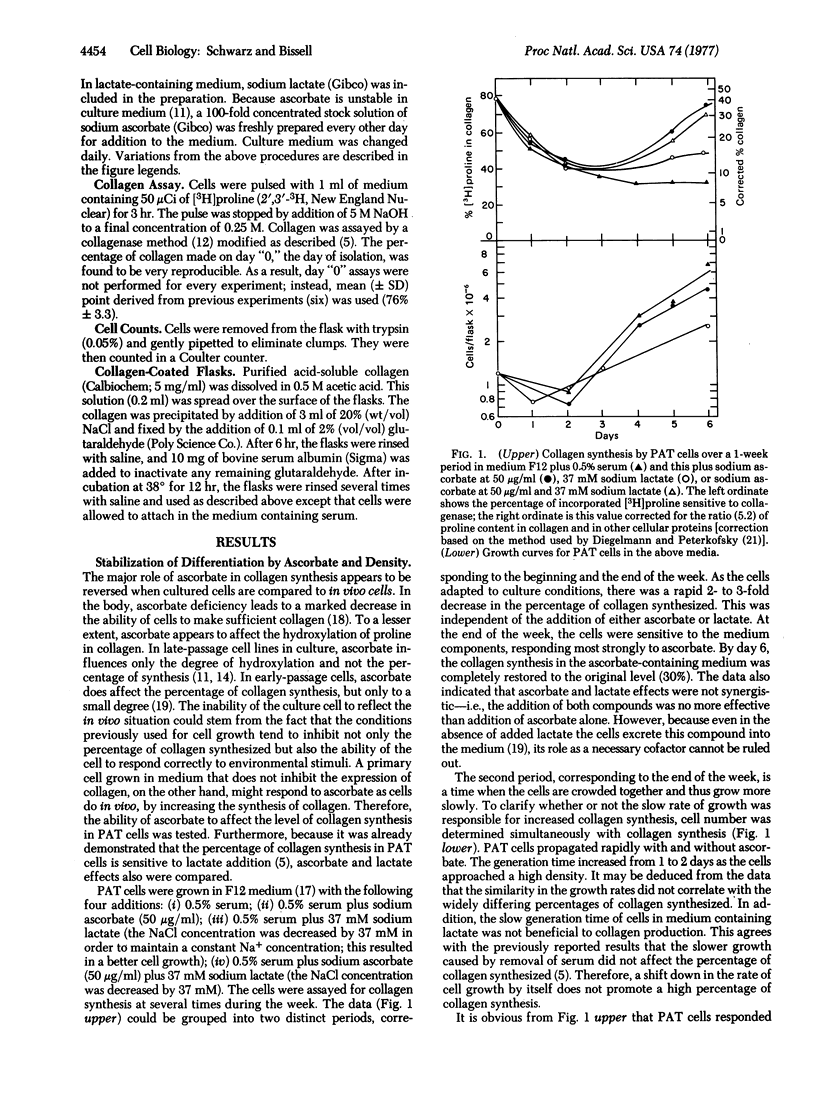
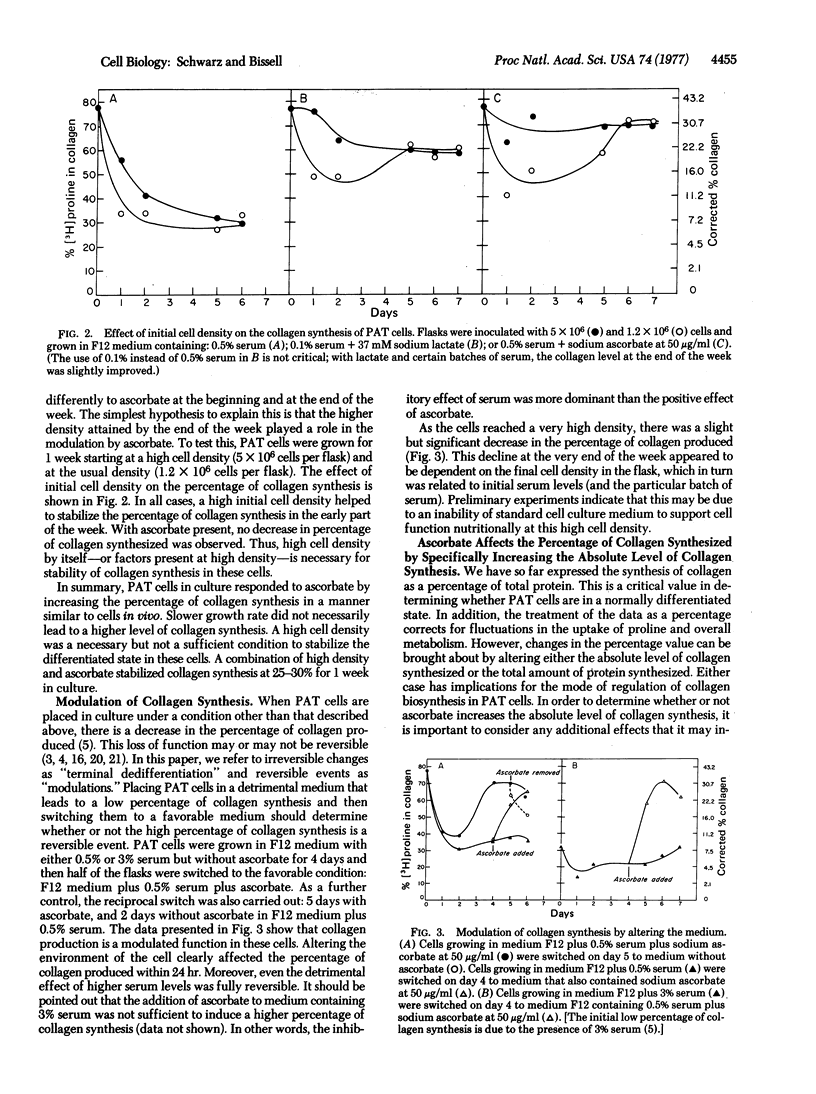
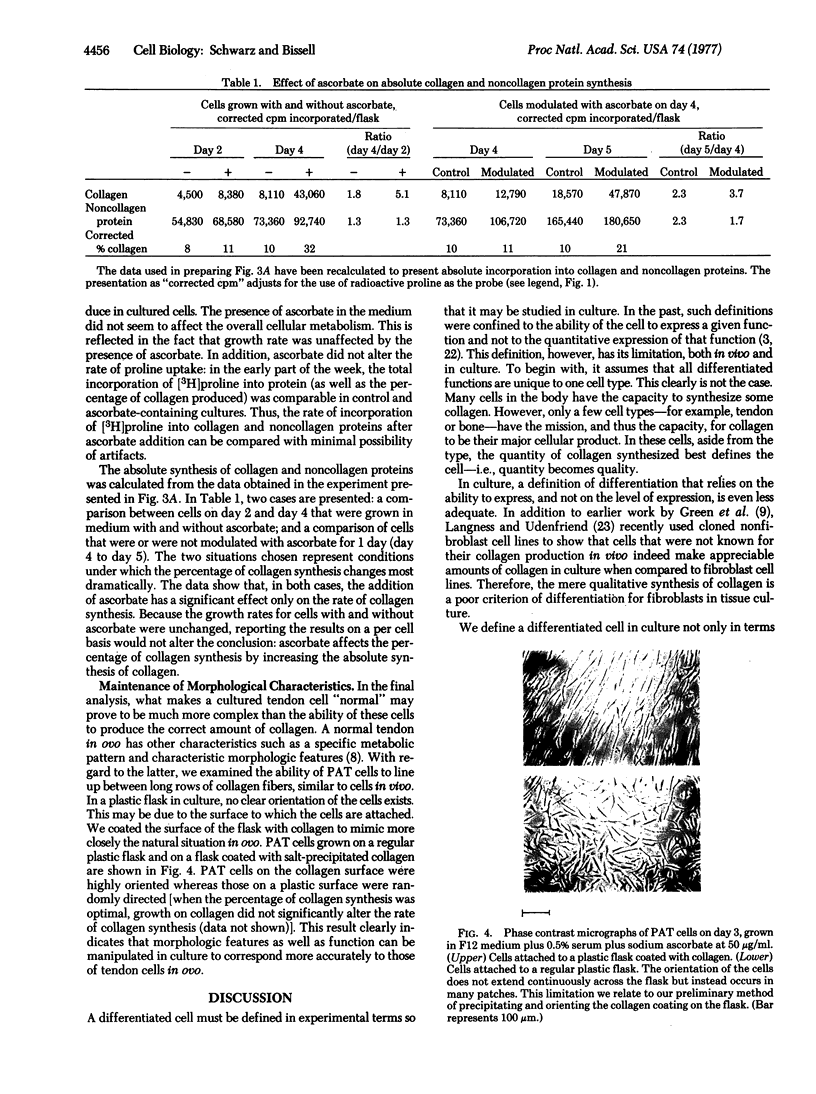
In an adequate environment, primary avian tendon cells are capable of retaining both the full expression of differentiated function and a correct morphological orientation for 1 week in culture. At high density and in the presence of ascorbate, they are fully stabilized in that they devote 25-30% of their total protein synthesis to collagen, a level comparable to that in tendon cells in ovo. However, either at low density or in medium without ascorbate, they synthesize collagen at only a third of this level. If plated on a collagen matrix, these cells will orient themselves in a manner similar to that of tendon cells in vivo. Furthermore, they are capable of fully modulating the percentage of collagen synthesis upon addition or removal of ascorbate and serum. The variation in the percentage of collagen produced is a result of alterations in collagen synthesis rather than of changes in total protein synthesis or hydroxylation of proline in collagen. Primary avian tendon cells, therefore, provide a suitable model for understanding the stability of the differentiated state, the mechanism of action of ascorbate, and the regulation of collagen biosynthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes M. J. Function of ascorbic acid in collagen metabolism. Ann N Y Acad Sci. 1975 Sep 30;258:264–277. doi: 10.1111/j.1749-6632.1975.tb29287.x. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Hatié C., Rubin H. Patterns of glucose metabolism in normal and virus-transformed chick cells in tissue culture. J Natl Cancer Inst. 1972 Aug;49(2):555–565. [PubMed] [Google Scholar]
- DAVIDSON E. H. DIFFERENTIATION IN MONOLAYER TISSUE CULTURE CELLS. Adv Genet. 1964;12:143–280. doi: 10.1016/s0065-2660(08)60416-2. [DOI] [PubMed] [Google Scholar]
- Diegelmann R. F., Peterkofsky B. Collagen biosynthesis during connective tissue development in chick embryo. Dev Biol. 1972 Jul;28(3):443–453. doi: 10.1016/0012-1606(72)90028-0. [DOI] [PubMed] [Google Scholar]
- EAGLE H. METABOLIC CONTROLS IN CULTURED MAMMALIAN CELLS. Science. 1965 Apr 2;148(3666):42–51. doi: 10.1126/science.148.3666.42. [DOI] [PubMed] [Google Scholar]
- Evans C. A., Peterkofsky B. Ascorbate-independent proline hydroxylation resulting from viral transformation of Balb 3T3 cells and unaffected by dibutyryl cAMP treatment. J Cell Physiol. 1976 Nov;89(3):355–367. doi: 10.1002/jcp.1040890302. [DOI] [PubMed] [Google Scholar]
- GREEN H., GOLDBERG B. COLLAGEN AND CELL PROTEIN SYNTHESIS BY AN ESTABLISHED MAMMALIAN FIBROBLAST LINE. Nature. 1964 Oct 24;204:347–349. doi: 10.1038/204347a0. [DOI] [PubMed] [Google Scholar]
- Green H., Goldberg B. Synthesis of collagen by mammalian cell lines of fibroblastic and nonfibroblastic origin. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1360–1365. doi: 10.1073/pnas.53.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Goldberg B., Todaro G. J. Differentiated cell types and the regulation of collagen synthesis. Nature. 1966 Nov 5;212(5062):631–633. doi: 10.1038/212631b0. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langness U., Udenfriend S. Collagen biosynthesis in nonfibroblastic cell lines. Proc Natl Acad Sci U S A. 1974 Jan;71(1):50–51. doi: 10.1073/pnas.71.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene C. I., Bates C. J. Ascorbic acid and collagen synthesis in cultured fibroblasts. Ann N Y Acad Sci. 1975 Sep 30;258:288–306. doi: 10.1111/j.1749-6632.1975.tb29289.x. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. B. Increased collagen synthesis in Kirsten sarcoma virus-transformed BALB 3T3 cells grown in the presence of dibutyryl cyclic AMP. Cell. 1974 Nov;3(3):291–299. doi: 10.1016/0092-8674(74)90144-5. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. Regulation of collagen secretion by ascorbic acid in 3T3 and chick embryo fibroblasts. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1343–1350. doi: 10.1016/0006-291x(72)90614-6. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B. The effect of ascorbic acid on collagen polypeptide synthesis and proline hydroxylation during the growth of cultured fibroblasts. Arch Biochem Biophys. 1972 Sep;152(1):318–328. doi: 10.1016/0003-9861(72)90221-4. [DOI] [PubMed] [Google Scholar]
- Schwarz R., Colarusso L., Doty P. Maintenance of differentiation in primary cultures of avian tendon cells. Exp Cell Res. 1976 Oct 1;102(1):63–71. doi: 10.1016/0014-4827(76)90299-8. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., Udenfriend S. Activation of prolyl hydroxylase in L-929 fibroblasts by ascorbic acid. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1090–1093. doi: 10.1073/pnas.70.4.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]



