Based on a positive opinion from the European Medicines Agency, a marketing authorization valid throughout the European Union (EU) was issued for axitinib (Inlyta) for the treatment of advanced renal cell carcinoma after failure of prior treatment with sunitinib or a cytokine. This paper summarizes the scientific review of the application leading to approval in the EU.
Keywords: Axitinib, Inlyta, Renal cell carcinoma, EMA, European Medicines Agency
Abstract
Axitinib is a tyrosine kinase inhibitor of vascular endothelial growth factor receptor 1 (VEGFR-1), VEGFR-2, and VEGFR-3. Based on the positive opinion from the European Medicines Agency (EMA), a marketing authorization valid throughout the European Union (EU) was issued for the treatment of advanced renal cell carcinoma (RCC) after failure of prior treatment with sunitinib or a cytokine. The demonstration of clinical benefit for axitinib was based on a phase III, randomized, open-label, multicenter study of axitinib compared with sorafenib in patients with advanced RCC after failure of a prior systemic first-line regimen containing one or more of the following agents: sunitinib, bevacizumab plus interferon-α, temsirolimus, or cytokines. In the primary analysis, a 2-month increase in median progression-free survival (PFS) was observed for axitinib compared with sorafenib (hazard ratio [HR]: 0.665; 95% confidence interval [CI]: 0.544–0.812; p < .0001). In the subgroup of patients with a prior cytokine-containing regimen, the increase in median PFS associated with axitinib was 5.4 months (updated analysis, HR: 0.519; 95% CI: 0.375–0.720; p < .0001). In the subgroup of patients with prior sunitinib treatment, the increase in median PFS was 1.4 months (updated analysis, HR: 0.736; 95% CI: 0.578–0.937; p = .0063). The analysis of overall survival showed no statistically significant survival benefit of axitinib over sorafenib in patients previously treated with cytokine-containing regimens (HR: 0.813; 95% CI: 0.556–1.191) or sunitinib (HR: 0.997; 95% CI: 0.782–1.270). The most common treatment-related adverse events associated with axitinib included diarrhea, hypertension, fatigue, nausea, decreased appetite, dysphonia, and palmar-plantar erythrodysesthesia. Most of these events were mild or moderate in severity. This paper summarizes the scientific review of the application leading to approval in the EU. The detailed scientific assessment report and product information, including the summary of product characteristics, are available on the EMA website (http://www.ema.europa.eu).
Implications for Practice:
Axitinib was approved in the European Union for the treatment of advanced renal cell carcinoma after failure of prior treatment with sunitinib or a cytokine. The approval is based on a controlled, randomized, pivotal study with 723 patients. A statistically significant improvement in progression-free survival was observed in the axitinib group compared with the sorafenib group in patients with prior sunitinib treatment (1.4 months) and with prior cytokine treatment (5.4 months). The most common side effects were diarrhea, hypertension, fatigue, dysphonia, nausea, decreased appetite, and palmar-plantar erythrodysesthesia (hand-foot) syndrome. The benefits were considered clinically relevant in patients who had failed prior treatment with sunitinib or a cytokine.
Introduction
Renal cell carcinoma (RCC) is the third leading urologic cancer. Approximately 30% of patients with RCC have metastatic disease at the time of diagnosis, and a significant proportion of patients with localized disease treated with curative nephrectomy relapse subsequently with metastatic disease. The most frequent locations of metastases are the lungs, mediastinum, bone, liver, and brain. The estimated number of deaths due to kidney cancer was about 35,000 persons in the European Union (EU; 27 member states) in 2012 [1].
Clear cell RCC is frequently associated with allelic loss on chromosome 3p and mutational inactivation of the von Hippel-Lindau (VHL) tumor suppressor gene, high levels of vascular endothelial growth factor (VEGF), and overexpression of their receptors. Consequently, the angiogenic pathway is a logical target of potential therapies for advanced RCC [2]. Until the development of agents that target tumor angiogenesis and other signaling pathways, systemic therapy with the cytokines interleukin 2 or interferon-α (IFN-α) was the main treatment for advanced RCC. Recently approved drugs for the treatment of RCC in the EU include sorafenib, sunitinib, everolimus, temsirolimus, bevacizumab in combination with IFN-α, and pazopanib.
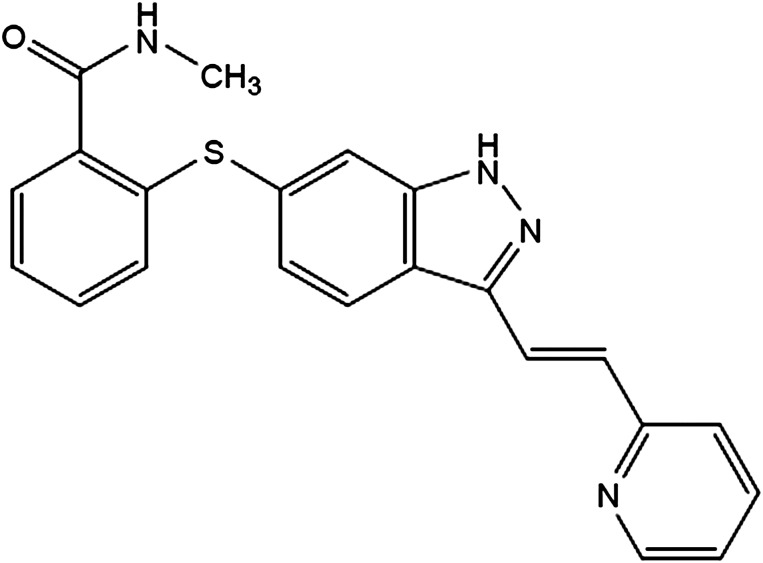
Axitinib (Inlyta; Pfizer Inc., New York, NY, http://www.pfizer.com) is an indazole derivative obtained by chemical synthesis (Fig. 1). Based on in vitro data, axitinib was a selective kinase inhibitor that appeared to be more potent on VEGF receptor (VEGFR) kinases and platelet-derived growth factor receptor kinases (including KIT) compared with other receptor tyrosine kinases and intracellular kinases. Axitinib has been shown to inhibit VEGF-mediated endothelial cell proliferation and survival. Axitinib inhibited the phosphorylation of VEGFR-2 in xenograft tumor vasculature that expressed the target in vivo and produced tumor growth delay, regression, and inhibition of metastases in many experimental models of cancer.
Figure 1.
Chemical structure of axitinib. Axitinib has the chemical name N-methyl-2-[3-((E)-2-pyridin-2-yl-vinyl)-1H-indazol-6-ylsulfanyl]-benzamide. The molecular formula is C22H18N4OS, and the molecular weight is 386.47 Da. Axitinib is a white to light yellow powder, weak base, nonhygrospic, classified as low solubility, high permeability [3].
Applicant Pfizer Ltd. submitted an application for marketing authorization for axitinib (Inlyta) to the European Medicines Agency (EMA). The review was conducted by the Committee for Medicinal Products for Human Use (CHMP), and a marketing authorization was granted in the EU on September 3, 2012, for the treatment of adult patients with advanced RCC after failure of prior treatment with sunitinib or a cytokine.
Nonclinical Aspects and Clinical Pharmacology
Major toxicity findings in mice and dogs following repeated dosing for up to 9 months were in the gastrointestinal, hematopoietic, reproductive, skeletal, and dental systems. Elevated systolic, diastolic, and mean arterial blood pressure was observed in mice and rats and possibly in dogs.
Axitinib was not mutagenic or clastogenic in conventional genotoxicity assays in vitro. Concerning reproduction and developmental toxicity, axitinib-related findings in the testes and epididymis included decreased organ weight, atrophy or degeneration, decreased numbers of germinal cells, hypospermia or abnormal sperm forms, and reduced sperm density and count. Findings in women included signs of delayed sexual maturity, reduced or absent corpora lutea, decreased uterine weights, and uterine atrophy at exposures approximately equivalent to the expected human exposure. Reduced fertility and embryonic viability were observed in female mice. Axitinib showed an increased occurrence of cleft palate malformations and skeletal variations, including delayed ossification, in fetuses and/or offspring at exposure levels in pregnant mice below the expected human exposure. In juvenile animals, reversible physeal dysplasia was observed in mice and dogs given axitinib for at least 1 month at exposure levels approximately 6-fold higher than the expected human exposure. Partially reversible dental caries were observed in mice treated for >1 month at exposure levels similar to the expected human exposure.
In humans, after oral administration, maximal plasma concentrations occurred within 4 hours (range of median time to peak concentration across studies: 2.5–4.1 hours). Mean absolute bioavailability was found to be 58% in the fasted state and 54% in the fed state. The plasma protein binding of axitinib at therapeutic concentrations was >99%. Axitinib is metabolized primarily in the liver by CYP3A4/5 and, to a lesser extent (<10%), by CYP1A2, CYP2C19, and UGT1A1.
Hepatobiliary elimination is the major route of elimination for axitinib. Approximately 20% of the administered dose is excreted renally as metabolites. In clinical studies with axitinib, the systemic exposure to axitinib was approximately 2-fold higher in subjects with moderate hepatic impairment (Child-Pugh class B) compared with subjects with normal hepatic function.
Clinical Efficacy
Pivotal study A4061032 (AXIS) was an open-label, multicenter, randomized controlled trial of axitinib compared with sorafenib in patients with advanced RCC after failure of treatment with one prior systemic therapy including sunitinib, bevacizumab plus IFN-α, temsirolimus, cytokine, or combination of these [4].
The study enrolled patients aged ≥18 years with histologically or cytologically confirmed diagnosis of RCC with a component of clear cell subtype and evidence of metastatic disease. Prior treatment must have contained one or more of the following agents: sunitinib, bevacizumab plus IFN-α, temsirolimus, or cytokine. Patients who had prior treatment of advanced RCC with more than one systemic first-line regimen, treatment with any neoadjuvant or adjuvant systemic therapy, or major surgery <4 weeks or radiation therapy <2 weeks prior to starting the study treatment were excluded from the study. Subjects were randomized at a 1:1 ratio to receive either axitinib (starting dose 5 mg b.i.d. with food) or sorafenib (starting dose of 400 mg b.i.d. without food). Treatment was administered continuously in 4-week cycles. The primary endpoint was progression-free survival (PFS) by blinded independent central review.
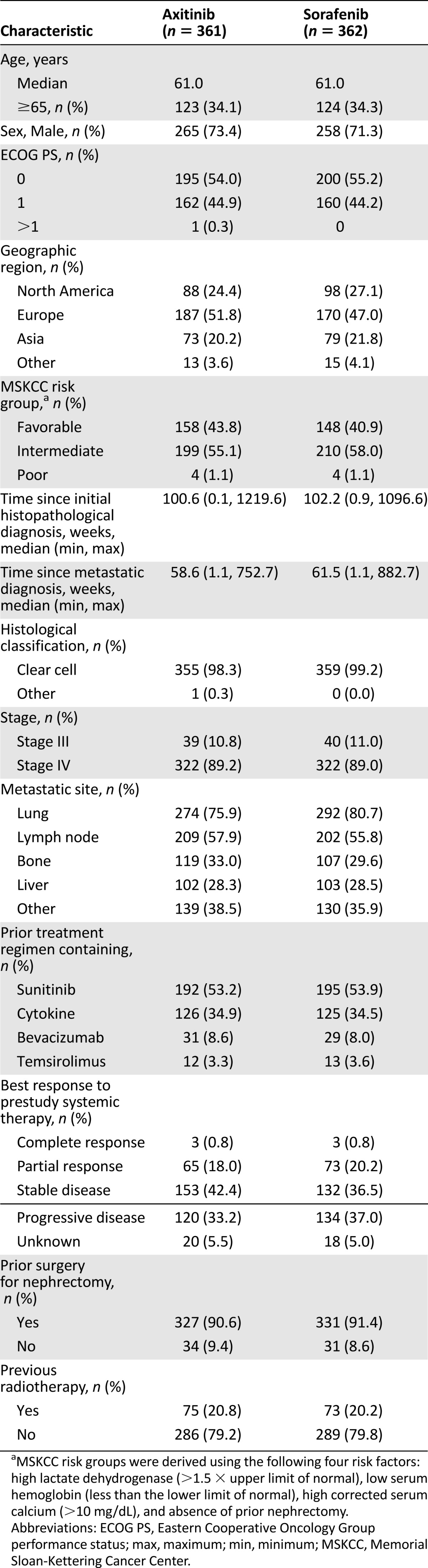
A total of 723 patients were randomized. The distribution of demographics and baseline characteristics and other important factors like previous malignancy and disease history were well balanced between groups. A predominance of men versus women and white versus other races characterized the study (Table 1).
Table 1.
Baseline demographic and disease characteristics by treatment (study A4061032)
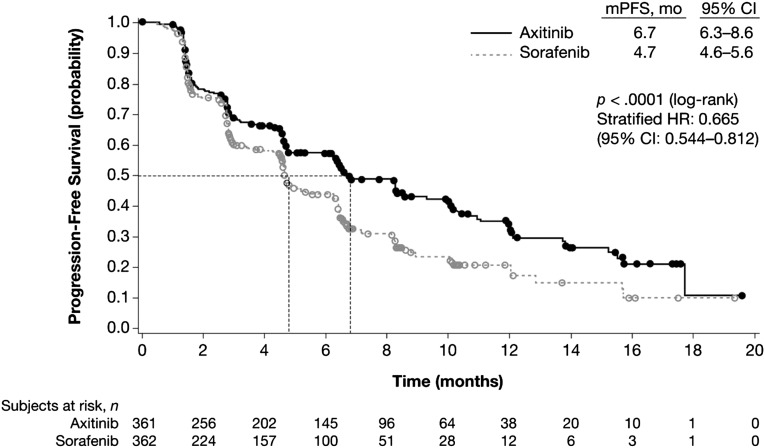
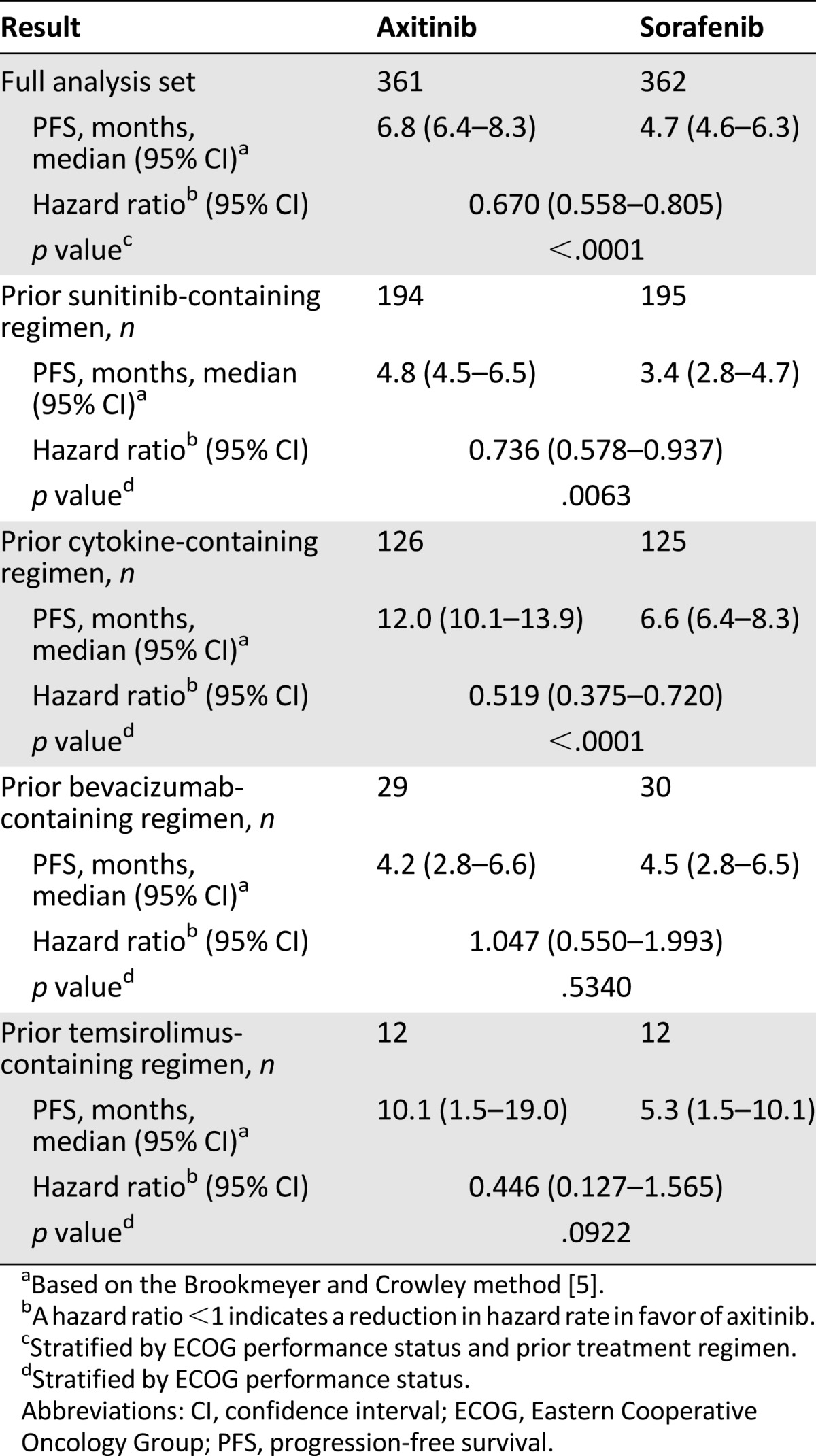
In the primary analysis (August 31, 2010), the median PFS was 6.7 months for the axitinib group and 4.7 months for the sorafenib group (hazard ratio [HR]: 0.665; 95% confidence interval [CI]: 0.544–0.812; p < .0001) (Fig. 2). The benefit in PFS was confirmed in an updated analysis (cutoff of June 3, 2011), showing median PFS of 6.8 months for the axitinib group versus 4.7 months for the sorafenib group (HR: 0.670; 95% CI: 0.558–0.805; p < .0001). In the updated analysis of PFS according to prespecified subgroups of prior treatment based on review by a blinded independent review committee (June 3, 2011), the difference in median PFS between the two groups in the prior sunitinib treated patients was 1.4 months (HR: 0.736; 95% CI: 0.578–0.937; p = .0063), whereas the difference was 5.4 months (HR: 0.519; 95% CI: 0.375–0.720; p < .0001) in the patients with prior cytokine treatment (Table 2).
Figure 2.
Study A4061032. Kaplan-Meier curves of progression-free survival by treatment, independent review committee assessment (full analysis set).
Abbreviations: CI, confidence interval; HR, hazard ratio; mPFS, median progression-free survival.
Table 2.
Summary of PFS by treatment and stratification factor, stratified analysis, independent review committee assessment (study A4061032)
In the full analysis set, median overall survival (OS) was 20.1 months versus 19.2 months for axitinib versus sorafenib, respectively (HR: 0.969; 95% CI: 0.800–1.174; p = .3744; cutoff of November 1, 2011). There was no survival benefit of axitinib over sorafenib in the prior sunitinib treatment group (HR: 0.997; 95% CI: 0.782–1.270), but a positive trend for OS was observed for axitinib over sorafenib in the prior cytokine treatment group (HR: 0.813; 95% CI: 0.555–1.191), with median OS of 29.4 months in the axitinib arm and 27.8 months in the sorafenib arm.
The analysis of objective response rate (ORR) showed a statistically significant improvement of 13.9% for axitinib compared with sorafenib in patients pretreated with cytokines. In the prior sunitinib treatment group, the difference in ORR between axitinib and sorafenib was 3.6%. The groups of patients previously treated with temsirolimus and bevacizumab plus IFN-α were very small (n = 24 and n = 59, respectively); therefore, no firm conclusions could be made regarding the efficacy in these subgroups. There were no differences between treatment groups in terms of patient-reported outcomes (Functional Assessment of Cancer Therapy-Kidney Symptom Index; EuroQol Group’s Self-Reported Health Status Measure) in the overall population.
The analysis of ORR showed a statistically significant improvement of 13.9% for axitinib compared with sorafenib in patients pretreated with cytokines. In the prior sunitinib treatment group, the difference in ORR between axitinib and sorafenib was 3.6%.
Clinical Safety
A total of 3,655 subjects (phase I–III studies) were evaluated for safety, including 2,507 (68.6%) who received at least one dose of axitinib. Updated data from 3,944 subjects treated in 42 clinical trials were also provided.
The most common adverse events reported in the axitinib group (in ≥20% subjects) were diarrhea, hypertension, fatigue, dysphonia, nausea, decreased appetite, and palmar-plantar erythrodysaesthesia (hand-foot) syndrome. Most of these events occurred with grade 1 or 2 severities (Table 3).
Table 3.
Treatment-emergent, treatment-related adverse events summarized by maximum severity grade for ≥5% (all grades; decreasing frequency) of subjects in either treatment group in study A4061032
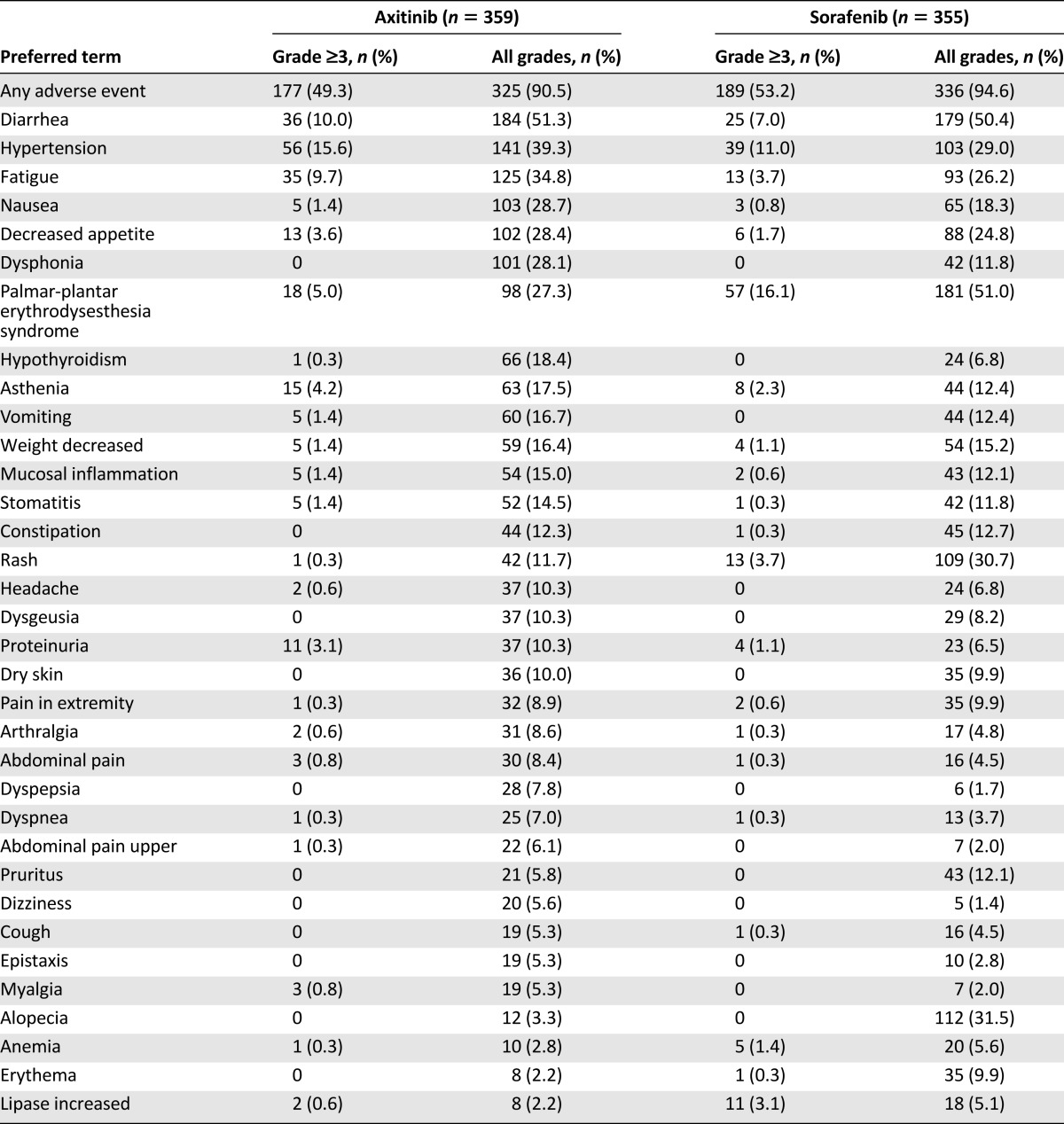
The most important serious adverse reactions reported in patients receiving axitinib were thromboembolic events, hemorrhage, gastrointestinal perforation and fistula formation, hypertensive crisis, and posterior reversible encephalopathy syndrome. In total, 36 deaths occurred in the axitinib arm versus 25 in the sorafenib arm. The majority of these events were due to progressive disease. Five events in each arm were considered treatment related. There is no indication that axitinib promotes disease progression or the development of new lesions.
Axitinib affected the incidence of hypertension and thyroid dysfunction, and sometimes aggravated these conditions if they were pre-existing. The hypertension reported during the study was largely manageable, but hypertension is still considered an unfavorable effect of axitinib.
There did not seem to be any clear signal of a clinically meaningful prolongation of the QT interval observed with axitinib; however, two patients had grade ≥3 QTc prolongation at cycle 1, day 15, and two additional patients had on-treatment increase in QTc >60 ms in the pivotal study. Consequently, to get the most optimal information about new suspected cases, applicant Pfizer included enhanced pharmacovigilance activities with use of a questionnaire to systematically collect follow-up data of individual case safety reports that can be associated with QT prolongation.
A risk management plan was submitted to address additional important potential risks (wound healing complications; congestive heart failure and cardiomyopathy; carcinogenicity; and drug interactions with CYP1A2, 2C8, and P-glycoprotein substrates) and important missing information (safety in pregnant and lactating women, in pediatric patients, in relevant malignancies, in patients with moderate and severe renal impairment, and in patients with severe hepatic impairment).
Discussion
An oncology scientific advisory group (SAG) was convened by the CHMP to provide clinical expert opinions on a number of issues. The relevance of sorafenib as a comparator had been questioned during the assessment. Sorafenib had been studied only in patients who were cytokine refractory and thus should be considered an experimental treatment in patients previously treated with a tyrosine kinase inhibitor (TKI). In theory, inclusion of a placebo arm would have been informative to assess the efficacy of both sorafenib and axitinib in these patients; however, many clinicians would have considered it unethical to use a placebo control at that point in time. The SAG agreed that, based on available knowledge at the time that the pivotal trial was designed and initiated, sorafenib must be considered as a reasonable active comparator.
The SAG acknowledged that the 1.4-month difference in PFS compared with sorafenib observed in the sunitinib group was small and clinically questionable; however, the difference was formally statistically significant, and the toxicity profile of axitinib was better than that of sorafenib. The SAG recommended that an indication in second-line metastatic RCC after failure of prior sunitinib or cytokine treatment could be supported; however, there was not adequate information on the use of axitinib after first-line bevacizumab or temsirolimus treatment.
The SAG expressed the view that although additional options in the treatment armamentarium for second-line advanced RCC are welcome, important questions such as whether TKIs or mammalian target of rapamycin (mTOR) inhibitors should be the preferred choice for second-line advanced RCC therapy have not been answered. The SAG also indicated that major shortcomings have existed in the concurrent development of multiple agents for advanced RCC, including axitinib. Biomarkers have not been identified to help define target patient populations for each compound, and comparative or combination trials have not been conducted to guide treatment choice and better define therapeutic regimens. Following the discussion at the SAG and further discussions with the CHMP, the applicant revised the claimed indication for axitinib, limiting it to treatment of advanced RCC after failure of prior treatment with sunitinib or a cytokine. The CHMP concluded by majority vote that the benefit-risk balance of axitinib was positive for patients who had received prior treatment with sunitinib or a cytokine.
The SAG expressed the view that although additional options in the treatment armamentarium for second-line advanced RCC are welcome, important questions such as whether TKIs or mTOR inhibitors should be the preferred choice for second-line advanced RCC therapy have not been answered.
The benefits and risks of axitinib have been assessed based on the AXIS trial. It is understood that in current practice, patients are more often treated with either pazopanib or sunitinib in first line and that these drugs are regarded as similar in terms of efficacy [6]. The benefits and risks of axitinib after failure of pazopanib have not been assessed. From a regulatory perspective, the approved indication is generally driven by the characteristics of the patients included in the pivotal studies. Although generalizations are sometimes possible on a case-by-case basis, in the case of axitinib, the CHMP felt it was important to reflect the actual treatment characteristics of the patients enrolled in the study. This approach is different from the more pragmatic approach taken by the U.S. Food and Drug Administration. In the U.S., the approved indication for axitinib is after failure of “one prior systemic therapy.”
The CHMP also recommended that the applicant should investigate suitable biomarkers to allow identification and selection of a more targeted population of patients who are most likely to benefit from treatment with axitinib. A minority of the CHMP members disagreed and considered that evidence for preferring TKIs instead of the approved mTOR inhibitor (everolimus) for second-line advanced RCC therapy was lacking.
Acknowledgments
The scientific assessment as summarized in this report is based on the marketing authorization application submitted by the applicant company and on important contributions from, among others, the rapporteur and corapporteur assessment teams, members of the Committee for Medicinal Products for Human Use, and additional experts. This publication is a summary of the European Public Assessment Report and the summary of product characteristics available on the European Medicines Agency (EMA) website. Healthcare professionals and interested readers are referred to the EMA website for up-to-date information on this marketing authorization (http://www.ema.europa.eu). The authors remain solely responsible for the opinions expressed in this publication.
Author Contributions
Manuscript writing: Kyriaki Tzogani, Venke Skibeli, Ingunn Westgaard, Marianne Dalhus, Hege Thoresen, Karsten Bruins Slot, Per Damkier, Kenneth Hofland, Jeanett Borregaard, Jens Ersbøll, Tomas Salmonson, Ronny Pieters, Richard Sylvester, Gerald Mickisch, Jonas Bergh, Francesco Pignatti
Disclosures
Gerald Mickisch: Roche, Novartis, Bayer, GlaxoSmithKline, Pfizer (H); Ronny Pieters: Coloplast, Hollister (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24:5601–5608. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 3. U.S. National Library of Medicine. Compound summary for CID6450551. Available at http://pubchem.ncbi.nlm.nih.gov/compound/Axitinib. Accessed July 1, 2014. [Google Scholar]
- 4.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 5. Brookmeyer R, Crowley JJ. A k-sample median test for censored data. J Am Stat Assoc 1982;77:433–440. [Google Scholar]
- 6.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]