A systematic review was performed to evaluate the antitumor effects and toxicity of ascorbate treatment. There is no high-quality evidence to suggest that ascorbate supplementation in cancer patients either enhances the antitumor effects of chemotherapy or reduces its toxicity. Given the high financial and time costs to patients of this treatment, high-quality placebo-controlled trials are needed.
Keywords: Vitamin C, Ascorbate, Cancer, Chemotherapy, Quality of life
Abstract
Background.
Many cancer patients receive supplemental ascorbate (vitamin C) in the belief that it synergizes the anticancer effects of chemotherapy and reduces its toxicity.
Methods.
A systematic review was performed to evaluate the antitumor effects and toxicity of ascorbate treatment. Medline (1946 to March 2014), EMBASE (1947 to March 2014), and the Cochrane central register (1993 to March 2014) were searched for randomized and observational studies.
Results.
Of 696 identified records, 61 full-text articles were screened and 34 were included. In total, 5 randomized controlled trials (RCTs) (n = 322), 12 phase I/II trials (n = 287), 6 observational studies (n = 7,599), and 11 case reports (n = 267) were identified. Because of study heterogeneity, no meta-analyses were performed. No RCTs reported any statistically significant improvements in overall or progression-free survival or reduced toxicity with ascorbate relative to control arm. Evidence for ascorbate’s antitumor effects was limited to case reports and observational and uncontrolled studies.
Conclusion.
There is no high-quality evidence to suggest that ascorbate supplementation in cancer patients either enhances the antitumor effects of chemotherapy or reduces its toxicity. Given the high financial and time costs to patients of this treatment, high-quality placebo-controlled trials are needed.
Implications for Practice:
Many cancer patients receive ascorbate (vitamin C) in conjunction with chemotherapy. There is no high-quality evidence to suggest that ascorbate either enhances the antitumor effects of chemotherapy or reduces its toxicity. Given the high financial and time costs of ascorbate, patients should be made aware of the paucity of data. Until high-quality placebo-controlled trials are completed, the use of ascorbate cannot be recommended.
Introduction
Since the 1950s, ascorbate (vitamin C) has been proposed to have anticancer effects [1]. Although epidemiological evidence suggested that ingestion of ascorbate-rich foods might have an association with reduced cancer incidence [2–5], this was not confirmed in randomized intervention trials [6–8]. As a cancer treatment, ascorbate also has a convoluted history. In 1974, Cameron and Campbell [9] treated patients with a variety of advanced cancers with “high” (10 g per day) doses of oral and i.v. ascorbate. Several responses were observed, and subsequently two case series of cancer patients were evaluated. The data suggested a possible survival benefit when advanced cancer patients’ treatment was supplemented with oral and i.v. ascorbate [10, 11]. One of the authors of these two series was Linus Pauling, a double Noble Prize winner who is regarded by some as one of the most influential scientists of the 20th century. Despite this, the published data were retrospective and were gathered in an uncontrolled, open label study. Two subsequent placebo-controlled trials using the same dose of oral ascorbate therapy were both negative [12, 13], and interest in the use of ascorbate in cancer patients declined. Subsequent preclinical and clinical studies regenerated interest in ascorbate’s potential role in both enhancing the anticancer effect of chemotherapy and reducing chemotherapy-induced side effects [14]. The biological rationale has been that high plasma concentrations of ascorbate can only be achieved with i.v. administration, and hence this route has been increasingly used [15, 16].
Many cancer patients currently receive supplemental oral and i.v. ascorbate in the belief that it synergizes the anticancer effects of chemotherapy and reduces its toxicity [17]. However, ascorbate is associated with significant costs to the patient, both financially (in the U.S. in 2007 sales of vitamin C alone reached $884 million [18, 19]) and in terms of time commitment to receive repeated infusions, as well as potential toxicity. This systematic review was performed to explore whether there is any evidence to confirm or refute a role for ascorbate treatment in the management of cancer patients.
Materials and Methods
Study Question and Inclusion Criteria
The study protocol was developed a priori among the coauthors. The systematic review was designed to summarize available information addressing the following research question framed in the population, intervention, comparator, outcome, and study design framework: “Based on randomized and observational data, does administration of oral or IV ascorbate in cancer patients demonstrate anti-tumour response or quality-of-life improvement compared to treatment with chemotherapy/standard therapy alone, placebo or as a standalone treatment?” The population of interest was humans with a current diagnosis of cancer of any type and stage. The intervention of interest was treatment with ascorbate (any dose and any route of administration i.e., oral, i.v., or both). Uncontrolled studies or controlled studies involving comparisons against no treatment, placebo, or other standard of care therapies were of interest. Outcomes of interest included overall survival, progression-free survival, validated measures of quality of life, validated markers of tumor response (e.g., Ki-67 proliferative index, tumor markers), and toxicity. Randomized controlled trials (RCTs) were of primary interest; however, as we anticipated a paucity of such data, all study designs were included in the initial search.
Literature Search
An experienced information specialist (R.S.) designed the electronic literature search to identify relevant evidence. Ovid Medline (1946 to March 2014), EMBASE (1947 to March 2014), and the Cochrane central register of controlled trials (1993 to March 2014) were searched to seek relevant citations. In addition, the reference lists of related and retrieved papers were reviewed to find additional relevant citations. The full literature search strategy for Medline is provided in supplemental online Appendix 1.
Study Screening and Selection
Two reviewers (C.J., M.C.) screened all citations that were retrieved from the literature search independently. The initial review consisted of screening titles and abstracts only, whereas the second step consisted of reviewing full-text articles, when available, to confirm study selection. After each stage, discrepancies were resolved by independent consultation with a third party (T.N.).
Data Collection and Quality Assessment
Data collection from the included studies was performed by two individual reviewers (C.J., M.C.) using a standardized data extraction template implemented in Microsoft Excel, and discrepancies were resolved by an independent party (T.N.). We collected the following information from each eligible study: year of publication, trial design, number of participants, participant details (type of malignancy, stage of malignancy), vitamin C intervention (route of administration, dose, dosing frequency, and duration of therapy), placebo or comparator arm details (drug name, dose, route, frequency and duration of therapy), any additional concurrent treatments, primary outcome, secondary outcome, outcome assessment details, overall survival, progression-free survival, response rates, quality-of-life data, toxicity data, safety data, and other outcomes were collected along with the published author conclusion. Study risk-of bias-assessment was assessed using the Jadad scale for controlled clinical trials [20] and the Newcastle-Ottawa scale for observational cohort or case control studies [21]. Two independent reviewers (C.J., M.C.) applied the scales to each relevant paper.
Data Analysis
We planned to carry out random-effect meta-analyses if there were opportunities to combine study data dependent upon judgments of the clinical and methodological homogeneity of included studies. Because of study heterogeneity in terms of delivery and dosing of ascorbate, different cancer types and mixed stages of the populations included, different outcome measures, and key study design differences, the authors judged that there were important clinical and treatment differences among studies that precluded the data from meta-analysis. Consequentially, a narrative approach to summarizing the data was used.
Results
Eligible Studies
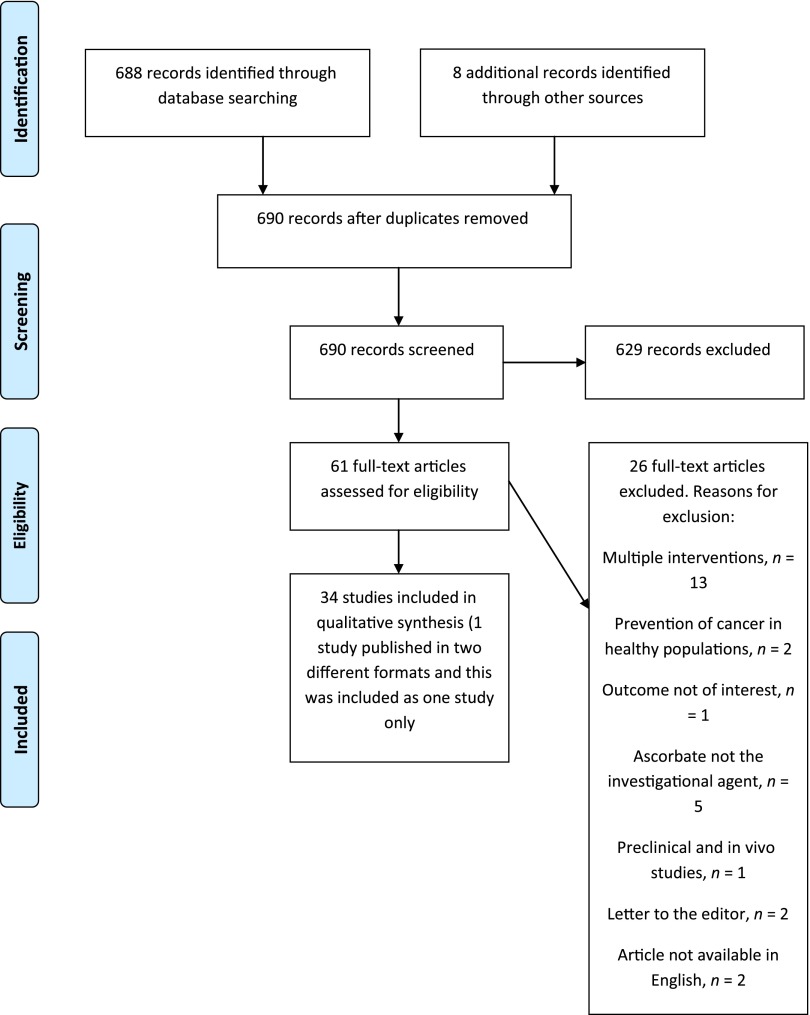
The electronic literature search identified 682 unique citations. Six duplicates were removed, and an additional eight citations were retrieved from other sources. Initial screening of titles and abstracts identified 61 citations that were considered eligible for full-text review. After screening of full-text reports, 26 studies were excluded, and 34 were retained; one case report was published in two different formats, and this case was included as only one study [22, 23]. A full list of excluded studies can be found in supplemental online Appendix 2. Figure 1 presents a PRISMA flow diagram summarizing the process of study selection [24]. Overall, 6 studies evaluated oral ascorbate (n = 3,694) [12, 13, 25–28], 16 studies evaluated i.v. ascorbate (n = 489) [29–44], and 13 studies combined oral and i.v. ascorbate use (n = 4,380) [9–11, 22, 23, 45–52].
Figure 1.
PRISMA flow diagram.
Studies of Oral Ascorbate
Characteristics
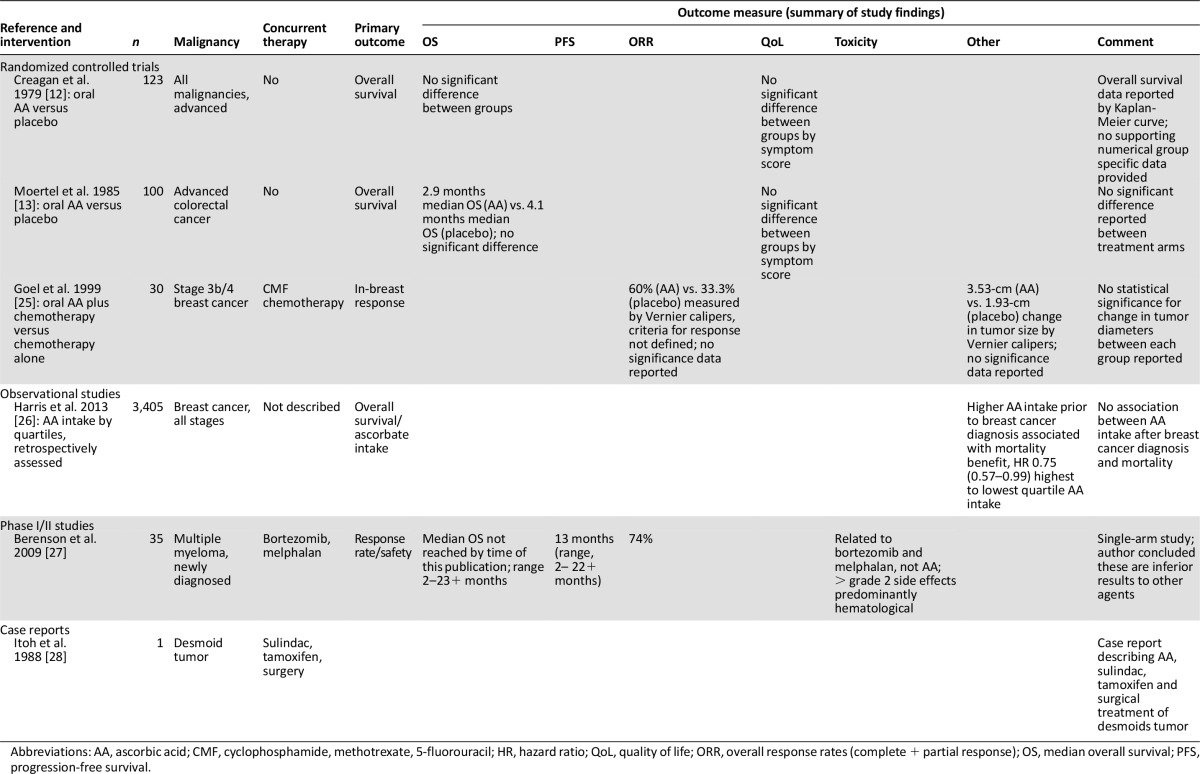
Six studies involving 3,694 patients were included [12, 13, 25–28] (Table 1). Three were randomized controlled trials [12, 13, 25], one was a phase II study [27], one was an observational study [26], and one was a case report [28]. Median year of publication was 1993 (range, 1979–2013). Two RCTs used a placebo arm as comparator [12, 13], one RCT used standard therapy plus ascorbate versus standard therapy alone [25], and the three other studies had no comparator arm [26–28]. Median sample size was 68 (range, 1–3,405). The studies included a range of patients including those with advanced cancers of all types, early stage breast cancer, multiple myeloma, and desmoid tumors. Three of the six studies used concurrent therapy (chemotherapy [25, 27], surgery [28], and endocrine therapy [28]). Five of the six studies documented the dose of ascorbate used [12, 13, 25, 27, 28], ranging from 1 g daily for 4 days of a 28-day cycle to 10 g daily, with a mean daily dose of 6.3 g. The primary outcome was overall survival in three studies [12, 13, 26], in-breast response in one study [25], and overall response and safety in one study [27]. The case report did not list its primary outcome [28]. Risk-of-bias assessment for oral ascorbate studies generally showed minimal risk of bias (supplemental online Appendix 3).
Table 1.
Studies of oral ascorbate
Results
Two of the six studies using oral ascorbate (2.5 g orally q.i.d. until progression/death) reported overall survival as the primary outcome [12, 13] (sample sizes of 123 and 100, respectively) (Table 1). Both of these studies were randomized, placebo-controlled studies. Neither study reported a statistically significant difference in overall survival associated with the ascorbate treatment arm nor in secondary outcomes of difference in quality-of-life or toxicity outcomes.
Two studies used response rates as their primary outcome of interest [25, 27]. One measured in-breast response, and the other measured overall response (a composite of complete and partial response) The first of these compared oral ascorbate (5 g orally b.i.d. daily for 84 days) given in combination with cyclophosphamide, methotrexate, and 5-fluorouracil chemotherapy to chemotherapy alone [25] (n = 30, 15 patients in each arm). This study reported data suggestive of a trend toward improved in-breast response with ascorbate (mean change in tumor diameter of 3.53 cm [±0.73] versus 1.93 cm [±0.77]) as measured using Vernier calipers. The paper reports a statistically significant change in tumor diameter, prechemotherapy to postchemotherapy. The diameter of each tumor was taken as the mean of the largest two diameters of that tumor. The study did not report results from a statistical significance test comparing the changes between groups but indicated that the combined ascorbate-and-chemotherapy group showed greater improvement than the chemotherapy-alone group. The study also reported change in tumor diameter by menopausal status in each treatment arm; however, there were no patients who were premenopausal in the ascorbate-plus-chemotherapy group, yet data were reported for this group.
The second study to report response rates used ascorbate in combination with bortezomib and melphalan in first line treatment of multiple myeloma [27] (n = 35). This single-arm study reported an overall response rate of 74% (using the European Group for Blood and Marrow Transplantation criteria [53]) with combination therapy and a median progression-free survival of 13 months (range, 2–22+ months). Harris et al. [26] reported findings from a retrospective observational study of breast cancer survivors (n = 3405). This study reported that higher levels of oral ascorbate intake prior to a diagnosis of breast cancer were associated with lower mortality from breast cancer (hazard ratio 0.75 [0.57–0.99]). Postdiagnosis intake of oral ascorbate was not associated with improved outcome. Finally, a case report by Itoh et al. [28] in a patient with a desmoid tumor reported a combination of ascorbate (1,500 mg orally daily), sulindac (400 mg daily), and tamoxifen (30 mg daily) used both preoperatively and as adjuvant treatment postsurgery. There was no regression in tumor size preoperatively using the combination, and the tumor recurred postoperatively.
Studies Using i.v. Ascorbate
Characteristics
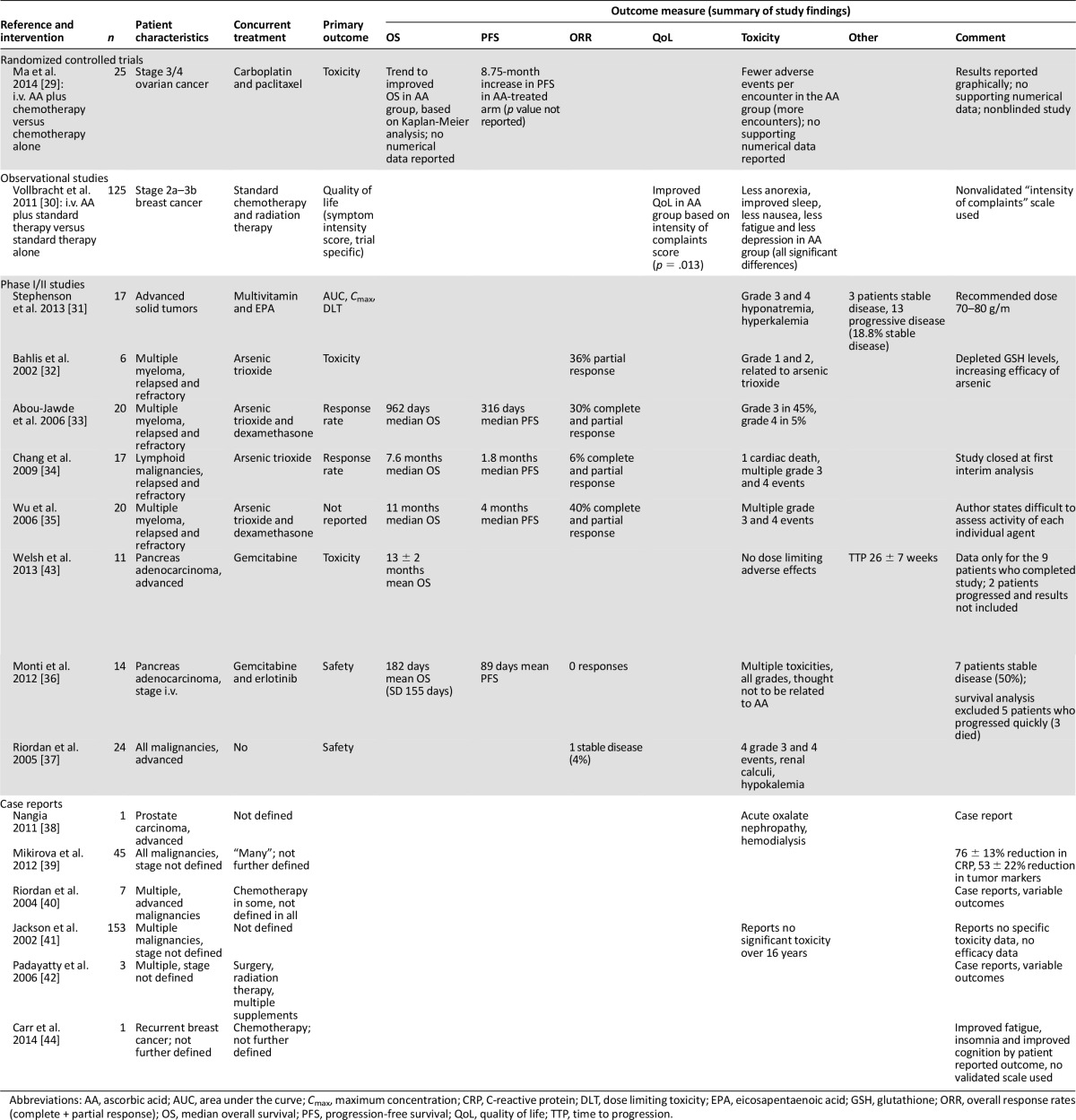
A total of 16 studies including 489 patients evaluated i.v. ascorbate (Table 2). One study was a randomized trial [29], eight were nonrandomized phase I or II studies [31–37, 43], one was a cohort study [30], and six were case reports [38–42, 44]. The median year of publication was 2006 (range, 2002–2014). One study used standard therapy alone as a comparator [29], one observational study compared patients who received i.v. ascorbate during their standard chemotherapy and radiation therapy for early stage breast cancer to patients who did not receive ascorbate therapy [30], and the other 14 studies had no comparator arm [31–44]. Median sample size for these studies was 17 (range, 1–153). There were 11 studies that enrolled patients diagnosed with advanced and metastatic disease [29, 31–38, 40, 43], whereas four studies did not define disease extent [39, 41, 42, 44], and one study was in early stage breast cancer [30]. Both hematological and solid organ malignancies were included, including four studies with patients with multiple myeloma or lymphoid malignancies [32–35]. Nine studies used i.v. ascorbate in combination with chemotherapy [29, 30, 32–36, 43, 44], one study used ascorbate in combination with surgery and radiation therapy [42], one study used ascorbate in combination with other multivitamins and nutritional supplements [31], and one study reported no additional therapy [37], whereas four studies did not report whether concurrent therapy was used or not [38–41]. The dose of ascorbate ranged from 500 mg i.v. daily to 100 g i.v. three times per week. One study dosed by plasma levels of ascorbate [43], one study did not define the dose of ascorbate given [41], and five studies were dose finding or had a primary endpoint of safety/toxicity [31, 32, 36, 37, 43]. One study’s primary endpoint was quality of life [30] (measured by symptom intensity score), and two studies used a primary endpoint of tumor response [33, 34]. Six studies were case reports and had no defined primary endpoint. Risk-of-bias assessment for i.v. ascorbate studies generally showed a high risk of bias (supplemental online Appendix 3).
Table 2.
Studies of i.v. ascorbate
Results
The results of the i.v. ascorbate studies are shown in Table 2. Ma et al. [29] reported a randomized trial of patients with stage 3 and 4 ovarian cancer receiving carboplatin and paclitaxel chemotherapy (n = 25). Patients were randomized to either i.v. ascorbate plus chemotherapy (n = 13) or chemotherapy alone (n = 12). The trial was not blinded. The primary outcome of toxicity was reported to show a lower number of average adverse events per encounter in the ascorbate group. The ascorbate group did have many more encounters than the control group; hence the denominator is not comparable. There were significantly fewer grade 1 and 2 average adverse events per encounter in the ascorbate group and no significant differences in terms of the incidence of toxicities of other grades. Full toxicity data were not presented. A trend toward an improvement in median overall survival was reported based on Kaplan-Meier analysis; however, there were no corresponding numerical data reported to provide additional insight. Median time for disease progression/relapse was reported as 25.5 months in the chemotherapy plus ascorbic acid arm and 16.75 months in the chemotherapy plus placebo arm (this difference did not reach statistical significance).
Vollbracht et al. [30] reported quality-of-life outcomes in an observational study for patients with breast cancer receiving standard chemotherapy and radiation therapy combined with i.v. ascorbate compared with matched comparisons who received standard chemotherapy and radiation therapy alone (n = 125, 53 in the ascorbate arm and 72 in the nonascorbate arm). Quality of life was measured by symptom intensity score (as designed by the trial, with no published reports of validation that we are aware of). Symptom intensity score was improved with i.v. ascorbate compared with patients who did not receive ascorbate during their treatment (p = .013). Toxicity was also reduced in the ascorbate group with less impairment to appetite (p = .046) and sleep (p = .005), less fatigue (p = .004), depression (p = .017), and nausea (p = .022) in the ascorbate group. Ascorbate was anecdotally reported to be well-tolerated.
Two studies assessing the benefits of i.v. ascorbate used response rate as the primary outcome [33, 34]. Abou-Jawde et al. [33] reported a single-arm study of i.v. ascorbate (1,000 mg i.v. daily for 5 days and then twice weekly for a maximum duration of 9 weeks) in combination with arsenic trioxide and dexamethasone in patients with relapsed and refractory multiple myeloma (n = 20). This combination reported a 30% response rate (complete and partial response). Overall, 10 of 20 patients developed grade 3 or 4 toxicity to combination treatment. Toxicity caused by ascorbate was not defined. Chang et al. [34] reported a similar study, conducted in a heavily pretreated population with lymphoid malignancies (n = 17), in which i.v. ascorbate (1,000 mg daily for 5 days and then twice weekly) was given with arsenic. A response rate of 6% was observed (complete and partial response by International Working Group criteria [54]), as well as severe toxicities that included multiple grade 3, 4, and 5 events. The trial was closed following the first interim analysis because of lack of activity.
Two studies were primarily dose-finding studies. Stephenson et al. [31] reported a phase I study in patients with advanced malignancies receiving i.v. ascorbate (n = 17). The recommended dose was 70–80 g/m2 3–4 times per week to obtain optimal peak plasma concentrations of ascorbate. The study reported a 19% stable disease rate and 81% progressive disease (“stable disease” and “progressive disease” were not defined in this publication), and only two patients completed the entire 4-week study period. Grade 3 and 4 metabolic toxicities were seen (hypernatremia, hypokalemia) related to ascorbate. Bahlis et al. [32] reported a dose-finding study using arsenic in combination with i.v. ascorbate in refractory myeloma patients to define dosing of arsenic (n = 6). He reported a dose of 0.25 mg/kg per day of arsenic as an appropriate dose. A partial response rate of 36% was observed (response defined by previously published criteria [55]), and there were no observed toxicities above grade 2 (NCI common toxicity criteria, version 2.0 [56]).
Four studies used safety and toxicity outcomes as their primary outcome. Two similar studies by Welsh et al. [43] (n = 11) and Monti et al. [36] (n = 14) reported toxicity in patients with advanced pancreas adenocarcinoma treated with chemotherapy and ascorbate. Toxicity was reported by both studies as predominantly related to gemcitabine chemotherapy and not secondary to ascorbate. Response rates and survival duration in both studies were reported only for patients who did not progress within the first month of treatment and therefore are not reflective of standard clinical trial reporting [57]. No partial or complete response was seen; 50% of patients had stable disease in the trial by Monti et al. [36], and mean overall survival 182 days (SD = 155 days). Welsh et al. [43] reported a 13 ± 2-month mean survival in the 9 patients analyzed (mean ± SEM).
A trial by Wu et al. [35] in refractory myeloma, used ascorbate 1,000 mg i.v. daily for 5 days and then twice weekly, in combination with arsenic trioxide (n = 20). This single-arm study was reported in letter format only. A median overall survival time of 11 months was reported [35].
The remaining six studies using i.v. ascorbate were case reports and case series [38–42, 44]. These papers included patients with a variety of malignancies and stages, some of whom received concurrent treatments and some of whom did not. Outcomes varied from study to study, including tumor responses, toxicity, and a number of other measures [39–42, 44] (Table 2). One study by Nangia et al. [38] reported acute oxalate nephropathy related to ascorbate administration requiring hemodialysis [38]. Another report of 153 separate cases over a 16-year period reported no adverse effects but did not report efficacy data [41]. A further case series of 45 patients with varying stages of malignancy receiving ascorbate ± concurrent therapy reported a decrease in C-reactive protein level with ascorbate in 76% of patients receiving ascorbate (±13%) [39].
Combined Oral and i.v. Ascorbate Studies
Characteristics
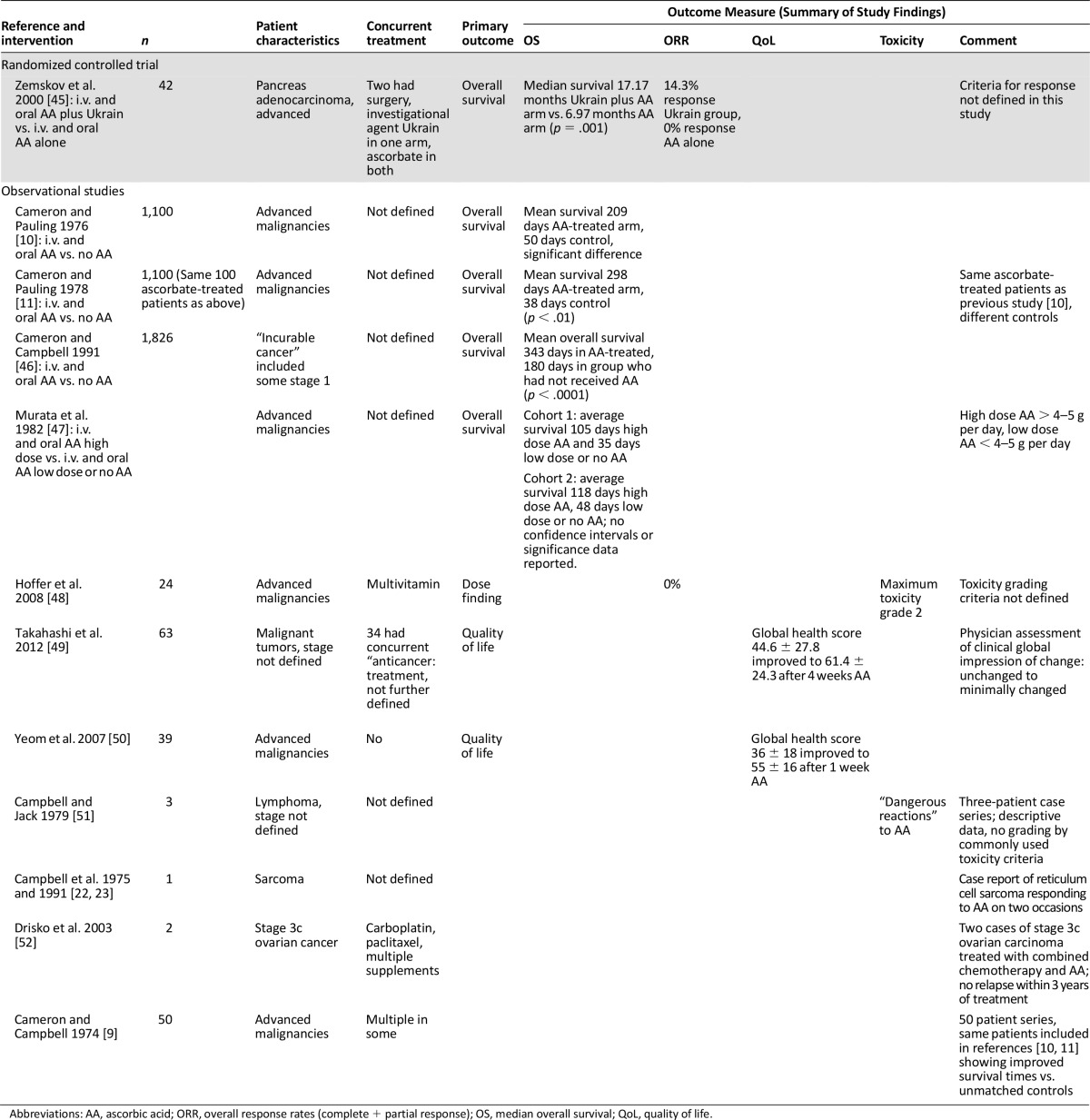
Twelve studies used both oral and i.v. ascorbate (n = 4,380 participants) (Table 3). One study was a randomized controlled trial [45], three were nonrandomized phase I or II studies [48–50], four were observational studies [10, 11, 46, 47], and four were case reports [9, 23, 51, 52]. The median year of publication was 1991 (range, 1974–2012). Five studies had a comparator arm [10, 11, 45–47]; in three studies this was a matched population who had not received ascorbate [10, 11, 46], in one study the comparison made was high dose versus low dose and no ascorbate [47], and in one study ascorbate was the control arm compared with ascorbate and a further investigational compound [45]. The median sample size was 46 (range, 1–1,826). Nine studies enrolled patients with advanced malignancies [10, 11, 45–48, 50–52], whereas three studies did not define the extent of disease [9, 23, 49]. A total of four studies used concurrent anticancer therapy [9, 45, 49, 52], two studies used concurrent multivitamins [48, 52], six studies did not define additional and concurrent treatments used [10, 11, 23, 46, 47, 51], and one study did not allow any additional treatment [50]. The dose of ascorbate ranged from 3 to 100 g of i.v. ascorbate with variable dose intensity. The oral dose ranged from 1 to 30 g daily. The primary outcome was survival in five studies [10, 11, 45–47], quality of life in two studies [49, 50] (the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire [EORTC QLQ C30] and global impression of clinical change), and safety in one study [48]. Four studies did not define a primary outcome [9, 23, 51, 52]. Risk-of-bias assessment for studies of combined oral and i.v. ascorbate generally showed a high risk of bias (supplemental online Appendix 3).
Table 3.
Studies of combined oral and i.v. ascorbate
Results
Of the twelve studies using both oral and i.v. ascorbate, five reported survival as their primary outcome [10, 11, 44–46]. Zemskov et al. [45] treated patients with advanced pancreatic cancer using i.v. (3 g on alternate days) and oral (800 mg t.i.d. alternate days) ascorbate as a control arm, whereas the investigational arm of their study used the agent Ukrain (a semi-synthetic alkaloid from Chelidonium majus L. and thiophosphoric acid triaziridide [45]) in combination with ascorbate. Median overall survival for the combination arm was 17.17 months compared to 6.97 months in the ascorbate-only arm (p = .001; methods of statistical analysis not reported). A response rate of 0% was seen in the ascorbate arm (response was not defined in this study).
Four observational studies were reported using both i.v. and oral ascorbate; two of these studies were by Cameron and Pauling and used the same 100 patients matched to 1,000 control patients and a further different 1,000 controls [10, 11]. All patients had incurable malignancies. Details of concurrent treatment were limited. Both studies reported improved survival in the ascorbate-treated arm (most common ascorbate treatment protocol was 10 g i.v. daily for 10 days and then 10 g orally daily thereafter); the original study reported mean survival as 209 days in the ascorbate-treated group versus 50 days for control, whereas the second study indicated durations of 298 days versus 38 days of mean survival [10, 11]. A further retrospective observational study by Cameron and Campbell [46] in patients with a variety of stages and types of malignancy reported improved survival in patients who had received ascorbate at any stage during their malignancy in three district general hospitals compared with those who had not received ascorbate during their malignancy. Mean overall survival was 343 days in the ascorbate-treated group versus 180 days in the control group (p < .0001 by log-rank test).
Murata et al. [47] reported an observational study of terminally ill patients who had received either low-dose (<4–5 g day; n = 69), high-dose (>4–5 g day; n = 50), or no ascorbate (n = 19) at two separate hospitals in Japan. The results were presented by hospital cohort, and patients were grouped as having received either high-dose ascorbate (>4–5 g per day), low-dose ascorbate (<4–5 g per day) or no ascorbate. The authors reported average survival times of 105 days versus 35 days for high- versus low-dose and no ascorbate in one hospital cohort and 118 versus 48 days in his second cohort of high- versus low-dose and no ascorbate [47]; no confidence intervals for survival times or statistical analysis was presented in this paper.
Two studies administering both oral and i.v. ascorbate reported quality-of-life data as their primary outcomes. Takahashi et al. [49] included patients with undefined stages and types of malignancies, many of whom were receiving concurrent treatment, and analyzed quality-of-life data after 4 weeks of ascorbate using EORTC QLQ C30, version 3 [58, 59] and the Clinical Global Impression of Change (CGIC) [60], a physician-assessed endpoint. Patient reported quality-of-life measures (EORTC QLQ C30) were improved after 4 weeks of ascorbate (mean 44.6 ± 27.8 before ascorbate, mean 61.4 ± 243 after 4 weeks of therapy; p < .01); however, physician assessment of well-being (CGIC) was reported as largely unchanged (35% patients unchanged at 4 weeks and 48% of patients minimally improved).
Yeom et al. [50] took patients with terminal malignancies and evaluated quality-of-life outcomes after 1 week of ascorbate therapy (10 g i.v. twice over 3 days and then 4 g orally for 1 week) using the quality-of-life measure EORTC QLQ C 30. Patients reported improved quality-of-life measures after 1 week of ascorbate therapy (global health score changing from 36 ± 18 initially to 55 ± 16 after 1 week of ascorbate therapy; p = .001). One dose-finding study by Hoffer et al. [48] used both oral and i.v. ascorbate. Patients were treated with up to 1.5 g/kg per day i.v. ascorbate 3 times per week along with oral ascorbate (500 mg b.i.d. orally on noninfusion days). Ascorbate was reported to be well-tolerated with the maximum toxicities being grade 2 (there was no reference as to what toxicity grading scale was used). No tumor response was seen. The four other studies using oral and i.v. ascorbate were case reports of a variety of malignancies [9, 22, 23, 51, 52]. Patients were treated both with ascorbate alone and in combination with chemotherapy. A variety of outcomes were reported. Toxicity was also reported, including ”dangerous” toxicity in the paper by Campbell and Jack [51]; no grading criteria were used to define this toxicity.
Discussion
The absolute extent of ascorbate use in patients with malignancy is unknown but widespread. One survey of 199 complementary medicine practitioners revealed that 172 of these practitioners delivered ascorbate to 11,233 patients in 2006 and 8,876 patients in 2008. The average dose given was 28 g every 4 days for 22 doses per person [18]. The same publication surveyed manufacturers of ascorbate in the U.S. and revealed that 750,000 vials (25 g per 50-mL vial) were sold in 2006 and 855,000 vials were sold in 2008 by those responding to the survey [18]. With the varying requirements for registration and licensure of practitioners of complementary medicine and the classification of oral ascorbate as a food by the Food and Drug Administration, data on the number of patients receiving ascorbate is difficult to accurately obtain. The anecdotal experience of the authors would suggest it is commonly used by patients while also receiving treatment for cancer at an academic cancer center.
This systematic review was performed to identify evidence related to the use of ascorbate in cancer patients and its impact on clinically relevant and validated outcomes. Our search identified a total of 5 randomized controlled trials (n = 322), 12 phase I/II studies (n = 287 participants), 6 observational studies (n = 7,599 participants), and 11 case reports (n = 267 participants) that assessed either oral ascorbate, intravenous ascorbate, or both. Many studies were small. Heterogeneity across included studies was considerable in terms of study design, patient characteristics, ascorbate treatment regimens, concurrent treatments administered, and outcomes measured. Based on our review, we have found no consistent evidence for an antitumor effect in terms of improved response rates or improved survival outcomes and also no evidence supporting an improvement in quality-of-life measures associated with ascorbate use in patients with malignancy in a controlled setting. We have found uncontrolled and anecdotal evidence for an antitumor effect and improved quality of life for ascorbate use in patients with malignancy.
We have found no consistent evidence for an antitumor effect in terms of improved response rates or improved survival outcomes and also no evidence supporting an improvement in quality-of-life measures associated with ascorbate use in patients with malignancy in a controlled setting.
Given the widespread use of i.v. ascorbate [17, 18], it was surprising how few trials there are evaluating its use. The trials identified were generally small, included a range of malignancies, were nonrandomized, lacked a control group, and often used a combination treatment of ascorbate with another agent. Evidence for i.v. ascorbate as an anticancer therapy is predominantly from observational and anecdotal studies [10, 11, 22, 46, 47]. We found no evidence for any statistically significant anticancer effect of i.v. ascorbate based on data from randomized controlled trials [29, 45].
There was evidence from nonrandomized studies suggesting a possible quality-of-life improvement (improvement in EORTC QLQ C30 measures) when i.v. ascorbate is given to cancer patients [49, 50]. There was also a reported improvement in a nonvalidated symptom score when ascorbate is combined with chemotherapy in breast cancer patients [30, 44]. However, again there are no randomized data to support this finding. The lack of double-blind placebo-controlled trials means that it is impossible to know how much effect on quality-of-life scores is related to the effects of the cancer, the chemotherapy, or other supportive care interventions. In addition, there is variable reporting of quality-of-life endpoints. We can therefore not exclude a possible placebo effect. Ascorbate therapy was reported as mostly well-tolerated and safe, but significant toxicities were reported including cardiac arrest (grade 4), renal toxicity (grade 3), and metabolic abnormalities (grade 3 and 4) based on data from both phase I/II studies and anecdotal reports of 113 patients.
To our knowledge, no previous review of ascorbate has studied both the oral and intravenous routes of administration. A recently published review of high dose intravenous ascorbate by Wilson et al. [61] suggests that evidence for a therapeutic effect is ambiguous and based on case series. A further systematic review of intravenous ascorbate in cancer concludes there may be evidence for improved cancer-related outcomes including quality of life when given to cancer patients, but high-quality evidence to confirm this is lacking [62]. This is in keeping with our findings in this review.
Study Limitations
We chose to include both oral and i.v. ascorbate use in our review. Pharmacokinetic studies of ascorbate suggest that much higher levels of serum ascorbate can be achieved by bypassing the oral route and usual mechanisms of homeostasis. It is perhaps this high level unachievable by oral administration that has the strongest scientific rationale for antitumor effect [16, 63–65]. However, as demonstrated, most studies were heterogeneous and incorporated both i.v. and oral use of ascorbate. Having included all relevant randomized trials, phase I/II trials, observational studies, and case reports may be seen by some as a limitation to this review based on the premise that the latter designs are considered less rigorous in terms of design. The paucity of randomized controlled studies, as well as the clinical and methodologic heterogeneity of the available literature, should be seen as limitations to the evidence base for ascorbate use.
Because of the lack of randomized controlled data, we attempted to further our evidence base by including these trials. In the controlled studies, we assessed for risk of bias; most studies scored poorly, failing to score well with appropriate randomization and blinding or selecting observational cohorts that were not well-matched or analyzed. There is a clear need for additional rigorous randomized controlled trials to establish the benefits and harms of ascorbate.
Conclusion
We have identified no consistent evidence supporting the existence of an anticancer effect of ascorbate when given either orally, intravenously, or in combination to cancer patients. There is weak evidence that ascorbate given intravenously may improve the quality of life of cancer patients and reduce the toxicity of chemotherapy. We have identified potential serious toxicity of intravenous ascorbate. There is also a lack of well-designed, randomized controlled trials exploring this question. If practitioners continue to offer and patients continue to pay for ascorbate therapy at the risk of toxicity and even death [66, 67], then high-quality placebo-controlled trials are needed. Until these trials are performed, the role of ascorbate therapy in cancer patients remains unsubstantiated. Patients should be honestly informed of this and only treated within the confines of high-quality clinical trials.
See http://www.TheOncologist.com for supplemental material available online.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Supplementary Material
Acknowledgments
Brian Hutton was partially funded by Grant R24 AT001293 from the National Center for Complementary and Alternative Medicine (NCCAM) of the U.S. National Institutes of Health. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or the U.S. National Institutes of Health.
Footnotes
For Further Reading: Yusuke Takagi, Yukio Hosomi, Kuniko Sunami et al. A Prospective Study of Shortened Vitamin Supplementation Prior to Cisplatin–Pemetrexed Therapy for Non-Small Cell Lung Cancer. The Oncologist 2014;19:1194–1199.
Implications for Practice: Routine supplementation with folic acid and vitamin B12, at least 1 week before the first pemetrexed administration, is necessary to reduce its toxicity, but the procedure can cause treatment delay. In daily practice, some patients experience disease progression before receiving planned treatment. Delayed start of pemetrexed-based chemotherapy can have a negative impact on patient outcomes because pemetrexed is an indispensable component of standard chemotherapy for non-small cell lung cancer. This study showed that the shortened vitamin supplementation before pemetrexed-based chemotherapy was well tolerated and retained antitumor efficacy, confirmed by the analysis of baseline total plasma homocysteine level.
Author Contributions
Conception/Design: Carmel Jacobs, Brian Hutton, Mark Clemons
Provision of study material or patients: Risa Shorr
Collection and/or assembly of data: Brian Hutton, Terry Ng, Risa Shorr, Mark Clemons
Data analysis and interpretation: Carmel Jacobs, Brian Hutton, Terry Ng, Risa Shorr, Mark Clemons
Manuscript writing: Carmel Jacobs, Brian Hutton, Terry Ng, Risa Shorr, Mark Clemons
Final approval of manuscript: Carmel Jacobs, Brian Hutton, Terry Ng, Risa Shorr, Mark Clemons
Disclosures
The authors indicated no financial relationships.
References
- 1.McCormick WJ. Cancer: A collagen disease, secondary to a nutritional deficiency. Arch Pediatr. 1959;76:166–171. [PubMed] [Google Scholar]
- 2.Block G. Epidemiologic evidence regarding vitamin C and cancer. Am J Clin Nutr. 1991;54(suppl):1310S–1314S. doi: 10.1093/ajcn/54.6.1310s. [DOI] [PubMed] [Google Scholar]
- 3.Loria CM, Klag MJ, Caulfield LE, et al. Vitamin C status and mortality in US adults. Am J Clin Nutr. 2000;72:139–145. doi: 10.1093/ajcn/72.1.139. [DOI] [PubMed] [Google Scholar]
- 4.Patterson RE, White E, Kristal AR, et al. Vitamin supplements and cancer risk: The epidemiologic evidence. Cancer Causes Control. 1997;8:786–802. doi: 10.1023/a:1018443724293. [DOI] [PubMed] [Google Scholar]
- 5.Gey KF. Vitamins E plus C and interacting conutrients required for optimal health: A critical and constructive review of epidemiology and supplementation data regarding cardiovascular disease and cancer. Biofactors. 1998;7:113–174. doi: 10.1002/biof.5520070115. [DOI] [PubMed] [Google Scholar]
- 6.Bjelakovic G, Nikolova D, Simonetti RG, et al. Antioxidant supplements for prevention of gastrointestinal cancers: A systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 7.Gaziano JM, Glynn RJ, Christen WG, et al. Vitamins E and C in the prevention of prostate and total cancer in men: The Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J Natl Cancer Inst. 2009;101:14–23. doi: 10.1093/jnci/djn438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron E, Campbell A. The orthomolecular treatment of cancer: II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact. 1974;9:285–315. doi: 10.1016/0009-2797(74)90019-2. [DOI] [PubMed] [Google Scholar]
- 10.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1976;73:3685–3689. doi: 10.1073/pnas.73.10.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc Natl Acad Sci USA. 1978;75:4538–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creagan ET, Moertel CG, O’Fallon JR, et al. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med. 1979;301:687–690. doi: 10.1056/NEJM197909273011303. [DOI] [PubMed] [Google Scholar]
- 13.Moertel CG, Fleming TR, Creagan ET, et al. High-dose vitamin C versus placebo in the treatment of patients with advanced cancer who have had no prior chemotherapy: A randomized double-blind comparison. N Engl J Med. 1985;312:137–141. doi: 10.1056/NEJM198501173120301. [DOI] [PubMed] [Google Scholar]
- 14.Du J, Cullen JJ, Buettner GR. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim Biophys Acta. 2012;1826:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padayatty SJ, Sun H, Wang Y, et al. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann Intern Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 17.Huebner J, Muenstedt K, Prott FJ, et al. Online survey of patients with breast cancer on complementary and alternative medicine. Breast Care (Basel) 2014;9:60–63. doi: 10.1159/000360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padayatty SJ, Sun AY, Chen Q, et al. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5:e11414. doi: 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nutrition Business Journal. An analysis of markets, trends, competition and strategy in the U.S. dietary supplement industry: NBJ's Supplement Business Report. Available at http://newhope360.com/site-files/newhope360.com/files/uploads/2011/10/TOCEXECSUMM110930.supp%20report%20FINAL-2.pdf. Accessed November 23, 2014.
- 20.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Wells GASB, O’Connell D, Peterson J et al. The newcastle-ottawa scale (nos) for assessing the quality of nonrandomised studies in meta-analyses. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 1, 2014.
- 22.Campbell A, Jack T, Cameron E. Reticulum cell sarcoma: Two complete ‘spontaneous’ regressions, in response to high-dose ascorbic acid therapy: A report on subsequent progress. Oncology. 1991;48:495–497. doi: 10.1159/000226988. [DOI] [PubMed] [Google Scholar]
- 23.Cameron E, Campbell A, Jack T. The orthomolecular treatment of cancer: III. Reticulum cell sarcoma: Double complete regression induced by high-dose ascorbic acid therapy. Chem Biol Interact. 1975;11:387–393. doi: 10.1016/0009-2797(75)90007-1. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goel SAS, Mandal A, Singhal K, et al. Emerging role of ascorbic acid in the management of advanced breast carcinoma as a chemosensitizer. Asian J Surg. 1999;22:333–336. [Google Scholar]
- 26.Harris HR, Bergkvist L, Wolk A. Vitamin C intake and breast cancer mortality in a cohort of Swedish women. Br J Cancer. 2013;109:257–264. doi: 10.1038/bjc.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berenson JR, Yellin O, Woytowitz D, et al. Bortezomib, ascorbic acid and melphalan (BAM) therapy for patients with newly diagnosed multiple myeloma: An effective and well-tolerated frontline regimen. Eur J Haematol. 2009;82:433–439. doi: 10.1111/j.1600-0609.2009.01244.x. [DOI] [PubMed] [Google Scholar]
- 28.Itoh H, Ikeda S, Oohata Y, et al. Treatment of desmoid tumors in Gardner’s syndrome. Report of a case. Dis Colon Rectum. 1988;31:459–461. doi: 10.1007/BF02552617. [DOI] [PubMed] [Google Scholar]
- 29.Ma Y, Chapman J, Levine M, et al. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci Transl Med. 2014;6:222ra218. doi: 10.1126/scitranslmed.3007154. [DOI] [PubMed] [Google Scholar]
- 30.Vollbracht C, Schneider B, Leendert V, et al. Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: Results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo. 2011;25:983–990. [PubMed] [Google Scholar]
- 31.Stephenson CM, Levin RD, Spector T, et al. Phase I clinical trial to evaluate the safety, tolerability, and pharmacokinetics of high-dose intravenous ascorbic acid in patients with advanced cancer. Cancer Chemother Pharmacol. 2013;72:139–146. doi: 10.1007/s00280-013-2179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, et al. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res. 2002;8:3658–3668. [PubMed] [Google Scholar]
- 33.Abou-Jawde RM, Reed J, Kelly M, et al. Efficacy and safety results with the combination therapy of arsenic trioxide, dexamethasone, and ascorbic acid in multiple myeloma patients: A phase 2 trial. Med Oncol. 2006;23:263–272. doi: 10.1385/MO:23:2:263. [DOI] [PubMed] [Google Scholar]
- 34.Chang JE, Voorhees PM, Kolesar JM, et al. Phase II study of arsenic trioxide and ascorbic acid for relapsed or refractory lymphoid malignancies: A Wisconsin Oncology Network study. Hematol Oncol. 2009;27:11–16. doi: 10.1002/hon.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu KL, Beksac M, van Droogenbroeck J, et al. Phase II multicenter study of arsenic trioxide, ascorbic acid and dexamethasone in patients with relapsed or refractory multiple myeloma. Haematologica. 2006;91:1722–1723. [PubMed] [Google Scholar]
- 36.Monti DA, Mitchell E, Bazzan AJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7:e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riordan HD, Casciari JJ, González MJ, et al. A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J. 2005;24:269–276. [PubMed] [Google Scholar]
- 38.Nangia SVM. Acute oxalate nephropathy from intravenous vitamin C therapy. Am J Kidney Dis. 2011;57:B71. [Google Scholar]
- 39.Mikirova N, Casciari J, Rogers A, et al. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. 2012;10:189. doi: 10.1186/1479-5876-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riordan HDR, Riordan NH, Jackson JA, et al. Intravenous vitamin C as a chemotherapy agent: A report on clinical cases. P R Health Sci J. 2004;23:115–118. [PubMed] [Google Scholar]
- 41.Jackson JA, Riordan HD, Bramhall NL, et al. Sixteen year history with high dose intravenous vitamin C treatment for various types of cancer and other diseases. J Orthomol Med. 2002;17:117–118. [Google Scholar]
- 42.Padayatty SJ, Riordan HD, Hewitt SM, et al. Intravenously administered vitamin C as cancer therapy: Three cases. CMAJ . 2006;174:937–942. doi: 10.1503/cmaj.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welsh JL, Wagner BA, van’t Erve TJ, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013;71:765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carr AC, Vissers MC, Cook J. Relief from cancer chemotherapy side effects with pharmacologic vitamin C. N Z Med J. 2014;127:66–70. [PubMed] [Google Scholar]
- 45.Zemskov VS, Procopchuk OL, Susak YM, et al. Ukrain (NSC-631570) in the treatment of pancreas cancer. Drugs Exp Clin Res. 2000;26:179–190. [PubMed] [Google Scholar]
- 46.Cameron E, Campbell A. Innovation vs. quality control: An ‘unpublishable’ clinical trial of supplemental ascorbate in incurable cancer. Med Hypotheses. 1991;36:185–189. doi: 10.1016/0306-9877(91)90127-k. [DOI] [PubMed] [Google Scholar]
- 47.Murata A, Morishige F, Yamaguchi H. Prolongation of survival times of terminal cancer patients by administration of large doses of ascorbate. Int J Vitam Nutr Res Suppl. 1982;23:103–113. [PubMed] [Google Scholar]
- 48.Hoffer LJ, Levine M, Assouline S, et al. Phase I clinical trial of I.V. ascorbic acid in advanced malignancy. Annals Oncol. 2008;19:1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi H, Mizuno H, Yanagisawa A. High-dose intravenous vitamin C improves quality of life in cancer patients. Personalized Medicine Universe. 2012;1:49–53. [Google Scholar]
- 50.Yeom CH, Jung GC, Song KJ. Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci. 2007;22:7–11. doi: 10.3346/jkms.2007.22.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell A, Jack T. Acute reactions to mega ascorbic acid therapy in malignant disease. Scott Med J. 1979;24:151–153. doi: 10.1177/003693307902400210. [DOI] [PubMed] [Google Scholar]
- 52.Drisko JA, Chapman J, Hunter VJ. The use of antioxidants with first-line chemotherapy in two cases of ovarian cancer. J Am Coll Nutr. 2003;22:118–123. doi: 10.1080/07315724.2003.10719284. [DOI] [PubMed] [Google Scholar]
- 53.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 54.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 55.Soignet SL, Maslak P, Wang Z-G, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 56.National Cancer Institute. Ctc v2.0. 1999. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed April 20, 2014.
- 57.CONSORT. The CONSORT statement. 2010. Available at http://www.consort-statement.org/consort-statement/overview0/. Accessed April 2, 2014.
- 58.Kobayashi K, Takeda F, Teramukai S, et al. A cross-validation of the European Organization for Research and Treatment of Cancer qlq-c30 (EORTC qlq-c30) for Japanese with lung cancer. Eur J Cancer. 1998;34:810–815. doi: 10.1016/s0959-8049(97)00395-x. [DOI] [PubMed] [Google Scholar]
- 59.Kato J, Nagahara A, Iijima K, et al. Evaluation of EORTC QLQ-C30 questionnaire in patients undergoing in-hospital chemotherapy for gastrointestinal cancer in Japan. J Gastroenterol Hepatol. 2008;23(suppl 2):S268–S272. doi: 10.1111/j.1440-1746.2008.05414.x. [DOI] [PubMed] [Google Scholar]
- 60.Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 61.Wilson MK, Baguley BC, Wall C, et al. Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac J Clin Oncol. 2014;10:22–37. doi: 10.1111/ajco.12173. [DOI] [PubMed] [Google Scholar]
- 62.Fritz HWL, Flower G, Kirchner L et al. Intravenous vitamin C and cancer: A systematic review. Poster presented at: Applied Research in Cancer Control; May 12, 2014; Toronto, ON, Canada. [Google Scholar]
- 63.Graumlich JF, Ludden TM, Conry-Cantilena C, et al. Pharmacokinetic model of ascorbic acid in healthy male volunteers during depletion and repletion. Pharm Res. 1997;14:1133–1139. doi: 10.1023/a:1012186203165. [DOI] [PubMed] [Google Scholar]
- 64.Bram S, Froussard P, Guichard M, et al. Vitamin C preferential toxicity for malignant melanoma cells. Nature. 1980;284:629–631. doi: 10.1038/284629a0. [DOI] [PubMed] [Google Scholar]
- 65.Casciari JJ, Riordan NH, Schmidt TL, et al. Cytotoxicity of ascorbate, lipoic acid, and other antioxidants in hollow fibre in vitro tumours. Br J Cancer. 2001;84:1544–1550. doi: 10.1054/bjoc.2001.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawton JM, Conway LT, Crosson JT, et al. Acute oxalate nephropathy after massive ascorbic acid administration. Arch Intern Med. 1985;145:950–951. [PubMed] [Google Scholar]
- 67.Campbell GD, Jr, Steinberg MH, Bower JD. Letter: Ascorbic acid-induced hemolysis in G-6-PD deficiency. Ann Intern Med. 1975;82:810. doi: 10.7326/0003-4819-82-6-810_1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.