The Institute of Medicine’s National Cancer Policy Forum convened a public workshop to examine the needs of the nearly 70,000 adolescents and young adults (AYAs) between the ages of 15 and 39 that are diagnosed with cancer each year. This article highlights potential action items to improve the care and outcomes for AYA patients with cancer.
Keywords: Adolescent, Young adult, Cancer survivorship, Psychosocial aspects, Fertility preservation, Adverse effects
Abstract
Cancer is the leading disease-related cause of death in adolescents and young adults (AYAs). This population faces many short- and long-term health and psychosocial consequences of cancer diagnosis and treatment, but many programs for cancer treatment, survivorship care, and psychosocial support do not focus on the specific needs of AYA cancer patients. Recognizing this health care disparity, the National Cancer Policy Forum of the Institute of Medicine convened a public workshop to examine the needs of AYA patients with cancer. Workshop participants identified many gaps and challenges in the care of AYA cancer patients and discussed potential strategies to address these needs. Suggestions included ways to improve access to care for AYAs, to deliver cancer care that better meets the medical and psychosocial needs of AYAs, to develop educational programs for providers who care for AYA cancer survivors, and to enhance the evidence base for AYAs with cancer by facilitating participation in research.
Implications for Practice:
Each year nearly 70,000 adolescents and young adults (AYAs) between the ages of 15 and 39 are diagnosed with cancer, but there are numerous challenges in providing optimal care for this population. The Institute of Medicine’s National Cancer Policy Forum convened a public workshop to examine the needs of this patient population. This article highlights potential action items to improve the care and outcomes for AYA patients with cancer.
Introduction
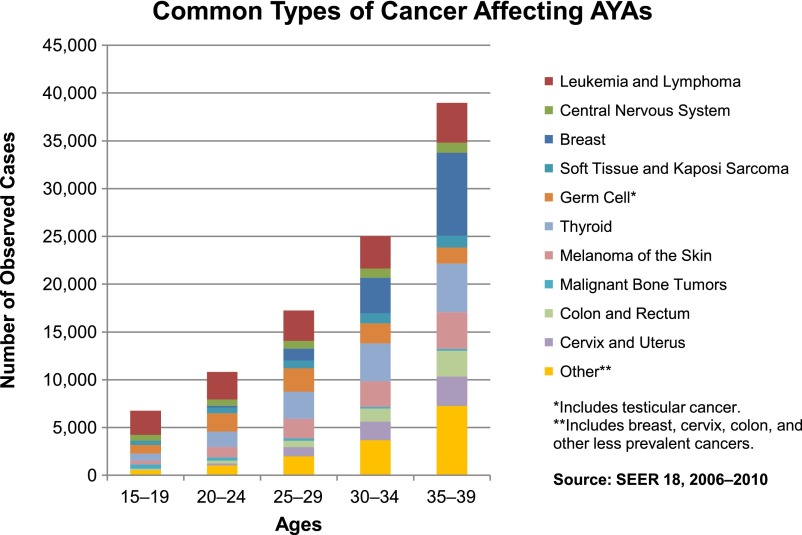
Cancer is the leading disease-related cause of death in adolescents and young adults (AYAs) [1]. Each year nearly 70,000 AYAs between the ages of 15 and 39 are diagnosed with cancer, approximately 6 times more than children under age 15. The prevalence of cancer histology varies by age (Fig. 1).
Figure 1.
The prevalence of cancer histology by age, 15–39 years.
Abbreviation: AYAs, adolescents and young adults.
In 2006, the National Cancer Institute (NCI) convened a progress review group to address the research and cancer care needs of AYAs [2]. A plan outlining strategies for implementation of these recommendations was developed in 2007 [3]. Since then, progress has been made in bringing attention to the special needs of AYAs with cancer (e.g., the growth of the LIVESTRONG Young Adult Alliance, now a separate entity known as Critical Mass: The Young Adult Cancer Alliance, and research efforts such as the NCI AYA HOPE study [4]). However, many challenges remain in providing optimal care for this population.
To facilitate discussion about gaps in care that AYA cancer patients confront and potential strategies and actions to improve the quality of their care, the National Cancer Policy Forum of the Institute of Medicine (IOM) convened a workshop titled Identifying and Addressing the Needs of Adolescents and Young Adults with Cancer in July 2013 [5]. In September 2013, the NCI also held a workshop to review data and identify research gaps in AYA oncology. The two workshops were organized to be complementary; the NCI workshop had a primary focus on epidemiology (incidence, survival, mortality) of select AYA cancers, as well as evidence related to basic biology and clinical trial enrollment, whereas the IOM workshop had a primary focus on quality of life issues, as well as late effects experienced by survivors of AYA cancers [6].
The National Cancer Policy Forum convenes representatives from government, industry, academia, and other stakeholders to discuss issues in science, medicine, public health, and policy relevant to the goals of reducing the burden of cancer. Their workshop included sessions on the unique risks and vulnerabilities of AYA patients across the lifespan, treatment and survivorship care planning for AYA patients, lifestyle management and behavioral health across the care continuum, and models of care. This article begins with a summary of the information presented at the workshop and ends with potential actions suggested by clinicians, researchers, and AYA cancer survivors participating in the workshop.
Examples of Gaps and Challenges in AYA Cancer Care
Preserving Fertility
As more people survive their cancer, more attention is being paid to the impact of certain chemotherapy, radiotherapy, or surgery treatments on future fertility [7, 8]. Thus, the emerging field of “oncofertility” research and care is focusing on preservation issues for children and AYAs with cancer and represents a signature area of needed care for AYAs [9]. The 2012 LIVESTRONG survey found that approximately one-quarter of AYAs took steps to preserve their fertility before their cancer treatment began [10]. The most common steps taken were sperm banking for men and egg or embryo preservation for women. Of those who tried to conceive a pregnancy after treatment, 65% of males and 58% of females were successful, and the majority of those pregnancies were conceived through natural means (i.e., without banked materials). The survey also assessed reasons for not using fertility preservation and found that although some people were not interested in having children, many did not know their fertility was at risk, did not have enough time or information to pursue fertility preservation options, or thought the costs of such preservation were too prohibitive. Advocacy groups and professional societies, including the American Medical Association, have stated that insurance companies should cover fertility preservation in cases in which the infertility is expected to occur as a result of cancer treatment, but not all insurance plans do so [11, 12]. LIVESTRONG Fertility helps cancer patients with the cost of fertility preservation.
Current costs for sperm banking range between $1,000 and $1,500, and annual storage costs range between $200 and $400. Another experimental option is to remove and freeze testicular tissue, although there is a possibility that cancer cells might be introduced with reimplanted tissue [13, 14]. In males receiving radiation therapy, gonadal shielding is also a common procedure to help preserve fertility.
Embryo preservation, the most common method for preserving the fertility of women undergoing cancer treatment, is expensive (currently, the national average is $12,400 per in vitro fertilization cycle, not including implantation costs and storage costs, which are approximately $300 annually) and requires sperm from a partner or donor. Also, the older a woman is at the time of the procedure, the less likely it is to be successful. It also takes a minimum of 2 weeks of preparation prior to egg retrieval because it requires ovarian hyperstimulation to create multiple follicles. Delay in initiation of chemotherapy for this procedure can be a concern to both patients and clinicians who wish to begin treatment as soon as possible. However, in breast cancer patients and other surgically treated conditions for which initial surgery may not occur for several weeks after diagnosis, there may be sufficient time to undertake egg retrieval [15].
Oocyte cryopreservation is similar to embryo preservation except that the egg is not fertilized; thus sperm from a partner or donor is not required. The American Society of Reproductive Medicine recently endorsed this approach for clinical use [16]. Although oocyte freezing is a newer procedure, the recent statistics show that in comparison with embryo freezing, the difference in success rates is minimal [17]. Another option is to remove ovarian strips, freeze them, and reimplant them later or use them for in vitro fertilization. This procedure can be done immediately and is the only option for prepubertal females. It is, however, controversial because of the concern that cancer cells, particularly in patients with hematological malignancies, might be reintroduced with the reimplanted tissue [18]. There also is not much evidence reported in the literature, with only approximately a dozen pregnancies known to have resulted from this procedure.
In female patients who receive radiation therapy, the ovaries can be protected with ovarian transposition and gonadal shielding. Various late effects of treatment, such as cardiovascular or pulmonary impairments [19], could also affect a woman’s ability to carry a pregnancy to term, so some cancer survivors may benefit from consultation with an obstetrician who specializes in high-risk pregnancies.
Guidelines from the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network recommend that care providers discuss fertility with AYA patients at the time of their cancer diagnosis and have an established referral mechanism in place [12, 20, 21]. For some patients, fertility interventions can also be considered post-treatment.
Psychosocial Aspects of AYA Cancer Diagnosis and Treatment
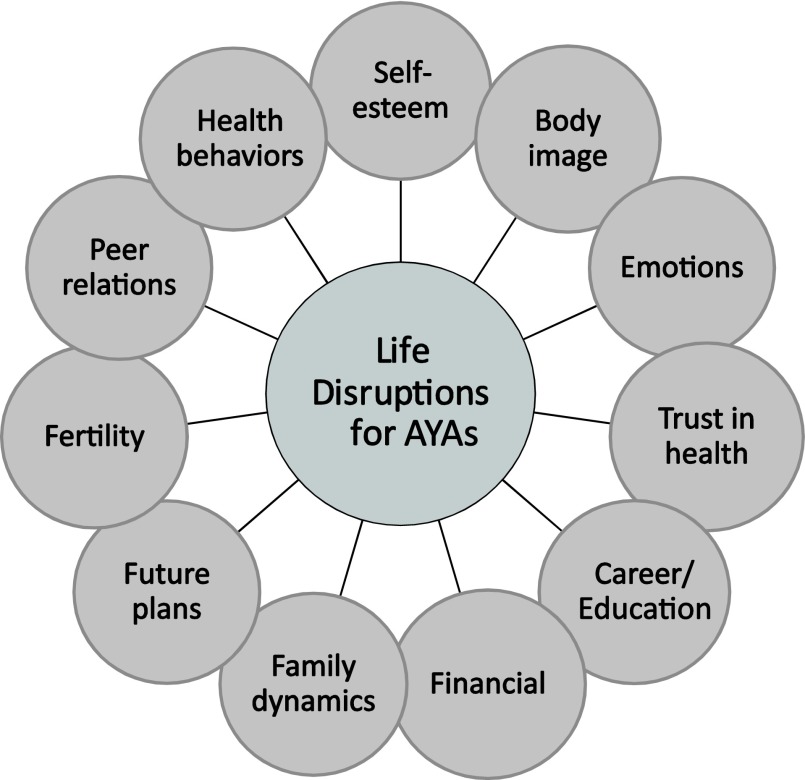
Adolescents and young adults are at a unique stage in their emotional, cognitive, and social development, which cancer often disrupts. Many younger AYAs are in the midst of taking steps to establish independence from their parents and to complete school or enter a desired career. Older AYAs are focused on ways to progress in the workforce toward a desired career goal, to find a life partner, and to raise a family. A cancer diagnosis often temporarily and sometimes permanently derails these plans (Fig. 2) [23].
Figure 2.
Possible life disruptions for AYA patients with cancer [5, 22].
Abbreviation: AYAs, adolescents and young adults.
The Adolescent and Young Adult Health Outcomes and Patient Experience (AYA HOPE) Study found that 41% of AYA cancer survivors reported an unmet need for counseling and other forms of psychosocial support 12 months after their cancer diagnoses. More than half of respondents reported six or more unmet information needs [24]. Another study found that the prevalence of psychological distress among AYAs with cancer varied over time following diagnosis (from 6% to 41%) but did not vary by the type of cancer or the prognosis [25]. Many AYA patients also report unmet practical support needs, such as assistance with health insurance, financial planning, transportation, childcare, and peer support, as well as unmet health care needs, including physical therapy and mental health services [24]. A 2010 National Health Interview Survey indicated that many patients are unaware of what psychosocial services are available [26]. Thus it is important to assess and address the unique psychosocial needs of each individual patient. Care providers can also help AYA patients and their family members determine the role of the family in the patients’ care.
Results from an online survey conducted by LIVESTRONG in 2012 found that most AYA respondents had to make some changes in their work life following a cancer diagnosis, such as taking time off from work or school, switching from full-time to part-time work, and changing to a less demanding job or to one with a more flexible work schedule. Many respondents also reported that their ability to perform mental or physical tasks and their overall productivity at work were affected by their cancer diagnosis. Caregivers for AYA patients (often parents) also frequently made work-life changes [10].
Data from the Behavioral Risk Factors and Surveillance System (BRFSS) survey have shown that, compared with age-matched peers without cancer, significantly fewer AYA cancer survivors report being employed, and significantly more AYA cancer survivors report being out of work [27]. In addition, a study based on a large national survey, the Medical Expenditure Panel Survey, evaluated employment and medical expenditures and found that the health and economic burden among adolescent and young adult cancer survivors is substantial, resulting in excess health care expenditures and lost productivity costs compared with adults without a history of cancer or to older adult cancer survivors [28]. Others respondents reported “job lock,” the inability of cancer survivors to pursue a career of choice, often because of the need to keep their current jobs to maintain employee benefits, including health insurance.
The AYA HOPE study surveyed AYAs who were in school or working when they were diagnosed with cancer and assessed how many were able to return to school or work between 15 and 35 months postdiagnosis. Most respondents felt cancer had an adverse impact on their schooling or work, but most were able to resume school or work within 35 months of diagnosis. Those who quit working completely directly after their diagnosis were the least likely to return to work [29].
Palliative Care
The recent IOM consensus study on the delivery of quality cancer care emphasized the importance of psychosocial support services and palliative care for all cancer patients as a component of initial and continuing care [30]. Given the high levels of distress in the AYA population, this need is even more critical [22]. Ideally, a palliative care specialist team consisting of a nurse, a social worker, a psychologist, a palliative care physician, and an oncologist works alongside the patient’s primary cancer care team and provides care in multiple venues, including the hospital, the clinic, and the home.
Cancer centers often offer a variety of models to meet the palliative care needs of patients including consultation services, targeting specific populations likely to benefit from palliative care and population-based education and policies. With attention to developmental stages and specific life disruptions, the palliative care needs of AYA patients should be routinely revisited and targeted to issues important to the symptom management for this population, such as increasing emotional coping skills, optimizing rehabilitation services, managing pain with attention to promotion of normal life activities, and peer support.
Studies are beginning to document the benefits of palliative care, which aims to improve the patients’ and family members’ quality of life by addressing physical, practical, emotional, and spiritual needs across the care continuum [31]. One study of older adults found that adding palliative care to standard care for metastatic lung cancer patients resulted in better quality of life, less anxiety and depression, the use of fewer hospital resources and chemotherapy, and longer life compared with standard care alone [32]. Based on this and other studies in the general population, ASCO has developed a provisional clinical opinion stating that combining standard oncology care and palliative care should be considered early in the course of illness for any patient with metastatic cancer or cancer with a high symptom burden [33].
Late and Long-Term Effects
There are three primary sources of data that can inform clinicians about the risks for long-term and late effects following AYA cancer [34]. The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort study of more than 35,000 long-term survivors of the eight most common cancer groups diagnosed prior to age 21 [35]. The CCSS has highly detailed treatment exposures and well characterized outcomes for adolescent cancer survivors diagnosed at ages 15–20 and so provides much information regarding the adolescent population. Much can also be extrapolated from CCSS reports for some cancers occurring in the young adult years, such as lymphoma, leukemia, or sarcoma. However, because the CCSS does not include survivors of adult-onset carcinomas, such as testicular, cervical, breast, or ovarian cancer, other sources of information are needed. Large national databases, such as Surveillance, Epidemiology, and End Results (SEER) [36] or BRFSS [26, 37], can be queried for outcomes of AYA survivors but have limited treatment information. Lastly, several studies have focused on specific cancer groups, such as Hodgkin lymphoma [38] and testicular cancer [39]. Recognizing the limitations of these sources and the heterogeneity of cancer groups, there are a few key points that should be understood regarding AYA cancer survivors.
First, long-term mortality, apart from mortality associated with the primary cancer, is elevated for many cancer survivors. The excess risk, largely related to second primary cancers (SPC) and cardiovascular disease (CVD), appears elevated for survivors of lymphoma, leukemia, testicular cancer, breast cancer, soft tissue and bone sarcoma, and brain tumors [36, 40–45]. Most second cancers are solid tumors arising in the radiation field [45–49]. Multiple cancer therapies are associated with a future CVD risk, including mediastinal irradiation, total body radiation, and anthracycline chemotherapy [45, 50, 51].
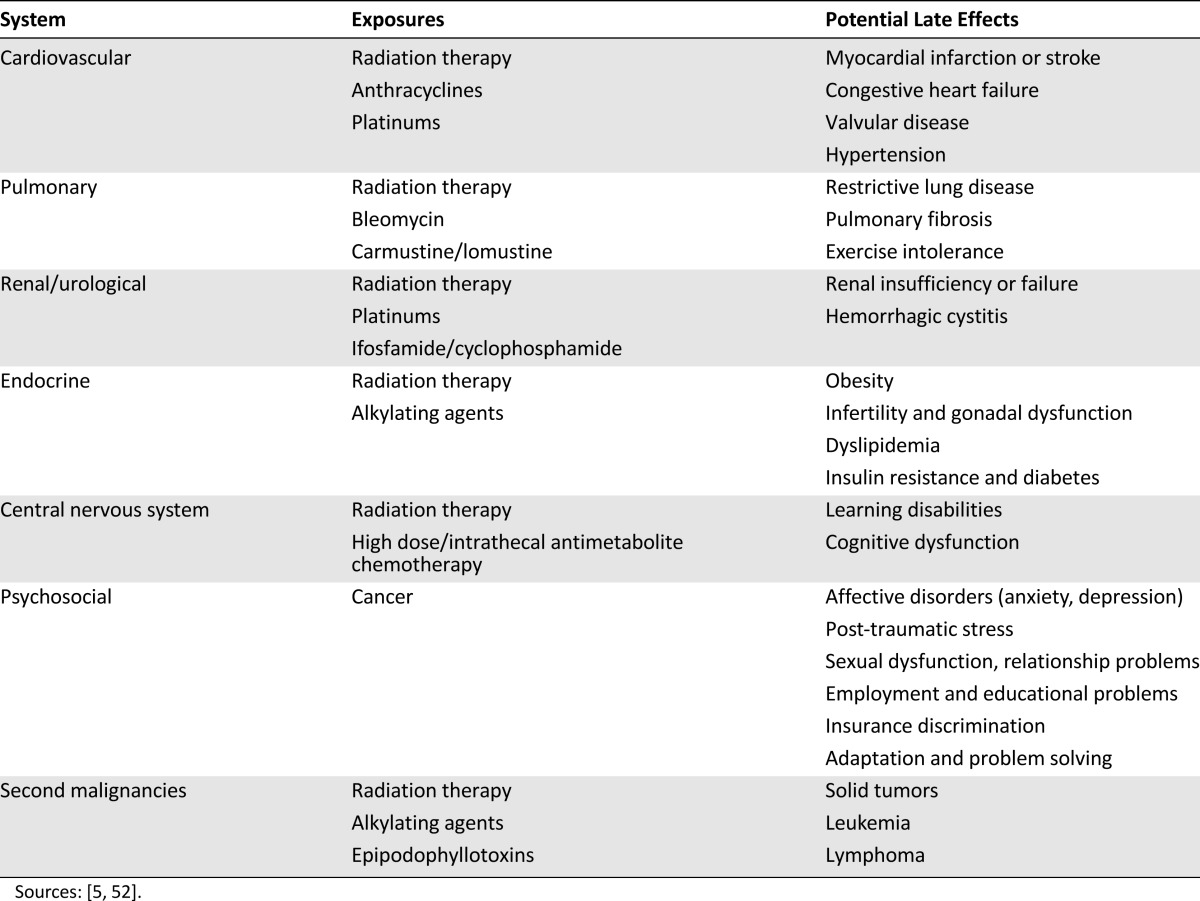
The risk for chronic health problems is similarly elevated (Table 1) and is largely due to treatment exposures, with risks potentiated by unhealthy lifestyle behaviors, presence of comorbidities, or underlying genetic factors [42, 50, 53–55]. Numerous studies from the CCSS have reported that survivors of adolescent cancer have an increased risk of serious and often life-threatening health conditions, including aforementioned SPC and CVD, but also including virtually all organ systems. It is very important, however, to point out that not all cancer survivors are at risk for widespread organ dysfunction. In fact, different organ systems are affected by very specific treatment exposures (e.g., anthracyclines and cardiomyopathy, alkylating agents and gonadal failure, cisplatin, and loss of hearing). Risk for CVD, SPCs, and other chronic health conditions have been confirmed for several groups of young adult cancers, including Hodgkin lymphoma and testicular cancer [38, 39, 41, 46, 48]. Using data from the 2009 BRFSS, Tai et al. [27] analyzed outcomes for 4,054 AYA survivors diagnosed between 15 and 29 years of age and compared them to similarly aged individuals who did not have a history of cancer. They reported that 14% of AYA cancer survivors had a history of CVD compared with 7% of individuals without cancer; the severity of CVD was unknown. In summary, many AYA cancer survivors have a lifelong elevated risk of chronic health problems as a consequence of the curative cancer therapy.
Table 1.
Potential late effects of cancer treatment, by system and exposure
Lifelong Survivorship Care
Fortunately, there is much that can be done to prevent or lessen the severity and impact of many of these chronic health problems and second cancers [34]. Previous IOM consensus reports on survivorship care strongly advocated that risk-based survivorship health care be available for all cancer survivors [56, 57]. Such care is lifelong and requires the integration of the cancer and survivorship experience in the overall health care needs of the individual [58]. A systematic plan for screening, surveillance, and prevention that incorporates risks based on the previous cancer, cancer therapy, genetic predispositions, lifestyle behaviors, and comorbid health conditions should be developed for all AYA cancer survivors.
A systematic plan for screening, surveillance, and prevention that incorporates risks based on the previous cancer, cancer therapy, genetic predispositions, lifestyle behaviors, and comorbid health conditions should be developed for all AYA cancer survivors.
Importantly, the long-term risks following some cancers are relatively low, and thus the intensity and frequency of follow-up care is minimal. On the other end of the spectrum, there are some particularly high risk populations (e.g., Hodgkin lymphoma, sarcoma, recipients of an allogeneic stem cell transplant) that should be followed by a clinician who is familiar with their risks. The National Comprehensive Cancer Network [59] and the Children’s Oncology Group [60] have published guidelines to assist in the care of AYA cancer survivors.
Lifestyle Management
AYA cancer survivors can also pursue various lifestyle interventions to lower their risk of cancer recurrence, new cancers, or the chronic conditions associated with treatment. Lifestyle interventions include adopting a healthy diet, regular physical activity, and tobacco and substance abuse reduction programs. Although much of the data comes from studies of survivors of pediatric cancers, there is some evidence that the diets of AYA cancer survivors are suboptimal. Studies find that only between 16 and 54% of such survivors have the recommended amount of fat in their diets [61–64], 86% are consuming more than the recommended daily intake of sugar [64], and a low percentage are eating 5 or more servings of fruits and vegetables per day [26, 61–63]. They are also less likely to meet physical activity guidelines than people in the general population, and they are less active and more sedentary than their siblings [26, 61–63, 65–68]. These behaviors are juxtaposed with observational evidence that physical activity not only improves quality of life but is also associated with prolonged survival for some types of cancer [69, 70].
There is a high demand for survivorship programs that focus on diet and exercise [61, 62], but relatively few interventions have been tailored to AYA cancer survivors. Distance-based delivery methods that use the internet and smart phone apps that are designed to match the developmental stage and interests of participants may be options to reach AYA patients [71].
Many lifestyle risk behaviors cluster together [72], and AYA cancer patients are also at an increased risk of substance abuse. Tobacco, alcohol, and drug use can exacerbate long-term treatment side effects [73–76], so it is important to develop evidence-based programs to address substance abuse among AYA cancer survivors. One such program was the Partnership for Health Program developed and tested by Emmons et al. [76], in which peer telephone counseling was provided to childhood cancer survivors and shown to reduce rates of smoking in this population. Effectively addressing mental health issues, which are often associated with substance abuse and lifestyle behavior change, might also be beneficial.
End-of-Life Care
Advance care planning is also a critical component of care for AYA patients with cancer. One study found that when adolescents were better informed about possible end-of-life decisions, there was better congruity between what the patient wanted and what the families thought the patient would want [77].
AYA patients with incurable disease experience many distressing physical and psychological symptoms, especially during the last month of life [78, 79]. Some of the psychological issues are unique to the AYA population, such as the grief associated with dying young, coming to terms with the meaning of their shortened lives, and determining their legacy [78, 79]. AYA patients with children have the additional challenge of preparing their children for the loss of a parent at a young age [80]. Thus, it is important to individualize support for these patients.
Moving Forward
Participants at the workshop made numerous suggestions for improving the care and outcomes for AYA patients (Panel 1). These suggestions focused on delivering cancer care that meets the medical and psychosocial needs of AYAs, improving access to care for AYAs, developing educational programs for providers who care for AYA cancer survivors, and enhancing the evidence base for AYAs with cancer by facilitating participation in research.
AYA Cancer Care Programs
Workshop participants noted numerous examples of programs for AYA cancer patients, but the content and organization of these programs vary. A consensus-based position statement that offered recommendations for quality care of AYA cancer patients was published in 2010 [33]. Another review published in 2010 described success stories and obstacles to overcome at six AYA cancer programs across the world, outlining the variability in environment, key elements, personnel, physical space, funding, and age range served [81]. Workshop participants noted numerous examples of programs for AYA cancer patients that have been initiated, but the content and organization of these programs still vary and have not been sufficiently described or studied to review their impact. This was recognized as a potential focus for future work.
Many lifestyle risk behaviors cluster together, and AYA cancer patients are also at an increased risk of substance abuse. Tobacco, alcohol, and drug use can exacerbate long-term treatment side effects, so it is important to develop evidence-based programs to address substance abuse among AYA cancer survivors.
One study of the psychosocial components of AYA cancer programs found that they provided a range of services, including patient/peer navigation, physical therapy, genetic counseling, palliative care, peer support, chaplaincy, nutritional counseling, and partnerships with community-based organizations. However, many programs lacked some important components. For example, none provided legal services, and most assessed the psychosocial needs of their AYA patients only when the need for such assessment was identified [82]. Another unpublished study found 23 self-identified AYA cancer programs in the U.S., of which 20 responded to a survey and phone interview to assess their services. Again, a wide variety of staffing, space, institutional support, and service components were found, with the most common elements being education and communication, fertility services, and psychosocial supports. Several workshop participants suggested that a clearinghouse for information on existing programs would help institutions that want to develop or expand AYA programs.
Access to Care
Provisions in the Affordable Care Act (ACA) facilitate health insurance coverage for some AYAs with cancer and thus access to care (Panel 2). In September 2010, the ACA required health plans that offer dependent coverage to allow young adults to remain on their parents’ insurance until age 26, and as of December 2011, more than 3 million young adults had gained health insurance through this provision [83]. As of April 2014, 28% of those enrolled through the ACA’s Health Insurance Marketplace were between the ages of 18 and 34 [84]. Other provisions of the ACA that should help AYAs diagnosed with cancer include those that require many health insurance plans to cover the costs of routine medical care provided within clinical trials, prohibit cost sharing for certain preventive services, prohibit annual or lifetime limits on essential health benefits, and ban exclusions for pre-existing conditions. The ACA also calls for expansion of Medicaid eligibility to people whose income is up to 133% of the federal poverty level, although many states have not yet participated in that expansion. Medicaid coverage is important to understand, because data from the CCSS indicate that cancer survivors are more likely to be covered by Medicaid than their siblings [85]. Overall, access enabled by the ACA should foster earlier diagnoses, better cancer care and survivorship care, and better surveillance for many AYAs with cancer. However, many young cancer survivors are unaware of these benefits and protections in the ACA [86], and workshop participants stressed the important role of care providers in disseminating this information.

Training Care Providers
Several workshop participants emphasized a need for education programs for medical and psychosocial care professionals on the particular needs of AYA patients with cancer, such as AYA-focused specialty training programs or fellowships, continuing education programs, support tools, or other initiatives. For example, additional medical training in fertility preservation or AYA survivorship care could be provided at the undergraduate level and at the graduate level, in primary care residencies, and in medical oncology fellowships. Web-based support tools for primary care providers might also be beneficial.
Further Research
Workshop participants noted many gaps in the evidence base to support the care of AYAs with cancer and advocated for research to fill those gaps. However, a number of research challenges were also described, including the heterogeneity of the AYA population (e.g., age and type of cancer), the difficulty of recruiting AYAs for studies, and the potential for bias in survey responses. The AYA population covers a large age range with a great deal of biological and developmental variability, so determining the best way to divide AYAs into subgroups for study and analysis can be challenging. Furthermore, differences in race and ethnicity, level of education and socioeconomic status, and cancer treatment can influence research outcomes and make it difficult to apply results from a particular subgroup to the group as a whole.
AYAs traditionally have a low participation rate in clinical trials. Data from an NCI Patterns of Care Study that focused on AYAs indicate that only approximately 14% of AYA cancer patients aged 15–39 enroll in clinical trials [87], compared with approximately 60% of pediatric cancer patients. Of those of ages 15–19, 34% were enrolled in clinical research, whereas only 3% of those from 35 to 39 were enrolled, similar to the rate for older adults with cancer. The AYA HOPE Study found that only 7% of the survey respondents participated in a clinical trial [88]. Providing incentives targeted to the AYA population and making participation in research easier could potentially increase accrual rates. An ongoing challenge, however, is the geographic distribution of AYA cancer survivors and the fact that many reside at a substantial distance from major referral centers where clinical trials may be available.
Some researchers have used online tools to recruit AYA participants for their studies because of the geographic distribution and evidence that the AYA age group relies on the internet for much of its information gathering and for interacting with others. However, online recruitment may be subject to bias, with women being more likely to respond than men. The use of online databases and data linkages could nonetheless be used more effectively to study the AYA cancer patients. For example, the Cancer Research Network, a group of managed care organizations, has health care delivery information about individuals of all ages with and without cancer [89]. This contrasts with SEER-Medicare data, which are derived from older adults and only includes information on cancer cases. ASCO also recently launched a large database for patient treatment data collected by ASCO physicians.
Conclusion
The IOM workshop provided an important synthesis of research and clinical practice-based perspectives on the needs of AYA patients diagnosed with cancer. The expert participants described many challenges that disproportionately affect AYA cancer survivors because of their life stage. Workshop participants stressed the need to leverage data sources to better understand and improve care needs, with specific emphasis on mitigating late and long-term effects, preserving fertility, addressing psychosocial issues, and improving quality of life.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
The responsibility for the content of this article rests with the authors and does not necessarily represent the views of the Institute of Medicine, its committees, its sponsors, or its convening activities and does not represent the official position of the Centers for Disease Control and Prevention or that of the National Cancer Institute. We thank the LIVESTRONG Foundation for generously cosponsoring the IOM workshop and Critical Mass: The Young Adult Cancer Alliance for supporting the workshop. The activities of the IOM’s National Cancer Policy Forum are supported by its sponsoring members, which currently include the Centers for Disease Control and Prevention, the National Cancer Institute, the Association of American Cancer Institutes, the American Association for Cancer Research, the American Cancer Society, the American Society of Clinical Oncology, the American Society of Hematology, the American Society for Radiation Oncology, AstraZeneca, Bristol-Myers Squibb, C-Change, the Cancer Support Community, the CEO Roundtable on Cancer, EMD Serono, Helsinn Group, the LIVESTRONG Foundation, the National Comprehensive Cancer Network, Novartis Oncology, the Oncology Nursing Society, and Sanofi Oncology. We thank the speakers and participants for their contributions to the workshop. We also thank Sarah Bender and Sara Tharakan for assistance with manuscript preparation.
Author Contributions
Conception/Design: Sharyl J. Nass, Lynda K. Beaupin, Wendy Demark-Wahnefried, Karen Fasciano, Patricia A. Ganz, Brandon Hayes-Lattin, Melissa M. Hudson, Brenda Nevidjon, Kevin C. Oeffinger, Ruth Rechis, Lisa C. Richardson, Nita L. Seibel, Ashley W. Smith
Provision of study material or patients: Sharyl J. Nass, Lynda K. Beaupin, Wendy Demark-Wahnefried, Karen Fasciano, Patricia A. Ganz, Brandon Hayes-Lattin, Melissa M. Hudson, Brenda Nevidjon, Kevin C. Oeffinger, Ruth Rechis, Lisa C. Richardson, Nita L. Seibel, Ashley W. Smith
Collection and/or assembly of data: Sharyl J. Nass, Lynda K. Beaupin, Wendy Demark-Wahnefried, Karen Fasciano, Patricia A. Ganz, Brandon Hayes-Lattin, Melissa M. Hudson, Brenda Nevidjon, Kevin C. Oeffinger, Ruth Rechis, Lisa C. Richardson, Nita L. Seibel, Ashley W. Smith
Data analysis and interpretation: Sharyl J. Nass, Lynda K. Beaupin, Wendy Demark-Wahnefried, Karen Fasciano, Patricia A. Ganz, Brandon Hayes-Lattin, Melissa M. Hudson, Brenda Nevidjon, Kevin C. Oeffinger, Ruth Rechis, Lisa C. Richardson, Nita L. Seibel, Ashley W. Smith
Manuscript writing: Sharyl J. Nass, Lynda K. Beaupin, Wendy Demark-Wahnefried, Karen Fasciano, Patricia A. Ganz, Brandon Hayes-Lattin, Melissa M. Hudson, Brenda Nevidjon, Kevin C. Oeffinger, Ruth Rechis, Lisa C. Richardson, Nita L. Seibel, Ashley W. Smith
Final approval of manuscript: Sharyl J. Nass, Lynda K. Beaupin, Wendy Demark-Wahnefried, Karen Fasciano, Patricia A. Ganz, Brandon Hayes-Lattin, Melissa M. Hudson, Brenda Nevidjon, Kevin C. Oeffinger, Ruth Rechis, Lisa C. Richardson, Nita L. Seibel, Ashley W. Smith
Disclosures
The authors indicated no financial relationships.
References
- 1.National Cancer Institute. A Snapshot of Adolescent and Young Adult Cancers. National Institutes of Health, 2013. Available at http://www.cancer.gov/researchandfunding/snapshots/pdf/AYA-Snapshot.pdf. Accessed September 20, 2013.
- 2.Adolescent and Young Adult Oncology Progress Review Group. Closing the Gap: Research and Care Imperatives for Adolescents and Young Adults with Cancer. National Institutes of Health, 2006. Available at http://planning.cancer.gov/library/AYAO_PRG_Report_2006_FINAL.pdf. Accessed September 20, 2013.
- 3.Young Adult Alliance LIVESTRONG. Closing the Gap: A Strategic Plan: Addressing the Recommendations of the Adolescent and Young Adult Oncology Progress Review Group. Lance Armstrong Foundation, 2007. Available at http://planning.cancer.gov/library/AYAO_PRG_Report_2006_FINAL.pdf. Accessed December 29, 2014.
- 4.NCI. Adolescent and Young Adult Health Outcomes & Patient Experience Study, 2014. Available at http://appliedresearch.cancer.gov/aya/. Accessed June 5, 2014.
- 5.Institute of Medicine . Identifying and Addressing the Needs of Adolescents and Young Adults with Cancer. Washington, D.C.: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 6.Next Steps for Adolescent and Young Adult Oncology: A Scientific Update. Available at http://ctep.cancer.gov/initiativesPrograms/nsayao_description.htm. Accessed November 13, 2014.
- 7.Kim CH, Jeon GH. Fertility preservation in female cancer patients. ISRN Obstet Gynecol. 2012;2012:807302. doi: 10.5402/2012/807302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams DH., 4th Fertility preservation in the male with cancer. Curr Urol Rep. 2013;14:315–326. doi: 10.1007/s11934-013-0345-6. [DOI] [PubMed] [Google Scholar]
- 9.Waimey KE, Duncan FE, Su HI, et al. Future directions in oncofertility and fertility preservation: A report from the 2011 Oncofertility Consortium Conference. J Adolesc Young Adult Oncol. 2013;2:25–30. doi: 10.1089/jayao.2012.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rechis R, Bann C, Arvey S et al. Unique Risks & Vulnerabilities of AYA Patients across the Lifespan, LIVESTRONG Foundation: AYA Survey Results. Presented at Institute of Medicine National Cancer Policy Forum/LIVESTRONG Workshop on Addressing the Needs of Adolescents and Young Adults with Cancer, Washington, D.C., 2013. Available at http://www.iom.edu/~/media/Files/Activity%20Files/Disease/NCPF/2013-JUL-15/2A_Rechis.pdf. Accessed September 10, 2013.
- 11.American Medical Association. AMA Adopts New Policies on First Day of Voting at Annual Meeting. June 17, 2013. Available at http://www.ama-assn.org/ama/pub/news/news/2013/2013-06-17-new-ama-policies-annual-meeting.page. Accessed September 20, 2013.
- 12.American Society of Clinical Oncology. Fertility Preservation, 2013. Available at http://www.asco.org/quality-guidelines/practice-improvement-resources/1401/1546. Accessed March 31, 2014.
- 13.Keros V, Hultenby K, Borgström B, et al. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22:1384–1395. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg JP, Li Y, Carlson CA, et al. Testicular tissue cryopreservation in prepubertal male children: An analysis of parental decision-making. Pediatr Blood Cancer. 2014;61:1673–1678. doi: 10.1002/pbc.25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baynosa J, Westphal LM, Madrigrano A, et al. Timing of breast cancer treatments with oocyte retrieval and embryo cryopreservation. J Am Coll Surg. 2009;209:603–607. doi: 10.1016/j.jamcollsurg.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Practice Committee of American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril. 2013;100:1214–1223. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Practice Committees of American Society for Reproductive Medicine. Society for Assisted Reproductive Technology Mature oocyte cryopreservation: A guideline. Fertil Steril. 2013;99:37–43. doi: 10.1016/j.fertnstert.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 18.Dolmans MM, Luyckx V, Donnez J, et al. Risk of transferring malignant cells with transplanted frozen-thawed ovarian tissue. Fertil Steril. 2013;99:1514–1522. doi: 10.1016/j.fertnstert.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Hadar A, Sheiner E, Press F, et al. Dilated cardiomyopathy in a pregnant woman after doxorubicin and radiotherapy for Hodgkin’s disease: A case report. J Reprod Med. 2004;49:401–403. [PubMed] [Google Scholar]
- 20.Coccia PF, Altman J, Bhatia S, et al. Adolescent and young adult oncology: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2012;10:1112–1150. doi: 10.6004/jnccn.2012.0117. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 22.Clark JK, Fasciano K. Young adult palliative care: Challenges and opportunities. Am J Hosp Palliat Care. 2013 doi: 10.1177/1049909113510394. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Bellizzi KM, Smith A, Schmidt S, et al. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer. 2012;118:5155–5162. doi: 10.1002/cncr.27512. [DOI] [PubMed] [Google Scholar]
- 24.Keegan THM, Lichtensztajn DY, Kato I, et al. Unmet adolescent and young adult cancer survivors information and service needs: A population-based cancer registry study. J Cancer Surviv. 2012;6:239–250. doi: 10.1007/s11764-012-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak M, Zebrack BJ, Meeske KA, et al. Trajectories of psychological distress in adolescent and young adult patients with cancer: A 1-year longitudinal study. J Clin Oncol. 2013;31:2160–2166. doi: 10.1200/JCO.2012.45.9222. [DOI] [PubMed] [Google Scholar]
- 26.Forsythe LP, Kent EE, Weaver KE, et al. Receipt of psychosocial care among cancer survivors in the United States. J Clin Oncol. 2013;31:1961–1969. doi: 10.1200/JCO.2012.46.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tai E, Buchanan N, Townsend J, et al. Health status of adolescent and young adult cancer survivors. Cancer. 2012;118:4884–4891. doi: 10.1002/cncr.27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guy GP, Jr, Ekwueme DU, Yabroff KR, et al. Economic burden of cancer survivorship among adults in the United States. J Clin Oncol. 2013;31:3749–3757. doi: 10.1200/JCO.2013.49.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons HM, Harlan LC, Lynch CF, et al. Impact of cancer on work and education among adolescent and young adult cancer survivors. J Clin Oncol. 2012;30:2393–2400. doi: 10.1200/JCO.2011.39.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IOM . Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, D.C.: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 31.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 32.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 33.Zebrack B, Mathews-Bradshaw B, Siegel S. Quality cancer care for adolescents and young adults: A position statement. J Clin Oncol. 2010;28:4862–4867. doi: 10.1200/JCO.2010.30.5417. [DOI] [PubMed] [Google Scholar]
- 34.Oeffinger KC, Tonorezos ES. The cancer is over, now what?: Understanding risk, changing outcomes. Cancer. 2011;117(suppl):2250–2257. doi: 10.1002/cncr.26051. [DOI] [PubMed] [Google Scholar]
- 35.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youn P, Milano MT, Constine LS, et al. Long-term cause-specific mortality in survivors of adolescent and young adult bone and soft tissue sarcoma: A population-based study of 28,844 patients. Cancer. 2014;120:2334–2342. doi: 10.1002/cncr.28733. [DOI] [PubMed] [Google Scholar]
- 37.Kirchhoff AC, Lyles CR, Fluchel M, et al. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer. 2012;118:5964–5972. doi: 10.1002/cncr.27537. [DOI] [PubMed] [Google Scholar]
- 38.van Eggermond AM, Schaapveld M, Lugtenburg PJ, et al. Risk of multiple primary malignancies following treatment of Hodgkin lymphoma. Blood. 2014;124:319–327. doi: 10.1182/blood-2013-10-532184. [DOI] [PubMed] [Google Scholar]
- 39.Altena R, Hummel YM, Nuver J, et al. Longitudinal changes in cardiac function after cisplatin-based chemotherapy for testicular cancer. Ann Oncol. 2011;22:2286–2293. doi: 10.1093/annonc/mdr408. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooning MJ, Aleman BM, van Rosmalen AJ, et al. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64:1081–1091. doi: 10.1016/j.ijrobp.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 44.Beard CJ, Travis LB, Chen MH, et al. Outcomes in stage I testicular seminoma: A population-based study of 9193 patients. Cancer. 2013;119:2771–2777. doi: 10.1002/cncr.28086. [DOI] [PubMed] [Google Scholar]
- 45.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 46.Oeffinger KC, Baxi SS, Novetsky Friedman D, et al. Solid tumor second primary neoplasms: Who is at risk, what can we do? Semin Oncol. 2013;40:676–689. doi: 10.1053/j.seminoncol.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217–2223. doi: 10.1200/JCO.2013.54.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Belt-Dusebout AW, Aleman BM, Besseling G, et al. Roles of radiation dose and chemotherapy in the etiology of stomach cancer as a second malignancy. Int J Radiat Oncol Biol Phys. 2009;75:1420–1429. doi: 10.1016/j.ijrobp.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 49.Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 50.Travis LB, Hill D, Dores GM, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97:1428–1437. doi: 10.1093/jnci/dji290. [DOI] [PubMed] [Google Scholar]
- 51.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oeffinger K. Long-Term and Late Effects Following AYA Cancer. Washington, D.C.: Presented at Institute of Medicine National Cancer Policy Forum/LIVESTRONG Workshop on Addressing the Needs of Adolescents and Young Adults with Cancer, 2013. Available at http://www.iom.edu/~/media/Files/Activity%20Files/Disease/NCPF/2013-JUL-15/3A_Oeffinger.pdf. Accessed September 10, 2013.
- 53.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 55.Travis LB, Gilbert E. Lung cancer after Hodgkin lymphoma: The roles of chemotherapy, radiotherapy and tobacco use. Radiat Res. 2005;163:695–696. [PubMed] [Google Scholar]
- 56.Childhood Cancer Survivorship IOM. Improving Care and Quality of Life. Washington, D.C.: The National Academies Press; 2003. [PubMed] [Google Scholar]
- 57.IOM . From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C.: The National Academies Press; 2005. [Google Scholar]
- 58.McCabe MS, Bhatia S, Oeffinger KC, et al. American Society of Clinical Oncology statement: Achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31:631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coccia PF, Pappo AS, Altman J, et al. Adolescent and young adult oncology, version 2.2014. J Natl Compr Canc Netw. 2014;12:21–32; quiz 32. doi: 10.6004/jnccn.2014.0004. [DOI] [PubMed] [Google Scholar]
- 60.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 61.Badr H, Paxton RJ, Ater JL, et al. Health behaviors and weight status of childhood cancer survivors and their parents: Similarities and opportunities for joint interventions. J Am Diet Assoc. 2011;111:1917–1923. doi: 10.1016/j.jada.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Demark-Wahnefried W, Werner C, Clipp EC, et al. Survivors of childhood cancer and their guardians. Cancer. 2005;103:2171–2180. doi: 10.1002/cncr.21009. [DOI] [PubMed] [Google Scholar]
- 63.Rabin C, Politi M. Need for health behavior interventions for young adult cancer survivors. Am J Health Behav. 2010;34:70–76. doi: 10.5993/ajhb.34.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robien K, Ness KK, Klesges LM, et al. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30:815–822. doi: 10.1097/MPH.0b013e31817e4ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bélanger LJ, Plotnikoff RC, Clark A, et al. Physical activity and health-related quality of life in young adult cancer survivors: A Canadian provincial survey. J Cancer Surviv. 2011;5:44–53. doi: 10.1007/s11764-010-0146-6. [DOI] [PubMed] [Google Scholar]
- 66.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: A comparison of long-term outcomes, health care utilization, and health-related behaviors from the childhood cancer survivor study. J Clin Oncol. 2005;23:6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 67.Florin TA, Fryer GE, Miyoshi T, et al. Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2007;16:1356–1363. doi: 10.1158/1055-9965.EPI-07-0048. [DOI] [PubMed] [Google Scholar]
- 68.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–1994. doi: 10.1002/cncr.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmes MD, Chen WY, Feskanich D, et al. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 70.Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 71.Rabin C, Simpson N, Morrow K, et al. Intervention format and delivery preferences among young adult cancer survivors. Int J Behav Med. 2013;20:304–310. doi: 10.1007/s12529-012-9227-4. [DOI] [PubMed] [Google Scholar]
- 72.Rebholz CE, Rueegg CS, Michel G, et al. Clustering of health behaviours in adult survivors of childhood cancer and the general population. Br J Cancer. 2012;107:234–242. doi: 10.1038/bjc.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clarke SA, Eiser C. Health behaviours in childhood cancer survivors: A systematic review. Eur J Cancer. 2007;43:1373–1384. doi: 10.1016/j.ejca.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: Role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–137. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 75.World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Available at http://www.dietandcancerreport.org/index.php. Accessed September 20, 2013.
- 76.Emmons KM, Puleo E, Mertens A, et al. Long-term smoking cessation outcomes among childhood cancer survivors in the Partnership for Health Study. J Clin Oncol. 2009;27:52–60. doi: 10.1200/JCO.2007.13.0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lyon ME, Jacobs S, Briggs L, et al. Family-centered advance care planning for teens with cancer. JAMA Pediatr. 2013;167:460–467. doi: 10.1001/jamapediatrics.2013.943. [DOI] [PubMed] [Google Scholar]
- 78.Wein S, Pery S, Zer A. Role of palliative care in adolescent and young adult oncology. J Clin Oncol. 2010;28:4819–4824. doi: 10.1200/JCO.2009.22.4543. [DOI] [PubMed] [Google Scholar]
- 79.Pritchard S, Cuvelier G, Harlos M, et al. Palliative care in adolescents and young adults with cancer. Cancer. 2011;117(suppl):2323–2328. doi: 10.1002/cncr.26044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rauch P, Muriel A. Raising an Emotionally Healthy Child When a Parent Is Sick. Boston: McGraw-Hill; 2006. [Google Scholar]
- 81.Ferrari A, Thomas D, Franklin AR, et al. Starting an adolescent and young adult program: Some success stories and some obstacles to overcome. J Clin Oncol. 2010;28:4850–4857. doi: 10.1200/JCO.2009.23.8097. [DOI] [PubMed] [Google Scholar]
- 82.Block R. 2013. AYA specific programs. Presentation to the Institute of Medicine National Cancer Policy Forum/LIVESTRONG Workshop on Addressing the Needs of Adolescents and Young Adults with Cancer, July 15–16, 2013, Washington, D.C. http://www.iom.edu/~/media/Files/Activity%20Files/Disease/NCPF/2013-JUL-15/4D_Block.pdf. Accessed September 10, 2013.
- 83.Department of Health and Human Services (ASPE Issue Brief) Number of Young Adults Gaining Insurance Due to the Affordable Care Act Now Tops 3 Million. June 2012. Available online at http://aspe.hhs.gov/aspe/gaininginsurance/rb.cfm. Accessed July 16, 2014.
- 84.Department of Health and Human Services (ASPE Issue Brief): Health Insurance Marketplace: Summary Enrollment Report for the Initial Annual Open Enrollment Period. May 1, 2012. Available at http://aspe.hhs.gov/health/reports/2014/MarketPlaceEnrollment/Apr2014/ib_2014Apr_enrollment.pdf. Accessed July 16, 2014.
- 85.Park ER, Li FP, Liu Y, et al. Health insurance coverage in survivors of childhood cancer: The Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:9187–9197. doi: 10.1200/JCO.2005.01.7418. [DOI] [PubMed] [Google Scholar]
- 86.Park ER, Kirchhoff AC, Zallen JP, et al. Childhood Cancer Survivor Study participants’ perceptions and knowledge of health insurance coverage: Implications for the Affordable Care Act. J Cancer Surviv. 2012;6:251–259. doi: 10.1007/s11764-012-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parsons HM, Harlan LC, Seibel NL, et al. Clinical trial participation and time to treatment among adolescents and young adults with cancer: Does age at diagnosis or insurance make a difference? J Clin Oncol. 2011;29:4045–4053. doi: 10.1200/JCO.2011.36.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harlan L. AYA HOPE Study: A Population-based Cohort Study of Adolesents and Young Adults with Cancer. Presented at the Institute of Medicine National Cancer Policy Forum/LIVESTRONG Workshop on Addressing the Needs of Adolescents and Young Adults with Cancer, Washington, D.C., 2013. Available at http://www.iom.edu/~/media/Files/Activity%20Files/Disease/NCPF/2013-JUL-15/2C_Harlan.pdf. Accessed September 10, 2013.
- 89.Nekhlyudov L, Greene SM, Chubak J, et al. Cancer research network: Using integrated healthcare delivery systems as platforms for cancer survivorship research. J Cancer Surviv. 2013;7:55–62. doi: 10.1007/s11764-012-0244-8. [DOI] [PubMed] [Google Scholar]