Abstract
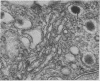
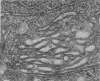
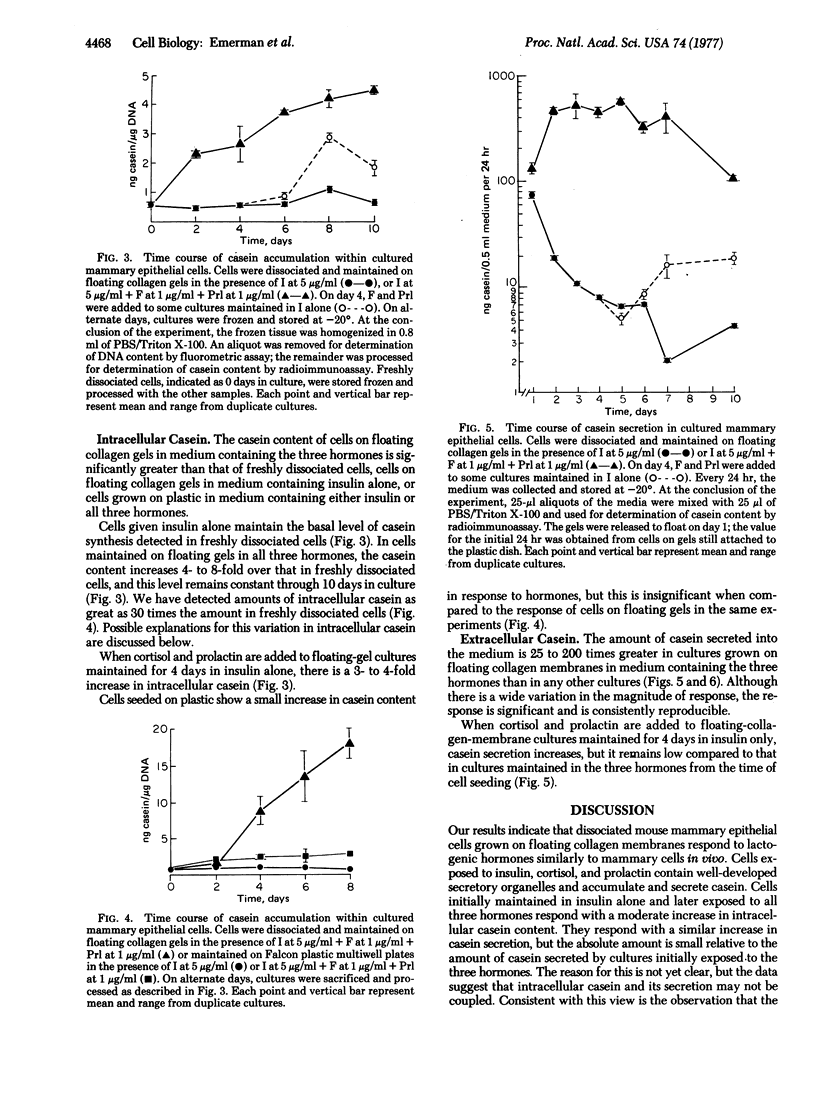
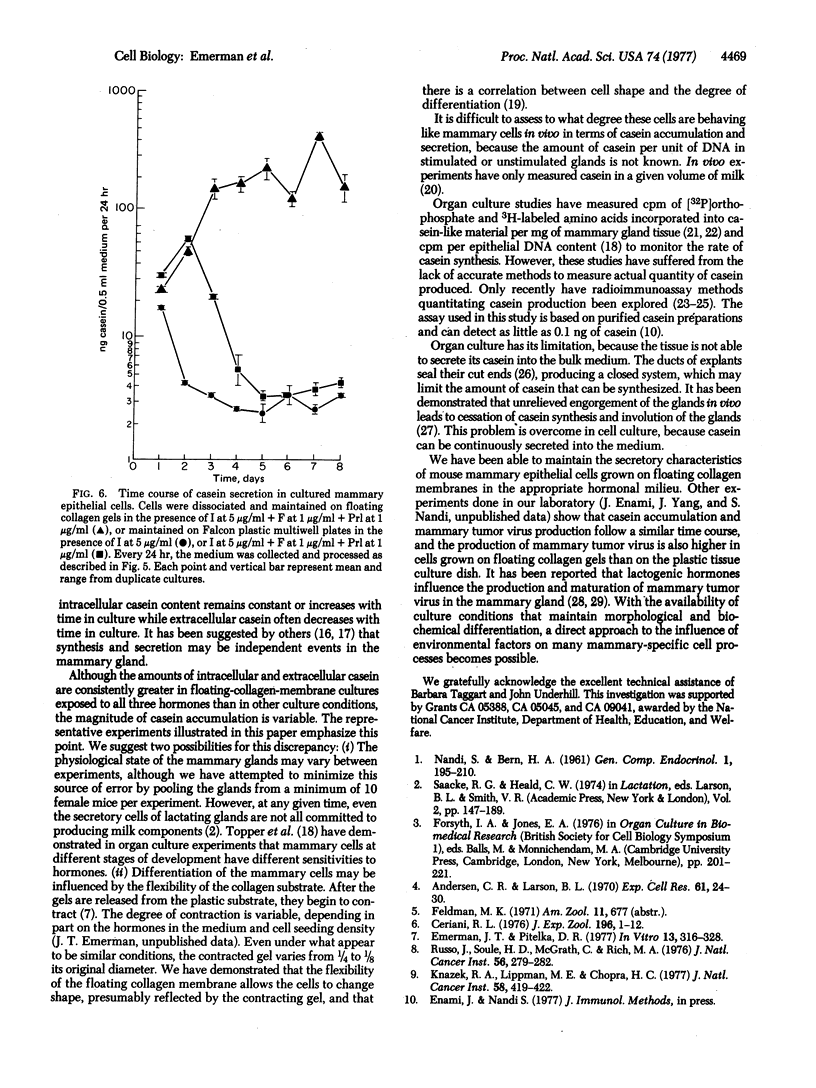
Cultured on floating collagen membranes in the presence of lactogenic hormones, dissociated normal mammary epithelial cells from prelactating mice acquire the ultrastructural and biochemical characteristics of differentiated mammary secretory cells in vivo. The cells on floating collagen membranes in medium containing insulin alone have sparse secretory organelles, and a small amount of casein can be detected in these cells with a sensitive radioimmunoassay. These cells resemble counterpart cells in early-pregnant mice. When the cells are exposed to insulin, cortisol, and prolactin, the secretory apparatus is elaborated and significant increases in intracellular and extracellular casein are observed. In this environment, the intracellular casein content is generally four to eight times greater than in freshly dissociated cells or cells cultured in insulin alone. The amount of casein secreted into the medium by floating-collagen-membrane cultures in the three hormones is from 25 to 200 times greater than that secreted by cultures in insulin alone. Cells cultured on plastic substrates in either hormone combination fail to show any increase in intracellular or extracellular casein. On floating collagen membranes, the cells differentiate in response to hormones as they do in vivo and in organ culture. This cell-culture system provides an opportunity to study direct effects of environmental factors on mammary differentiation at the cellular level.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen C. R., Larson B. L. Comparative maintenance of function in dispersed cell and organ cultures of bovine mammary tissue. Exp Cell Res. 1970 Jul;61(1):24–30. doi: 10.1016/0014-4827(70)90253-3. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D. Quantitation of mouse mammary tumor virus (MTV) virions by radioimmunoassay. J Immunol. 1973 Dec;111(6):1722–1729. [PubMed] [Google Scholar]
- Ceriani R. L. Hormone induction of specific protein synthesis in midpregnant mouse mammary cell culture. J Exp Zool. 1976 Apr;196(1):1–12. doi: 10.1002/jez.1401960102. [DOI] [PubMed] [Google Scholar]
- Chatterton R. T., Harris J. A., Wynn R. M. Lactogenesis in the rat: an ultrastructural study of the initiation of the secretory process. J Reprod Fertil. 1975 Jun;43(3):479–484. doi: 10.1530/jrf.0.0430479. [DOI] [PubMed] [Google Scholar]
- Delouis C., Denamur R. Induction of lactose synthesis by prolactin in rabbit mammary gland extracts. J Endocrinol. 1972 Feb;52(2):311–319. doi: 10.1677/joe.0.0520311. [DOI] [PubMed] [Google Scholar]
- Emerman J. T., Pitelka D. R. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 1977 May;13(5):316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L. Human alpha-lactalbumin: measurement in serum and in breast cancer organ cultures by radioimmunoassay. Science. 1975 Oct 17;190(4211):276–278. doi: 10.1126/science.1179206. [DOI] [PubMed] [Google Scholar]
- Knazek R. A., Lippmann M. E., Chopra H. C. Formation of solid human mammary carcinoma in vitro. J Natl Cancer Inst. 1977 Feb;58(2):419–422. doi: 10.1093/jnci/58.2.419. [DOI] [PubMed] [Google Scholar]
- McGrath C. M., Blair P. B. Immunofluorescent localization of mammary tumor virus antigens in mammary tumor cells in culture. Cancer Res. 1970 Jul;30(7):1963–1968. [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- NANDI S., BERN H. A. The hormones responsible for lactogenesis in BALB/cCrgl mice. Gen Comp Endocrinol. 1961 Sep;1:195–210. doi: 10.1016/0016-6480(61)90029-6. [DOI] [PubMed] [Google Scholar]
- Pickett P. B., Pitelka D. R., Hamamoto S. T., Misfeldt D. S. Occluding junctions and cell behavior in primary cultures of normal and neoplastic mammary gland cells. J Cell Biol. 1975 Aug;66(2):316–332. doi: 10.1083/jcb.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose H. N., McGrath C. M. Alpha-lactalbumin production in human mammary carcinoma. Science. 1975 Nov 14;190(4215):673–675. doi: 10.1126/science.1188362. [DOI] [PubMed] [Google Scholar]
- Russo J., Soule H. D., McGrath C., Rich M. A. Reexpression of the original tumor pattern by a human breast carcinoma cell line (MCF-7) in sponge culture. J Natl Cancer Inst. 1976 Feb;56(2):279–282. doi: 10.1093/jnci/56.2.279. [DOI] [PubMed] [Google Scholar]
- Terry P. M., Ball E. M., Ganguly R., Banerjee M. R. An indirect radioimmunoassay for mouse casein using 125I-labeled antigen. J Immunol Methods. 1975 Dec;9(2):123–134. doi: 10.1016/0022-1759(75)90102-7. [DOI] [PubMed] [Google Scholar]
- Topper Y. J., Oka T., Owens I. S., Vonderhaar B. K. Some aspects of mouse mammary gland development from maturity to early pregnancy. In Vitro. 1972 Nov-Dec;8(3):228–236. doi: 10.1007/BF02619503. [DOI] [PubMed] [Google Scholar]
- WELLINGS S. R., DEOME K. B. Electron microscopy of milk secretion in the mammary gland of the C3H/Crgl mouse. III. Cytomorphology of the involuting gland. J Natl Cancer Inst. 1963 Feb;30:241–267. [PubMed] [Google Scholar]