Immunotoxins are a novel class of antibody-based therapeutics currently in clinical development. This review of the field will help physicians better inform patients about the potential benefits and toxicities of these experimental treatments.
Keywords: Antibody conjugate, Recombinant immunotoxin, Vascular leak syndrome, Antidrug antibody
Abstract
Immunotoxins are a novel class of antibody-conjugated therapeutics currently in clinical development for a variety of malignancies. They consist of an antibody-based targeting domain fused to a bacterial toxin payload for cell killing. Immunotoxins kill cells by inhibiting protein synthesis, a unique mechanism of action that is toxic to both dividing and nondividing cells. Recent advances in the design and administration of immunotoxins are overcoming historical challenges in the field, leading to renewed interest in these therapeutics.
Implications for Practice:
Immunotoxins are a novel class of antibody-based therapeutics currently in clinical development. A review of the field will help physicians better inform patients about the potential benefits and toxicities of these experimental treatments.
Introduction
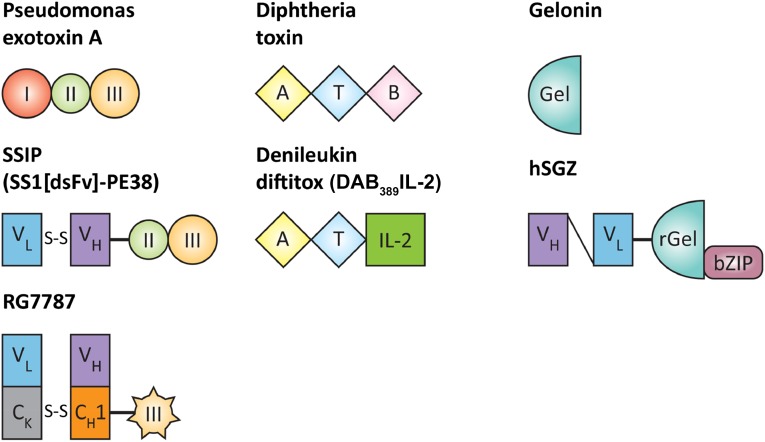
Immunotoxins combine the specificity of antibody therapeutics with the cell-killing power of some of nature’s most toxic proteins. They are chimeric molecules that consist of a protein toxin fused to a targeting moiety. The targeting domain is most commonly the antigen-binding fragment of a monoclonal antibody. The antibody brings the toxin to a cancer cell, then the toxin enters and kills the cell. Structures of several modern immunotoxins are depicted in Figure 1. These molecules are synthesized by recombinant DNA techniques and contain only those portions of the antibody and toxin needed to kill a target cell. The native receptor domain of the toxin is replaced by the antibody. The two are recombinantly fused by a peptide bond. Portions of the toxin that are not essential for cytotoxic activity or processing are deleted from the sequence. Point mutations can be created in the native toxins to improve activity, reduce off-target toxicity, or limit immunogenicity. The modular nature of immunotoxins allows for extensive recombinant tailoring.
Figure 1.
Structure of select toxins and immunotoxins. Native Pseudomonas exotoxin A contains three domains: domain I (binding), domain II (unknown function), and domain III (catalytic domain). In the SS1P immunotoxin, domain I is replaced by a double-stranded Fv (VL and VH) that targets mesothelin. The engineered disulfide bond links VL and VH. The RG7787 immunotoxin uses a humanized Fab fragment and lacks PE domain II. Diphtheria toxin also contains three domains: catalytic, transmembrane, and binding. In denileukin diftitox, the B domain is replaced by human IL-2 to permit binding to cells bearing the IL-2 receptor. Gelonin is a plant toxin with n-glycosidase activity that inhibits ribosomal activity to halt protein synthesis. It consists of a single domain. In the hSGZ immunotoxin, recombinant gelonin is fused to a single-chain Fv (VL and VH) that binds fibroblast growth factor receptor 14-kDa protein (Fn14), and a bZIP domain that increases activity of the immunotoxin by allowing dimerization.
Abbreviations: A, catalytic domain; B, binding domain; Gel, gelonin; IL-2, interleukin-2; rGel, recombinant gelonin; T, transmembrane domain.
The first immunotoxins were made in the early 1980s when monoclonal antibodies reacting with cancer cells became widely available. Protein toxins from a variety of plants and several bacteria were investigated. These areas have been extensively reviewed elsewhere [1]. We will focus on agents that have properties suitable for clinical development or that are already in clinical use. Our group has focused on the use of Pseudomonas exotoxin A (PE) to make immunotoxins. We have previously reported that immunotoxins targeting CD22 can cause complete remissions in patients with refractory hairy cell leukemia (HCL) [2]. In addition, we recently found that recombinant immunotoxins targeting the protein mesothelin produced major tumor regressions in some patients with advanced chemotherapy-resistant mesothelioma [3]. In this review, we summarize the current state of the immunotoxin field, analyze the advantages and disadvantages of immunotoxins compared with antibody-drug conjugates (ADCs) and radioimmunotherapies, and discuss future directions.
Immunotoxin Mechanism of Action
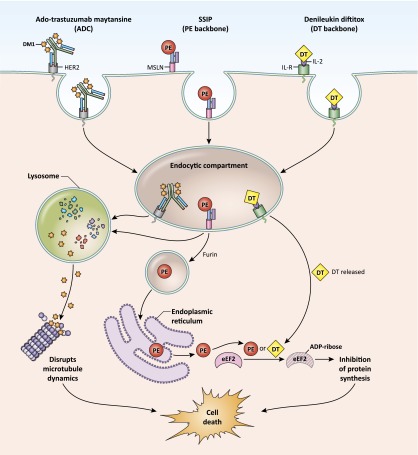
The plant and bacterial toxins used in immunotoxins kill cells by halting cellular protein synthesis. Intracellular delivery to the cytosol is required for antitumor activity. After the immunotoxin targeting moiety binds to the cancer cell surface, the molecule is internalized to the endocytic compartment. As shown in Figure 2, processing and trafficking of these molecules is target and toxin specific but ultimately results in delivery of the enzymatically active portion of the toxin to the cytosol. The bacterial toxins diphtheria toxin (DT) and PE irreversibly modify and inactivate eukaryotic elongation factor 2 (eEF2), a critical component of the protein synthesis machinery [4, 5]. Plant toxins such as gelonin and ricin also arrest protein synthesis but do so by inactivating the ribosome instead of eEF2 [6, 7]. These toxin-mediated modifications stimulate the apoptotic pathway, leading to cell death.
Figure 2.
Delivery of ADCs and immunotoxins. ADCs and immunotoxins bind to partners (HER-2, MSLN, or IL-R) on the cell surface and are internalized into an endocytic compartment. The ADC ado-trastuzumab maytansine traffics to lysosomes, where the maytansine chemotherapeutic is released from the antibody structure, allowing drug penetration into the cytosol, disruption of microtubule dynamics, and cell death (left). In endosomes, the modified PE toxin is cleaved from SS1P by the furin protease (middle). PE then undergoes retrograde transport through the Golgi to the endoplasmic reticulum. The method for egress from the endoplasmic reticulum to the cytosol is unknown. The toxin catalyzes irreversible ADP ribosylation of eEF2, leading to global inhibition of protein synthesis and cell death. The T domain (Fig. 1) of DT forms a pore in the membrane of the endosome, allowing transit of DT into the cytoplasm (right). Like PE, DT also catalyzes inhibitory modification of eEF2.
Abbreviations: ADC, antibody-drug conjugate; ADP-ribose, adensodine diphosphate ribose; DT, diphtheria toxin; eEF2, eukaryotic elongation factor 2; IL-2, interleukin-2; IL-R, interleukin-2 receptor; MSLN, mesothelin; PE, Pseudomonas exotoxin A.
Comparison With Antibody-Drug Conjugates
ADCs consist of a monoclonal antibody chemically attached to a highly toxic chemotherapy agent with an insufficient therapeutic window for use in traditional systemic therapy. Antibody targeting localizes the drug to the tumor but limits its deposition elsewhere, increasing antitumor activity and decreasing systemic toxicity [8, 9]. Both ADCs currently approved by the U.S. Food and Drug Administration (FDA) and most of those in development use antitubulin chemotherapeutics. Once inside the cell, these drugs disrupt microtubule organization, leading to mitotic arrest and cell death (Fig. 2). Clinical development of ADCs has been vigorous over the past few years because of their decreased toxicity compared with standard chemotherapy and their effectiveness against some refractory tumors.
Immunotoxins have several favorable properties not shared by ADCs. First, the novel immunotoxin mechanism of action translates into a nonoverlapping toxicity profile, allowing for easy combination with standard of care agents. Second, unlike traditional chemotherapeutics and those used in ADCs, immunotoxins can effectively kill quiescent, nondividing cells. In addition, immunotoxins appear to have little cross-resistance with other agents and have demonstrated activity in chemorefractory patients. Finally, although ADCs can cause off-target toxicity due to inappropriate payload dissociation from the chemical linker that joins the chemotherapy to the antibody, modern recombinant immunotoxins do not have this issue. The recombinant peptide linkers that join toxin to antibody in immunotoxins require the action of specific intracellular proteases to unlink.
The same general principles apply when selecting targets for ADCs and for immunotoxins. First, antigens must be expressed on the cancer cell surface for good antitumor efficacy. The antibody must trigger internalization of the molecule with suitable kinetics. Second, for both therapeutic types, the antigens must have strong differential expression between normal and tumor cells to limit on-target off-tumor toxicity. This requirement is more stringent for immunotoxins than for ADCs because immunotoxins kill even quiescent, antigen-expressing cells. Many targets suitable for ADCs are not suitable for immunotoxin development. The very successful anti-Her2 monoclonal antibody trastuzumab, for example, and the subsequent ADC ado-trastuzumab maytansine rarely cause hepatotoxicity. In contrast, development of the Her-2-targeted immunotoxin erb-38 was halted early in phase I testing because of marked hepatotoxicity in all of the first six patients [10]. Further investigations identified very low-level Her-2 expression on the surface of hepatocytes that had not been appreciated in prior studies. Although the exquisite sensitivity of hepatocytes to immunotoxins that is highlighted by this example suggests that this class of therapeutics could be highly effective against naturally chemoresistant hepatocellular carcinoma, it also illustrates how low-level antigen expression in vital organs can affect the safety profile of an immunotoxin.
Comparison With Radioimmunotherapy Agents
Monoclonal antibodies can also be tagged with radioactive agents for use as anticancer therapeutics. This strategy concentrates therapeutic radioactivity at sites of disease; however, because the radioactive cargo does not require cell internalization for activity, these agents produce significant toxic bystander effects on neighboring non-neoplastic, radiosensitive tissues. Two radiolabeled anti-CD20 monoclonal antibodies have been approved by the FDA for treatment of refractory non-Hodgkin lymphoma [11, 12]. Both increase the depth and length of clinical response but produce profound myelosuppression that can persist for months. This myelosuppressive toxicity overlaps with that of standard chemotherapy agents used to treat this disease, making combination difficult, unlike the situation with immunotoxins. No successful radioimmunotherapy agents have been developed for treating solid tumors. These tumors are generally much less sensitive to radiation than lymphocytes, and current targeting technology delivers insufficient radioisotope to solid tumors for adequate antitumor effect [13].
Overcoming Historical Obstacles to Clinical Use of Immunotoxins
The first successful in vivo use of an immunotoxin was described in Nature in 1981 [14]. The technology developed rapidly, and the first clinical trials in solid tumor patients followed [15, 16]. Unfortunately, these early immunotoxins lacked a sufficient therapeutic window to merit further clinical development. The dose-limiting toxicity for these agents was a vascular leak syndrome (VLS) characterized by generalized edema, weight gain, hypoalbuminemia, and orthostatic hypotension. No clinical responses were observed, even at dose levels that caused life-threatening VLS. In addition, only a few doses could be given successfully because all patients on study rapidly developed antidrug antibodies. Additional trials of other immunotoxins in various patient populations produced similar results, and interest in immunotoxins waned. Over the past few years, significant strides have been made to overcome immunotoxin-induced vascular leak syndrome and immunogenicity such that immunotoxins are again being considered as promising anticancer therapeutics.
Over the past few years, significant strides have been made to overcome immunotoxin-induced vascular leak syndrome and immunogenicity such that immunotoxins are again being considered as promising anticancer therapeutics.
Vascular Leak Syndrome
Immunotoxin-mediated damage to endothelial cells is presumed to be responsible for VLS because induction of VLS requires an enzymatically active toxin molecule. Mutant toxins that lack enzymatic activity or that fail to traffic to the cytosol do not cause VLS, suggesting that VLS is an off-target effect of the active molecule [17]. One study suggested that toxins contain short amino acid motifs that bind endothelial cells and demonstrated that the ricin toxin could bind to endothelial cells using these motifs [18]. Subsequent studies showed that modification or deletion of these sequences reduced toxin-induced VLS [19, 20]. Most recently, we reported that deletion of the near entirety of PE domain II can prevent VLS while preserving on-target cytotoxicity. This deletion does not affect two of the three putative endothelial binding motifs previously identified [21]. Further studies will be required to determine the minimal sequence responsible for PE-induced VLS.
Clinically, severity of VLS depends on the immunotoxin administered and varies among patients. Multiple clinical trials with ricin-based toxins have reported severe and even fatal cases of VLS [22–25], whereas studies with PE-based immunotoxins over the past 15 years have reported only mild VLS [2, 3, 10, 26, 27]. Clinical factors may also be important. In a retrospective study of Hodgkin’s lymphoma patients who had received ricin-based immunotoxin therapy, VLS was found to be more frequent and more severe in patients with a history of prior radiation therapy [28]. No similar studies have been reported for solid tumor patients or for patients administered immunotoxins with PE or DT backbones.
For our immunotoxin clinical trials using PE-based agents, we have found that simple supportive measures can significantly lessen VLS. All patients on our studies receive premedication with dexamethasone, which has been shown to diminish severity of VLS [29, 30]. In addition, bolus intravenous fluids are administered before each immunotoxin dose to transiently increase intravascular volume. Hypotension was not observed when these supportive measures were applied. Hypoalbuminemia accompanied by dependent edema still occurs in most patients; however, rapid spontaneous normalization of fluid status occurs in the majority following each treatment cycle. A few patients require short-term treatment with a loop diuretic to normalize fluid status. This mild, self-limited VLS has not proven a barrier to clinical development.
Immunogenicity
Immunotoxins are immunogenic molecules. Host anti-mouse antibodies against the antibody portion of the immunotoxin can be avoided by humanizing this portion of the molecule. Unsurprisingly, the toxin domain is even more immunogenic. Patients with hematologic malignancies have impaired immune reactions secondary to their tumor and generally develop antidrug antibodies later in treatment or not at all [2]. In contrast, almost all solid tumor patients rapidly develop anti-immunotoxin antibodies after treatment administration. Several immunosuppressive regimens have been tested in this population. Administration of a single intravenous dose of cyclophosphamide prior to immunotoxin therapy was found to be ineffective [31]. Similarly, pretreatment with oral cyclosporine A did not reduce anti-immunotoxin antibody formation [32]. Suppression of antibody response by rituximab was also examined. All patients had undetectable peripheral B cells when immunotoxin was administered but developed anti-immunotoxin antibodies despite this [33].
Recently, a novel lymphocyte-depleting regimen consisting of pentostatin plus cyclophosphamide was shown to be effective in delaying formation of neutralizing anti-immunotoxin antibodies in solid tumor patients. The pentostatin plus cyclophosphamide regimen was originally designed to combat host-versus-graft reactivity in major histocompatibility complex-mismatched allogeneic bone marrow transplant patients. This regimen depletes T and B cells while largely sparing myeloid cells. It also causes marked durable suppression of T-cell effector function disproportionate to what one would expect based on numerical depletion alone [34]. The combination was tested as a preparative regimen preceding immunotoxin treatment in preclinical studies with mice [35] and then in patients with malignant mesothelioma [3]. In the clinical pilot study, 8 of 10 patients could receive repeated cycles of immunotoxin before development of anti-immunotoxin neutralizing antibodies. This breakthrough allows repetitive administration of immunotoxins to solid tumor patients for the first time.
Immunotoxins in the Clinic
A number of immunotoxins that have been investigated in recent or ongoing clinical trials are listed in Table 1. Others shown in Table 2 have shown promise in the preclinical setting and may shortly begin testing. Additional information about these agents is provided below.
Table 1.
Immunotoxins in the clinic

Table 2.
Immunotoxins in preclinical testing

Immunotoxins for Hematologic Malignancies
Denileukin Diftitox (ONTAK, DAB389IL-2)
The FDA granted initial approval of denileukin diftitox in 2001 for treatment of cutaneous T-cell lymphoma (CTCL). This immunotoxin contains a traditional DT backbone; however, the targeting domain of denileukin diftitox does not contain an antibody. Instead, recombinant human interleukin-2 (IL-2) is fused to the C-terminus of the toxin. The ligand targets the molecule to cells that express the IL-2 receptor (IL-2R). IL-2R is transiently expressed on activated T cells but is constitutively present in a number of hematologic malignancies, including CTCL, making it a good therapeutic target. Testing of denileukin diftitox in a single-arm phase III trial of patients with recurrent IL-2R-positive CTCL demonstrated a 30% response rate with a median duration of response of 6.9 months [36]. A later randomized, placebo-controlled trial confirmed improved the response rate with this agent [37]. In practice, denileukin diftitox is used infrequently because of poor tolerability. Side effects include flu-like symptoms (e.g., fever, fatigue, rigors, diarrhea, and nausea), infusion-related events, disruption of color vision, VLS with hypotension, and frequent grade 3–4 hypoalbuminemia. Supportive measures that can be used to ameliorate side effects have been described previously [38].
LMB-2 (anti-Tac[Fv]-PE38)
LMB-2 also targets IL-2R. The high-affinity IL-2R consists of three subunits. The antibody fragment in LMB-2 selectively binds the α-subunit, called CD25 or Tac. This is fused to PE38, a modified PE toxin developed by our laboratory. LMB-2 was tested in patients with relapsed CD25-positive hematologic malignancies in a phase I clinical trial. Partial responses were observed in patients with HCL, CTCL, chronic lymphocytic leukemia (CLL), adult T-cell leukemia (ATL), and Hodgkin’s disease. One patient with HCL developed a durable complete response [39, 40]. Currently, LMB-2 is being investigated in combination with fludarabine and cyclophosphamide for patients with relapsed ATL. Complete responses have been observed in otherwise treatment-refractory disease [27].
Moxetumomab Pasudotox
CD22 is a B-cell differentiation antigen expressed on mature B cells and by many B-cell malignancies. Moxetumomab pasudotox consists of a high-affinity anti-CD22 Fv fused to PE38. In phase I testing, moxetumomab pasudotox was studied in 28 patients with chemotherapy-refractory HCL. No dose-limiting toxicity was observed up to 50 μg/kg, the maximum dose tested. The overall response rate was 86%, and 46% of patients achieved complete remission. These responses have proven durable in all but one patient [2]. Based on these results, a single-arm phase III registration trial investigating the efficacy of moxetumomab pasudotox for relapsed and refractory HCL recently opened (ClinicalTrials.gov identifier NCT01829711). This immunotoxin is also under evaluation for childhood and adult acute lymphoblastic leukemia. A 24% complete response rate was reported in treatment-refractory pediatric patients, with the molecule demonstrating activity in a total of 67% of patients [41]. No results are available yet from the adult trial.
A-dmDT390-bisFv(UCHT1)
The mature T-cell receptor is a heterodimer composed of CD3 chains γ, δ, ε, and ζ [42]. A-dmDT390-bisFv(UCHT1) is a bivalent immunotoxin that consists of a DT fragment bound to two single-chain anti-CD3ε Fv fragments. It targets T cells. It was tested in patients with CTCL (11 patients) and 1 patient with peripheral T-cell lymphoma in a phase I study. Two patients achieved complete remissions lasting >15 months, and 4 patient experienced partial remissions [43]. A phase II trial is currently accruing for patients with previously treated CTCL. Preference is being given to patients with stage IB and IIB disease because the responses were observed in this subgroup.
DT2219ARL
CD19, like CD22 described above, is a B-cell differentiation antigen and a validated target for antibody-based therapies, given the clinical success of rituximab. DT2219ARL is a DT-based molecule that binds both CD19 and CD22 through a complex single-chain design that includes two oppositely oriented Fv fragments linked in series. This allows effective application to more tumor types compared with molecules binding either target alone [44]. A phase I study in patients with relapsed or refractory B-lineage leukemia or lymphoma is ongoing. The trial was temporarily halted after a dose-limiting lower extremity weakness was observed but has since reopened. No data are available on outcomes in the initial cohort of patients.
HuM195-Gelonin
HuM195 is a humanized monoclonal antibody that targets CD33. CD33 was originally thought to be a myeloid-specific marker; however, some natural killer and T-cell subsets also express this leukocyte-differentiation antigen [45]. Importantly, many acute myelogenous leukemias (AMLs) have strong CD33 surface expression [46]. The HuM195-gelonin immunotoxin was tested in a phase I study in patients with relapsed or refractory AML, chronic myelogenous leukemia in accelerated blast phase, and defined subgroups of myelodysplastic syndrome. No vascular leak syndrome and little immunogenicity were observed, but there were no responses. This study is the first published using a gelonin-based immunotoxin. Although efficacy was limited with this particular construct, the safety results suggest that gelonin has a more favorable toxicity profile than the related plant toxin ricin and warrants further clinical investigation.
Immunotoxins for Solid Tumors
SS1P
SS1P was developed in our laboratory. It is the only immunotoxin currently being clinically tested as a systemic agent in solid tumor patients. SS1P consists of the same PE38 fragment found in moxetumomab pasudotox and LMB-2 but uses the SS1 anti-mesothelin (anti-MSLN) antibody for targeting. MSLN is a cell-surface glycoprotein normally expressed only in mesothelial cells that line the pleura, pericardium, and peritoneum. The protein is not critical because mesothelin knockout mice develop normally and have no discernible phenotype [47]. Mesothelin is also robustly expressed by many solid tumors including mesothelioma [48], pancreatic adenocarcinoma [49, 50], nonmucinous ovarian cancer [51], gastric cancer [52, 53], cholangiocarcinoma [54], lung adenocarcinoma [55], cervical cancer [56], and triple-negative-type breast cancer [57]. These properties make MSLN one of the few antigens with sufficient differential expression to allow safe targeting of solid tumor malignancies.
Two phase I trials of single-agent SS1P were performed, each examining a different administration schedule. Efficacy and toxicity profile were similar [26, 58]. The large majority of patients developed antidrug antibodies by the end of their first cycle, resulting in nontherapeutic drug levels if any additional cycles were given. Dose-limiting toxicities included a self-limited on-target pleuritis, presumably from damage to the normal pleura caused by targeting MSLN. Later experience demonstrated that this toxicity could be managed effectively by premedication with steroids and prompt administration of narcotic pain medications at the first signs of onset. A self-limited mild vascular leak syndrome and fatigue were also noted. Efficacy was limited; one radiographic response was documented, and one patient had improvement in ascites. These studies again demonstrated the need for an effective means to suppress the host immune reaction to the therapeutic.
SS1P was next tested in patients with newly diagnosed malignant mesothelioma in combination with standard cisplatin and pemetrexed [59]. Toxicity of the combination in this phase I study was similar to that observed with the individual agents. No reduction from the established single-agent dose of SS1P was required for safe combination. Fatigue was the dose-limiting toxicity. Overall, 60% of patients (12 of 20) achieved a partial response, although the hematologic suppression caused by the chemotherapy failed to delay development of neutralizing antidrug antibodies. This compares favorably to the response rate (41.3%) reported for cisplatin and pemetrexed alone in this patient population [60]. Randomized studies would be required to determine whether addition of a single cycle of SS1P to standard chemotherapy improves patient outcomes.
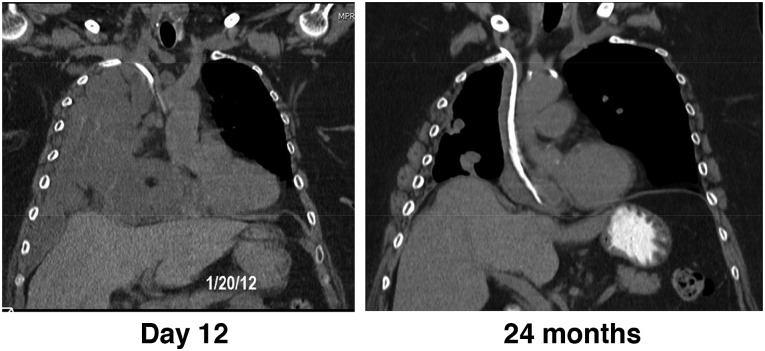
Most recently, SS1P was tested in a pilot study in combination with the pentostatin plus cyclophosphamide immunosuppressive regimen [3]. As described above, lymphocyte depletion allowed patients to receive more than one cycle of SS1P before development of neutralizing antibodies. In this study, 3 of 10 patients with advanced, treatment-refractory mesothelioma experienced major responses that persisted for >18 months (Fig. 3). Interestingly, one of these patients experienced a delayed response that was first evident months after treatment. This was preceded by a dramatic increase in fluorodeoxyglucose uptake on positron emission tomography scan with no concordant tumor growth seen on simultaneous computed tomography, consistent with infiltration by activated immune cells. Moreover, several additional patients who had previously progressed on chemotherapy developed partial responses to the same chemotherapy when it was administered after the immunotoxin regimen. It is interesting to speculate that these observations may be the result of regimen activation of a previously tumor-tolerant immune system. Understanding the mechanism of these responses is an active area of investigation in our laboratory. This regimen has been advanced into phase II studies and is currently accruing patients with pleural and peritoneal mesothelioma. Phase II pancreatic and lung adenocarcinoma cohorts are also planned.
Figure 3.
Durable response with SS1P plus lymphocyte-depleting regimen. Coronal computed tomography images of a responding patient 12 days after initiation of therapy on an SS1P, pentostatin, and cyclophosphamide clinical trial with durable response continuing at 24 months, more than 16 months after the last treatment.
RG7787
This mesothelin-targeted immunotoxin is being developed collaboratively between our laboratory and Roche (Basel, Switzerland, http://www.roche.com) with the goal of engineering a molecule that can be given repeatedly to solid tumor patients without coadministration of immunosuppressive agents. It contains a humanized Fab version of the SS1 antibody and a newly developed PE fragment that has been modified to reduce immunogenicity. Fv RNA of B cells from study patients who had developed neutralizing antibodies following treatment with PE-based therapy were used to pan for antigens in the catalytic domain of PE by a phage-display assay. Reactive phages were then tested in competitive assays for binding to a series of mutated PE fragments bearing alanine substitutions at residues that contain large polar amino acids in the native protein [61]. These bulky residues were known to be important in B-cell recognition and generation of immune response in mice [62]. Using this strategy, six reactive epitopes were identified in the catalytic domain. We also identified seven alanine point mutations that could ablate these B-cell epitopes without destroying PE catalytic activity. Two additional epitopes were removed by deletion of the near entirety of noncatalytic domain II. This modification also improved the therapeutic window of the molecule because PE fragments bearing this modification do not cause vascular leak syndrome in animal models and thus can be given at much higher doses than previous-generation PE molecules, like SS1P. As a single agent, RG7787 shrinks tumors in xenograft models of MSLN-expressing lung [63], gastric, and triple-negative breast cancers [64]. In addition, combination with paclitaxel resulted in marked in vivo synergy and induced complete responses in a xenograft model of pancreatic cancer [65]. Clinical testing of RG7787 is scheduled to begin in early 2015.
As a single agent, RG7787 shrinks tumors in xenograft models of MSLN-expressing lung, gastric, and triple-negative breast cancers. In addition, combination with paclitaxel resulted in marked in vivo synergy and induced complete responses in a xenograft model of pancreatic cancer.
hSGZ-fibroblast growth factor receptor 14-kDa protein (Fn14) is a highly-inducible cell surface receptor for the tumor necrosis factor-related TWEAK ligand. It is frequently upregulated following injury and in disease states. Many solid tumor malignancies express Fn14 [66–68]. The hSGZ immunotoxin consists of a single-chain humanized anti-Fn14 Fab fused to recombinantly modified gelonin, a ribosome inhibiting plant toxin (Fig. 1) [69]. In mouse xenograft models of melanoma and in Her-2-positive breast cancer, hSGZ delayed tumor progression [69, 70]. No major toxicities were observed in these studies, although Fn14 is known to be expressed at low levels in several vital organs [71, 72]. Fn14 could prove to be an interesting target.
Oportuzumab Monatox by Intravesicular Administration
The immunotoxin oportuzumab monatox uses a single-chain anti-EpCAM antibody fused to PE. Intravesicular administration was tested in patients with urothelial carcinoma in situ who were intolerant or refractory to bacillus Calmette-Guérin. This delivery method limits systemic toxicity by delivering the immunotoxin directly to the site of the tumor and avoids issues with immunogenicity of the circulating molecule [73]. No dose-limiting toxicity was observed in a phase I study [74]. The immunotoxin was advanced into phase II testing in which 44% of patients achieved a complete response and 16% continued to maintain this response at 1 year [75]. Successful therapy in this setting has the potential to delay or spare these patients from cystectomy and the decreased quality of life that goes with it.
VB6-845
This is an anti-EpCAM immunotoxin intended for use in solid tumor malignancies. The backbone consists of deBouganin plant toxin fused to a humanized Fab. Preclinical studies have suggested that this recombinant toxin is significantly less immunogenic than other payloads. Studies in mice demonstrated efficacy against EpCAM-positive solid tumors. VB6-845 caused severe vascular leak syndrome in rats at high doses but appeared to be better tolerated in cynomolgus monkeys [76]. A phase I study was opened in 2007 but closed for nonmedical reasons.
Convection-Enhanced Drug Delivery to Central Nervous System Tumors
Local delivery of immunotoxins to malignant brain tumors has shown potential in early clinical trials. In these trials, convection-enhanced drug delivery (CED) is typically used. This technique uses interstitial microinfusion through surgically placed catheters to deliver immunotoxin to the tumor bed and surrounding affected tissue. Immunotoxins targeting tumor-associated antigens or surface proteins particularly enriched in the tumor versus the normal brain tissue are used. These targets have included transferrin receptor (found in Tf-CRM107) [77], IL-13 receptor (in cintredekin besudotox) [78], epidermal growth factor receptor (EGFR; in TP-38) [79], and the mutant EGFR variant EGFRvIII (in MR1-1). Toxicities observed with local treatment are generally neurologic and low grade. Promising responses, including a few complete responses in glioblastoma, have been seen in a minority of patients. Variability in effectiveness has been attributed to difficulty in consistently achieving effective catheter placement, resulting in variable immunotoxin delivery to the tumor site. In addition, heterogeneity of immunotoxin target expression in patient tumors may also play a role. A recent review has examined this literature in depth [80].
D2C7-(scdsFv)-PE38KDEL
The majority of glioblastomas multiforme overexpress EGFR and a constitutively active mutant form called variant III (EGFRvIII) [81]. D2C7 is a new antibody that reacts with both forms. A PE toxin payload is used. In orthotopic mouse models of glioblastoma, treatment with D2C7-(scdsFv)-PE38KDEL resulted in dramatic increases in survival time compared with previously developed immunotoxins that could bind only EGFR or EGFRvIII [82]. This immunotoxin is a promising candidate for clinical development using CED.
Conclusion
Immunotoxins are exciting therapeutics that are once again receiving attention as potential antineoplastic agents now that previous problems with toxicity and immunogenicity are being solved. Recent studies of immunotoxins have produced promising results in patients with relapsed and refractory malignancies. Immunotoxins have a unique mechanism of action and nonoverlapping toxicity compared with standard chemotherapies and could be ideal molecules for combination therapy.
Acknowledgment
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, and Center for Cancer Research.
Author Contributions
Conception/Design: Christine Alewine, Raffit Hassan, Ira Pastan
Manuscript writing: Christine Alewine, Raffit Hassan, Ira Pastan
Final approval of manuscript: Christine Alewine, Raffit Hassan, Ira Pastan
Disclosures
Ira Pastan: Roche Pharmaceuticals (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Antignani A, Fitzgerald D. Immunotoxins: The role of the toxin. Toxins (Basel) 2013;5:1486–1502. doi: 10.3390/toxins5081486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreitman RJ, Tallman MS, Robak T, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30:1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassan R, Miller AC, Sharon E, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5:208ra147. doi: 10.1126/scitranslmed.3006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier RJ. Effect of diphtheria toxin on protein synthesis: Inactivation of one of the transfer factors. J Mol Biol. 1967;25:83–98. doi: 10.1016/0022-2836(67)90280-x. [DOI] [PubMed] [Google Scholar]
- 5.Weldon JE, Pastan I. A guide to taming a toxin—recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278:4683–4700. doi: 10.1111/j.1742-4658.2011.08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stirpe F, Olsnes S, Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980;255:6947–6953. [PubMed] [Google Scholar]
- 7.Walsh MJ, Dodd JE, Hautbergue GM. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence. 2013;4:774–784. doi: 10.4161/viru.26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 10.Pai-Scherf LH, Villa J, Pearson D, et al. Hepatotoxicity in cancer patients receiving erb-38, a recombinant immunotoxin that targets the erbB2 receptor. Clin Cancer Res. 1999;5:2311–2315. [PubMed] [Google Scholar]
- 11.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 12.Davies AJ, Rohatiner AZ, Howell S, et al. Tositumomab and iodine I 131 tositumomab for recurrent indolent and transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2004;22:1469–1479. doi: 10.1200/JCO.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 13.Pouget JP, Navarro-Teulon I, Bardiès M, et al. Clinical radioimmunotherapy—the role of radiobiology. Nat Rev Clin Oncol. 2011;8:720–734. doi: 10.1038/nrclinonc.2011.160. [DOI] [PubMed] [Google Scholar]
- 14.Blythman HE, Casellas P, Gros O, et al. Immunotoxins: Hybrid molecules of monoclonal antibodies and a toxin subunit specifically kill tumour cells. Nature. 1981;290:145–146. doi: 10.1038/290145a0. [DOI] [PubMed] [Google Scholar]
- 15.Spitler LE, del Rio M, Khentigan A, et al. Therapy of patients with malignant melanoma using a monoclonal antimelanoma antibody-ricin A chain immunotoxin. Cancer Res. 1987;47:1717–1723. [PubMed] [Google Scholar]
- 16.Weiner LM, O’Dwyer J, Kitson J, et al. Phase I evaluation of an anti-breast carcinoma monoclonal antibody 260F9-recombinant ricin A chain immunoconjugate. Cancer Res. 1989;49:4062–4067. [PubMed] [Google Scholar]
- 17.Kuan CT, Pai LH, Pastan I. Immunotoxins containing Pseudomonas exotoxin that target LeY damage human endothelial cells in an antibody-specific mode: Relevance to vascular leak syndrome. Clin Cancer Res. 1995;1:1589–1594. [PubMed] [Google Scholar]
- 18.Baluna R, Rizo J, Gordon BE, et al. Evidence for a structural motif in toxins and interleukin-2 that may be responsible for binding to endothelial cells and initiating vascular leak syndrome. Proc Natl Acad Sci USA. 1999;96:3957–3962. doi: 10.1073/pnas.96.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smallshaw JE, Ghetie V, Rizo J, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol. 2003;21:387–391. doi: 10.1038/nbt800. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Song S, Kou G, et al. Treatment of hepatocellular carcinoma in a mouse xenograft model with an immunotoxin which is engineered to eliminate vascular leak syndrome. Cancer Immunol Immunother. 2007;56:1775–1783. doi: 10.1007/s00262-007-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weldon JE, Xiang L, Zhang J, et al. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol Cancer Ther. 2013;12:48–57. doi: 10.1158/1535-7163.MCT-12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LoRusso PM, Lomen PL, Redman BG, et al. Phase I study of monoclonal antibody-ricin A chain immunoconjugate Xomazyme-791 in patients with metastatic colon cancer. Am J Clin Oncol. 1995;18:307–312. doi: 10.1097/00000421-199508000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Lynch TJ, Jr, Lambert JM, Coral F, et al. Immunotoxin therapy of small-cell lung cancer: A phase I study of N901-blocked ricin. J Clin Oncol. 1997;15:723–734. doi: 10.1200/JCO.1997.15.2.723. [DOI] [PubMed] [Google Scholar]
- 24.Fidias P, Grossbard M, Lynch TJ., Jr A phase II study of the immunotoxin N901-blocked ricin in small-cell lung cancer. Clin Lung Cancer. 2002;3:219–222. doi: 10.3816/clc.2002.n.006. [DOI] [PubMed] [Google Scholar]
- 25.Avarbock AB, Loren AW, Park JY, et al. Lethal vascular leak syndrome after denileukin diftitox administration to a patient with cutaneous gamma/delta T-cell lymphoma and occult cirrhosis. Am J Hematol. 2008;83:593–595. doi: 10.1002/ajh.21180. [DOI] [PubMed] [Google Scholar]
- 26.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–5149. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 27.Kreitman RJ, Singh R, Stetler-Stevenson M, et al. Regression of adult T-cell leukemia with anti-CD25 recombinant immunotoxin LMB-2 preceded by chemotherapy. Blood. 2011;118:2575a. [Google Scholar]
- 28.Schindler J, Sausville E, Messmann R, et al. The toxicity of deglycosylated ricin A chain-containing immunotoxins in patients with non-Hodgkin’s lymphoma is exacerbated by prior radiotherapy: A retrospective analysis of patients in five clinical trials. Clin Cancer Res. 2001;7:255–258. [PubMed] [Google Scholar]
- 29.Siegall CB, Liggitt D, Chace D, et al. Prevention of immunotoxin-mediated vascular leak syndrome in rats with retention of antitumor activity. Proc Natl Acad Sci USA. 1994;91:9514–9518. doi: 10.1073/pnas.91.20.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foss FM, Bacha P, Osann KE, et al. Biological correlates of acute hypersensitivity events with DAB(389)IL-2 (denileukin diftitox, ONTAK) in cutaneous T-cell lymphoma: Decreased frequency and severity with steroid premedication. Clin Lymphoma. 2001;1:298–302. doi: 10.3816/clm.2001.n.005. [DOI] [PubMed] [Google Scholar]
- 31.Oratz R, Speyer JL, Wernz JC, et al. Antimelanoma monoclonal antibody-ricin A chain immunoconjugate (XMMME-001-RTA) plus cyclophosphamide in the treatment of metastatic malignant melanoma: Results of a phase II trial. J Biol Response Mod. 1990;9:345–354. [PubMed] [Google Scholar]
- 32.Selvaggi K, Saria EA, Schwartz R, et al. Phase I/II study of murine monoclonal antibody-ricin A chain (XOMAZYME-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J Immunother Emphasis Tumor Immunol. 1993;13:201–207. doi: 10.1097/00002371-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Hassan R, Williams-Gould J, Watson T, et al. Pretreatment with rituximab does not inhibit the human immune response against the immunogenic protein LMB-1. Clin Cancer Res. 2004;10:16–18. doi: 10.1158/1078-0432.ccr-1160-3. [DOI] [PubMed] [Google Scholar]
- 34.Mariotti J, Taylor J, Massey PR, et al. The pentostatin plus cyclophosphamide nonmyeloablative regimen induces durable host T cell functional deficits and prevents murine marrow allograft rejection. Biol Blood Marrow Transplant. 2011;17:620–631. doi: 10.1016/j.bbmt.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mossoba ME, Onda M, Taylor J, et al. Pentostatin plus cyclophosphamide safely and effectively prevents immunotoxin immunogenicity in murine hosts. Clin Cancer Res. 2011;17:3697–3705. doi: 10.1158/1078-0432.CCR-11-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen E, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 2001;19:376–388. doi: 10.1200/JCO.2001.19.2.376. [DOI] [PubMed] [Google Scholar]
- 37.Prince HM, Duvic M, Martin A, et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:1870–1877. doi: 10.1200/JCO.2009.26.2386. [DOI] [PubMed] [Google Scholar]
- 38.McCann S, Akilov OE, Geskin L. Adverse effects of denileukin diftitox and their management in patients with cutaneous T-cell lymphoma. Clin J Oncol Nurs. 2012;16:E164–E172. doi: 10.1188/12.CJON.E164-E172. [DOI] [PubMed] [Google Scholar]
- 39.Kreitman RJ, Wilson WH, White JD, et al. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18:1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 40.Kreitman RJ, Wilson WH, Robbins D, et al. Responses in refractory hairy cell leukemia to a recombinant immunotoxin. Blood. 1999;94:3340–3348. [PubMed] [Google Scholar]
- 41.Wayne AS, Bhojwani D, Silverman LB, et al. A novel anti-CD22 immunotoxin, moxetumomab pasudotox: Phase I study in pediatric acute lymphoblastic leukemia (ALL) Blood. 2011;118:248a. doi: 10.1182/blood-2017-02-749101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dave VP. Role of CD3ε-mediated signaling in T-cell development and function. Crit Rev Immunol. 2011;31:73–84. doi: 10.1615/critrevimmunol.v31.i1.70. [DOI] [PubMed] [Google Scholar]
- 43.Frankel AE. Anti-CD3 immunotoxin to induce remissions in cutaneous T-cell lymphoma patients. J Clin Oncol. 2012;30(suppl):2505a. [Google Scholar]
- 44.Vallera DA, Chen H, Sicheneder AR, et al. Genetic alteration of a bispecific ligand-directed toxin targeting human CD19 and CD22 receptors resulting in improved efficacy against systemic B cell malignancy. Leuk Res. 2009;33:1233–1242. doi: 10.1016/j.leukres.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernández-Caselles T, Martínez-Esparza M, Pérez-Oliva AB, et al. A study of CD33 (SIGLEC-3) antigen expression and function on activated human T and NK cells: Two isoforms of CD33 are generated by alternative splicing. J Leukoc Biol. 2006;79:46–58. doi: 10.1189/jlb.0205096. [DOI] [PubMed] [Google Scholar]
- 46.Drexler HG. Classification of acute myeloid leukemias—a comparison of FAB and immunophenotyping. Leukemia. 1987;1:697–705. [PubMed] [Google Scholar]
- 47.Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol. 2000;20:2902–2906. doi: 10.1128/mcb.20.8.2902-2906.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang K, Pai LH, Pass H, et al. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol. 1992;16:259–268. doi: 10.1097/00000478-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: Identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res. 2001;7:3862–3868. [PubMed] [Google Scholar]
- 50.Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–845. [PubMed] [Google Scholar]
- 51.Hassan R, Kreitman RJ, Pastan I, et al. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- 52.Einama T, Homma S, Kamachi H, et al. Luminal membrane expression of mesothelin is a prominent poor prognostic factor for gastric cancer. Br J Cancer. 2012;107:137–142. doi: 10.1038/bjc.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baba K, Ishigami S, Arigami T, et al. Mesothelin expression correlates with prolonged patient survival in gastric cancer. J Surg Oncol. 2012;105:195–199. doi: 10.1002/jso.22024. [DOI] [PubMed] [Google Scholar]
- 54.Ordóñez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol. 2003;27:1418–1428. doi: 10.1097/00000478-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Ho M, Bera TK, Willingham MC, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13:1571–1575. doi: 10.1158/1078-0432.CCR-06-2161. [DOI] [PubMed] [Google Scholar]
- 56.Chang K, Pastan I, Willingham MC. Frequent expression of the tumor antigen CAK1 in squamous-cell carcinomas. Int J Cancer. 1992;51:548–554. doi: 10.1002/ijc.2910510408. [DOI] [PubMed] [Google Scholar]
- 57.Tchou J, Wang LC, Selven B, et al. Mesothelin, a novel immunotherapy target for triple negative breast cancer. Breast Cancer Res Treat. 2012;133:799–804. doi: 10.1007/s10549-012-2018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kreitman RJ, Hassan R, Fitzgerald DJ, et al. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer. 2014;120:3311–3319. doi: 10.1002/cncr.28875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 61.Liu W, Onda M, Lee B, et al. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc Natl Acad Sci USA. 2012;109:11782–11787. doi: 10.1073/pnas.1209292109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Onda M, Beers R, Xiang L, et al. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA. 2008;105:11311–11316. doi: 10.1073/pnas.0804851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niederfellner G, Bauss F, Imhof-Jung S et al. RG7787 - a novel de-immunized PE based fusion protein for therapy of mesothelin-positive solid tumors [abstract 4510]. Presented at: American Association for Cancer Research annual meeting; April 5–9, 2014; San Diego, CA. [Google Scholar]
- 64.Alewine C, Xiang L, Yamori T, et al. Efficacy of RG7787, a next generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol Cancer Ther. 2014;13:2653–2661. doi: 10.1158/1535-7163.MCT-14-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollevoet K, Mason-Osann E, Liu XF, et al. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther. 2014;13:2040–2049. doi: 10.1158/1535-7163.MCT-14-0089-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Culp PA, Choi D, Zhang Y, et al. Antibodies to TWEAK receptor inhibit human tumor growth through dual mechanisms. Clin Cancer Res. 2010;16:497–508. doi: 10.1158/1078-0432.CCR-09-1929. [DOI] [PubMed] [Google Scholar]
- 67.Willis AL, Tran NL, Chatigny JM, et al. The fibroblast growth factor-inducible 14 receptor is highly expressed in HER2-positive breast tumors and regulates breast cancer cell invasive capacity. Mol Cancer Res. 2008;6:725–734. doi: 10.1158/1541-7786.MCR-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H, Marks JW, Hittelman WN, et al. Development and characterization of a potent immunoconjugate targeting the Fn14 receptor on solid tumor cells. Mol Cancer Ther. 2011;10:1276–1288. doi: 10.1158/1535-7163.MCT-11-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou H, Ekmekcioglu S, Marks JW, et al. The TWEAK receptor Fn14 is a therapeutic target in melanoma: Immunotoxins targeting Fn14 receptor for malignant melanoma treatment. J Invest Dermatol. 2013;133:1052–1062. doi: 10.1038/jid.2012.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou H, Hittelman WN, Yagita H, et al. Antitumor activity of a humanized, bivalent immunotoxin targeting fn14-positive solid tumors. Cancer Res. 2013;73:4439–4450. doi: 10.1158/0008-5472.CAN-13-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng SL, Guo Y, Factor VM, et al. The Fn14 immediate-early response gene is induced during liver regeneration and highly expressed in both human and murine hepatocellular carcinomas. Am J Pathol. 2000;156:1253–1261. doi: 10.1016/S0002-9440(10)64996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desplat-Jégo S, Varriale S, Creidy R, et al. TWEAK is expressed by glial cells, induces astrocyte proliferation and increases EAE severity. J Neuroimmunol. 2002;133:116–123. doi: 10.1016/s0165-5728(02)00368-5. [DOI] [PubMed] [Google Scholar]
- 73.Goldberg MR, Heimbrook DC, Russo P, et al. Phase I clinical study of the recombinant oncotoxin TP40 in superficial bladder cancer. Clin Cancer Res. 1995;1:57–61. [PubMed] [Google Scholar]
- 74.Kowalski M, Entwistle J, Cizeau J, et al. A phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCGrefractory and BCG-intolerant patients. Drug Des Devel Ther. 2010;4:313–320. doi: 10.2147/DDDT.S14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kowalski M, Guindon J, Brazas L, et al. A phase II study of oportuzumab monatox: An immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guérin. J Urol. 2012;188:1712–1718. doi: 10.1016/j.juro.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 76.Entwistle J, Brown JG, Chooniedass S, et al. Preclinical evaluation of VB6-845: An anti-EpCAM immunotoxin with reduced immunogenic potential. Cancer Biother Radiopharm. 2012;27:582–592. doi: 10.1089/cbr.2012.1200.271. [DOI] [PubMed] [Google Scholar]
- 77.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 78.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sampson JH, Akabani G, Archer GE, et al. Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10:320–329. doi: 10.1215/15228517-2008-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chandramohan V, Sampson JH, Pastan I, et al. Toxin-based targeted therapy for malignant brain tumors. Clin Dev Immunol. 2012;2012:480429. doi: 10.1155/2012/480429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chandramohan V, Bao X, Keir ST, et al. Construction of an immunotoxin, D2C7-(scdsFv)-PE38KDEL, targeting EGFRwt and EGFRvIII for brain tumor therapy. Clin Cancer Res. 2013;19:4717–4727. doi: 10.1158/1078-0432.CCR-12-3891. [DOI] [PMC free article] [PubMed] [Google Scholar]