Abstract
Background.
The approved capecitabine regimen as monotherapy in metastatic breast cancer (MBC) is 1,250 mg/m2 twice daily for 2 weeks on and 1 week off (Cint). Dose modifications are often required because of severe hand-foot syndrome (HFS). We tested a continuous regimen with a lower daily dose but a similar cumulative dose in an attempt to reduce the severity of adverse events (AEs) while maintaining efficacy.
Methods.
We randomized 195 patients with HER-2/neu-negative MBC to capecitabine 800 mg/m2 twice daily throughout the 21-day cycle (Ccont) or to Cint to assess noninferiority in the percentage of patients free of progression at 1 year. Secondary endpoints included efficacy and safety. Associations between polymorphisms in capecitabine metabolism-related genes and drug response were assessed.
Results.
The percentage of patients free of progression at 1 year was 27.3% with Cint versus 25.3% with Ccont (difference of −2.0%; 95% confidence interval: −15.5% to 11.5%, exceeding the 15% deemed noninferior). Differences regarding other efficacy variables were also not found. Grade 3–4 HFS was the most frequent AE (41.1% in Cint vs. 42.3% in Ccont). Grade 3–4 neutropenia, thrombocytopenia, diarrhea, and stomatitis were more frequent with Cint. A 5′ untranslated region polymorphism in the carboxylesterase 2 gene was associated with HFS. One polymorphism in cytidine deaminase and two in thymidine phosphorylase were associated with survival.
Conclusion.
Our study was unable to show noninferiority with the continuous capecitabine regimen (Ccont) compared with the approved intermittent regimen (Cint). Further investigation is required to improve HFS. Polymorphisms in several genes might contribute to interindividual differences in response to capecitabine.
Author Summary
Discussion
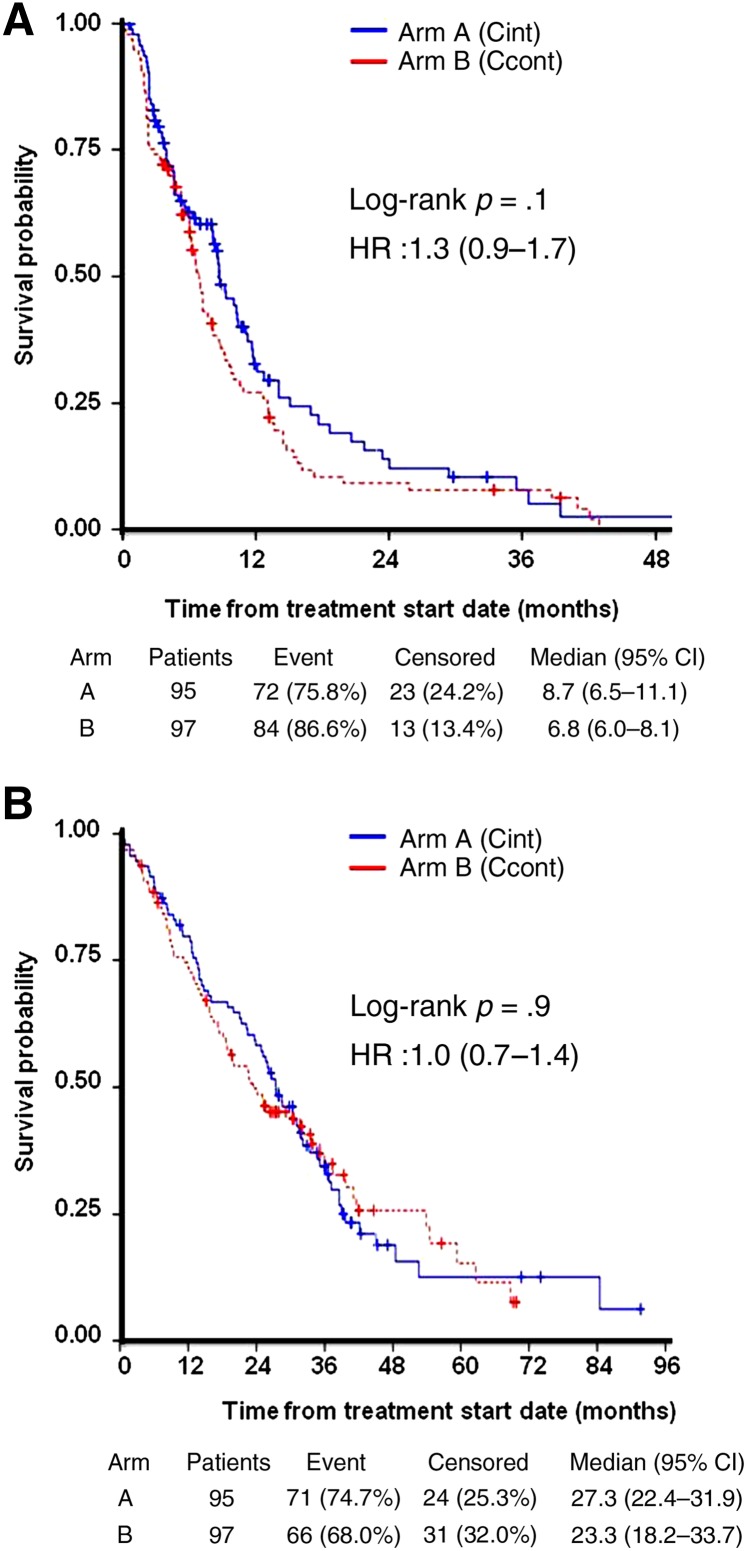
In this patient population (Table 1), continuous, lower daily doses of capecitabine were not shown to be noninferior in efficacy to the standard schedule despite maintaining the same cumulative dose and dose intensity (Fig. 1). The percentage of patients free of progression at 1 year were 27.3% with 1,250 mg/m2 twice daily for 2 weeks on and 1 week off versus 25.3% with 800 mg/m2 twice daily throughout the 21-day cycle (difference of −2.0%; 95% confidence interval: −15.5 to 11.5%), meaning that the margin deemed noninferior by the study design (15%) was exceeded. Median progression-free survival (PFS) and overall survival (OS) were numerically superior (although nonsignificant) with the approved intermittent administration schedule. Hand-foot syndrome (HFS) was not different between arms.
Table 1.
Baseline patient and tumor characteristics
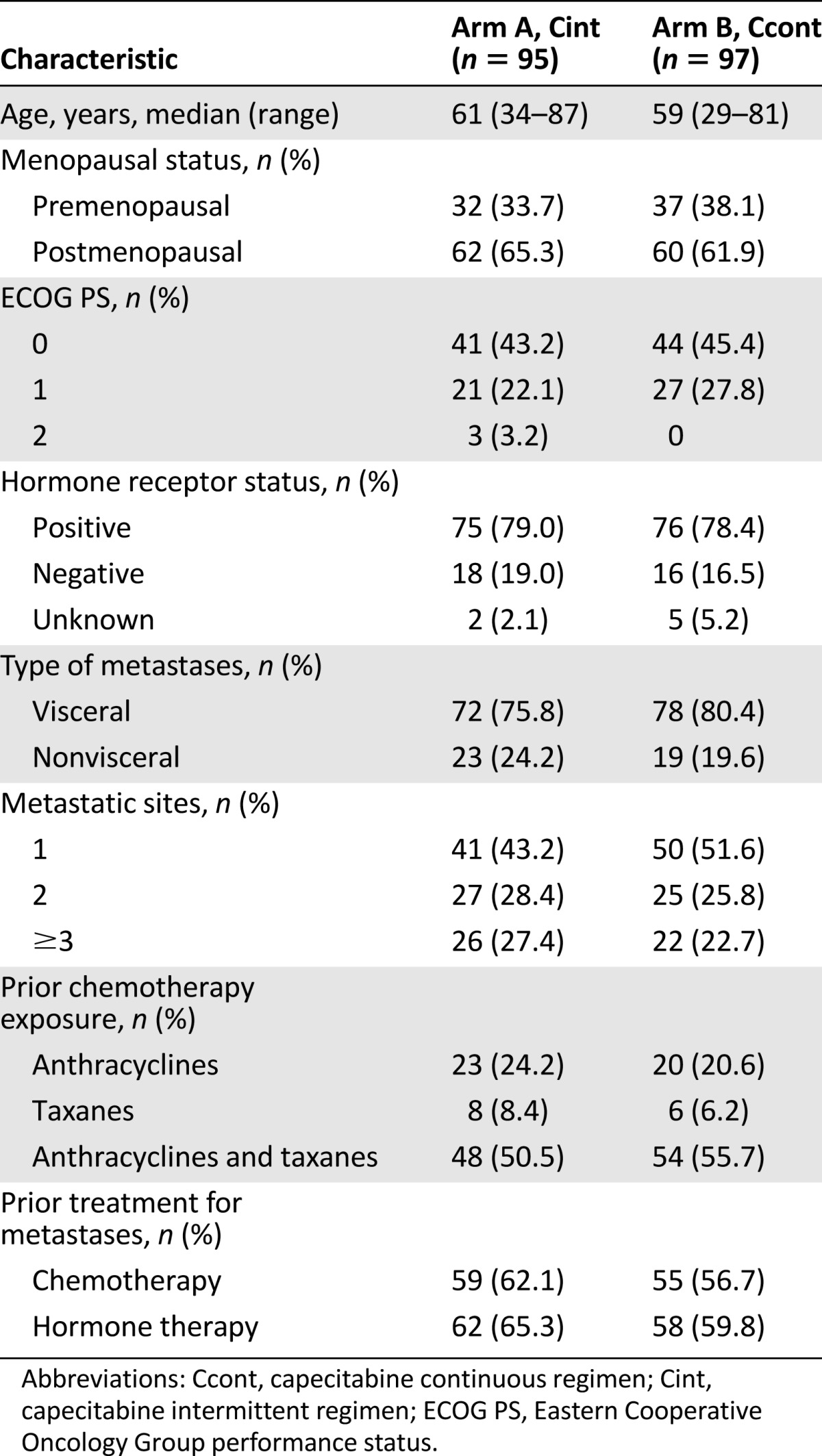
Figure 1.
Kaplan-Meier analysis of time to progression (A) and overall survival (B) in the intention-to-treat population.
Abbreviations: Ccont, capecitabine continuous doses; CI, confidence interval; Cint, capecitabine intermittent doses; HR, hazard ratio.
The greater incidence of severe adverse events (AEs) resulted in a larger percentage of patients requiring dose reductions with the approved intermittent regimen (67.4%) compared with the experimental continuous administration regimen (52.6%); however, patients in both arms received similar dose intensity. Stockler et al. [1] compared the classical cyclophosphamide, methotrexate, and fluorouracil (CMF) regimen with two different capecitabine schedules: an intermittent regimen with lower doses (1,000 mg/m2 twice daily for 2 weeks on and 1 week off) and continuous administration of very low doses (650 mg/m2 twice daily). Both capecitabine regimens showed similar PFS (6 months), response rates, and OS and achieved improved OS versus CMF. Despite the greater frequency of severe AEs, the rate of dose reduction and median duration of treatment were equivalent for the two arms. These data disagree with our study results, perhaps because of the use of a higher dose of capecitabine in our trial or the different criteria used to assess disease progression (strict Response Evaluation Criteria in Solid Tumors in ours vs. the need for palliative radiation or change in chemotherapy in the trial by Stockler et al. [1]).We found an association of HFS intensity and rs11075646 polymorphism in CES2. Ribelles et al. [2] previously described an association of the G allele of rs11075646 with capecitabine efficacy but not with HFS. We also found one polymorphism in CDD (rs2072671) and two in TP (rs11479, rs470119) associated with survival.
In conclusion, our study suggests that the schedule of capecitabine used in the treatment of MBC matters. The role of polymorphism in some genes involved in the metabolism of capecitabine should be further elucidated.
Supplementary Material
Acknowledgments
We thank all participating patients, clinicians, and local research staff and Pivotal staff for the collection, review, and analysis of the data. Genotyping was provided by Centro Nacional de Genotipado - Instituto de Salud Carlos III (CeGen-ISCIII; http://www.cegen.org). Thanks to Roger Milne from Centro Nacional de Investigaciones Oncológicas for statistical support. We also thank F. Hoffman-La Roche Ltd. for funding this study and Patricia Ortega, funded by F. Hoffmann-La Roche Ltd., for her contribution to this manuscript as medical writer.
Footnotes
Access the full results at: Martin-14-379.theoncologist.com
ClinicalTrials.gov Identifier: NCT00418028
Sponsor(s): Miguel Martin
Principal Investigator: Miguel Martín
IRB Approved: Yes
Author disclosures and references available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.