In the pivotal phase III MPACT trial, nab-paclitaxel plus gemcitabine demonstrated improved survival compared with gemcitabine alone. This analysis of the phase III study confirms the survival benefit of nab-paclitaxel plus gemcitabine across many prespecified prognostic factors and the broad utility of this regimen for patients with advanced pancreatic cancer.
Keywords: Pancreatic cancer, nab-Paclitaxel, Gemcitabine, Prognostic factors
Abstract
Background.
nab-Paclitaxel in combination with gemcitabine has emerged as a new treatment option for patients with metastatic pancreatic cancer (MPC), based on superiority over gemcitabine demonstrated in the phase III MPACT trial. Previously, Karnofsky performance status (KPS) score and the presence of liver metastases were shown to be predictive of survival with nab-paclitaxel plus gemcitabine treatment. This analysis sought to further explore the relationship between clinical characteristics and survival in the MPACT trial and to identify potential predictors of overall survival and progression-free survival in patients with MPC.
Materials and Methods.
Cox regression models adjusted for stratification factors and a stepwise multivariate analysis of prespecified baseline prognostic factors were performed.
Results.
Treatment effect was significantly associated with survival, with a similar magnitude of reduction in risk of death compared with the previously reported primary analysis. Treatment effect consistently favored nab-paclitaxel plus gemcitabine across the majority of the prespecified factors. In addition to KPS score and presence of liver metastases, age and number of metastatic sites were independent prognostic factors of overall and progression-free survival. Baseline carbohydrate antigen 19-9 was not found to be an independent prognostic factor of survival in this analysis.
Conclusion.
The results of this analysis confirm broad utility of nab-paclitaxel plus gemcitabine for the treatment of MPC. In addition, these findings suggest that KPS score, presence of liver metastases, age, and number of metastatic sites are important predictors of survival that may be useful when making treatment decisions and designing future clinical trials.
Implications for Practice:
In the pivotal phase III MPACT trial, nab-paclitaxel plus gemcitabine demonstrated improved survival compared with gemcitabine alone, a standard therapy for advanced pancreatic cancer. This analysis of the phase III study confirms the survival benefit of nab-paclitaxel plus gemcitabine across many prespecified prognostic factors and the broad utility of this regimen for patients with advanced pancreatic cancer.
Introduction
The worldwide incidence of all-stage pancreatic cancer is 2.4%, and the mortality rate is 4% [1]. Most patients are diagnosed with advanced disease, which carries a 5-year survival rate of only 2% [2]. Only three phase III trials comparing a new treatment combination with single-agent gemcitabine for advanced pancreatic cancer had positive results [3–5]. Recent data from the phase III international MPACT study revealed significant improvements in all efficacy outcomes for nab-paclitaxel plus gemcitabine over gemcitabine alone in patients with metastatic pancreatic adenocarcinoma [5]. In that study, nab-paclitaxel plus gemcitabine versus gemcitabine produced median overall survival (OS) of 8.5 versus 6.7 months (hazard ratio [HR]: 0.72; 95% confidence interval [CI]: 0.62–0.83; p < .001), median progression-free survival (PFS) of 5.5 versus 3.7 months (HR: 0.69; 95% CI: 0.58–0.82; p < .001), and an overall response rate of 23% versus 7% (response rate ratio: 3.19; 95% CI: 2.18–4.66; p < .001). Treatment with nab-paclitaxel plus gemcitabine resulted in more grade ≥3 neutropenia, fatigue, and neuropathy compared with gemcitabine but similar incidences of grade ≥3 anemia and thrombocytopenia. Based on these findings, nab-paclitaxel plus gemcitabine has become a new option for the first-line treatment of metastatic pancreatic cancer (MPC).
Previous studies have identified factors prognostic of survival outcomes with gemcitabine in patients with MPC, including carbohydrate antigen 19-9 (CA19-9), age >60 years, weight loss, absence of metastases, stage, and serum carcinoembryonic antigen [6–8]. In the MPACT study, a Cox regression analysis using the trial stratification factors as covariates was used to identify factors predictive of survival [5]. This preliminary analysis revealed that Karnofsky performance status (KPS) score and the presence or absence of liver metastases were independent predictors of survival. In addition, a significant treatment effect was observed with nab-paclitaxel plus gemcitabine versus gemcitabine alone (HR: 0.71; 95% CI: 0.61–0.83; p < .001). The current analysis was performed to further characterize the relationship between baseline clinical characteristics and survival in the MPACT patient population. In addition, Cox modeling of the original data set from the MPACT study using an expanded set of prespecified baseline prognostic factors was conducted to provide further insight into factors that may be prognostic of survival in patients with pancreatic cancer.
Materials and Methods
Patients
We performed an analysis of the phase III MPACT study. Patients and methods were reported previously [5]. Briefly, eligible adults had a KPS score of ≥70, no previous chemotherapy for metastatic disease, and histologically or cytologically confirmed metastatic adenocarcinoma of the pancreas that was measurable according to Response Evaluation Criteria in Solid Tumors version 1.0 [9]. Patients were also required to have adequate hematologic, hepatic, and renal function.
Study Design
Eligible patients were randomized 1:1 to receive intravenous nab-paclitaxel 125 mg/m2 followed by intravenous gemcitabine 1,000 mg/m2 on days 1, 8, 15, 29, 36, and 43 or gemcitabine 1,000 mg/m2 weekly for 7 of 8 weeks (cycle 1). All patients were administered treatment on days 1, 8, and 15 of each 28-day cycle in subsequent cycles. Patients were stratified according to KPS score, presence or absence of liver metastases, and geographic region. Treatment continued until disease progression or unacceptable toxicity. Serial measurements of CA19-9 levels were performed at baseline and every 8 weeks thereafter. Patients were followed for survival until death or study closure. The primary endpoint was OS. Independently assessed PFS was a secondary endpoint; PFS was also assessed by investigators.
Statistical Analyses
All efficacy analyses were performed on the intent-to-treat (ITT) population, which was composed of all enrolled patients. Overall survival, the primary endpoint of the study, was analyzed by the Kaplan-Meier method. As specified by the study protocol, the treatment effect on OS was assessed for statistical significance by log-rank test stratified by the prespecified randomization stratification criteria of geographic region (North America vs. other), baseline KPS score (70–80 vs. 90–100), and presence of liver metastases (yes vs. no). The associated HR and two-sided 95% CI were estimated using a stratified Cox proportional hazards model. Survival data for patients who were lost to follow-up were censored at the last date at which they were known to be alive. The data cutoff for the original OS analysis was September 17, 2012.
As prespecified in the statistical analysis plan, the potential influence of the following prognostic factors on overall survival was assessed: age (<65 and ≥65 years), sex, KPS score (70–80 and 90–100), location of primary pancreatic cancer (head and other), peritoneal carcinomatosis (yes and no), presence of liver metastases (yes and no), presence of pulmonary metastases (yes and no), geographic region, presence of biliary stent at baseline (yes and no), previous Whipple procedure (yes and no), number of metastatic sites (1, 2, 3, and >3), stage at diagnosis (IV and other), CA19-9 level (within normal limit, upper limit of normal [ULN] to <59 × ULN, and ≥59 × ULN).
Three additional analyses were performed: a Cox regression model with stratified factors as covariates; a stepwise Cox regression model with all factors listed above except CA19-9; and a stepwise Cox regression model with all factors, including CA19-9. An additional stratified analysis of OS was conducted using stratification factors, including geographic region, derived baseline KPS score, and liver metastasis status in which the derivation of baseline KPS score and liver metastasis was based on the clinical database (not as randomized). A multivariate analysis of OS was conducted using a Cox proportional hazards model to evaluate treatment effect adjusted for stratification factors. A stepwise multivariate analysis (with a significance level for entry of .20 and a significance level for stay of .10) was performed to evaluate treatment effect and to identify possible predictors for OS by including all prognostic factors in the model. The goal of the stepwise regression was to identify independent prognostic factors. A consequence of this method is that if two factors were related, the effect of the first may have rendered any effect of the second nonsignificant. In such a case, one of the two factors may not have met the stay criteria and would have been rejected.
A multivariate analysis of PFS was conducted using a Cox proportional hazards model to evaluate treatment effect adjusted for stratification factors. A stepwise model similar to the one described for OS was applied to PFS to identify the potential influence of prognostic factors on PFS, using the same factors as described for OS. Progression-free survival was compared between the two treatment arms using a nonstratified log-rank test; a p value was provided. The HR and two-sided 95% CI were estimated using a Cox proportional hazards model.
Results
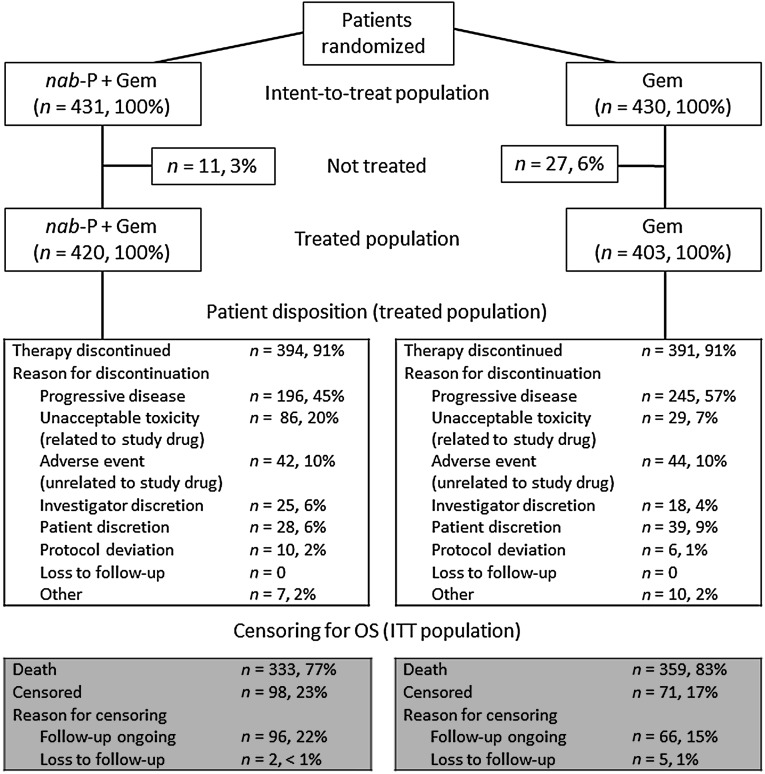
Overall, 861 patients underwent randomization from May 2009 through April 2012 at 151 community and academic centers in 11 countries (Fig. 1). A total of 431 and 430 patients, respectively, were randomly assigned to receive nab-paclitaxel plus gemcitabine or gemcitabine (ITT population) [5]. Selected baseline prognostic characteristics were well balanced between the arms (supplemental online Table 1).
Figure 1.
Consolidated Standards of Reporting Trials diagram. One patient was randomized to Gem but was treated with nab-P plus Gem. In the ITT analysis, this patient was analyzed as randomized. In all analyses of the treated population, the patient was analyzed as treated.
Abbreviations: Gem, gemcitabine; ITT, intent-to-treat; nab-P, nab-paclitaxel; OS, overall survival.
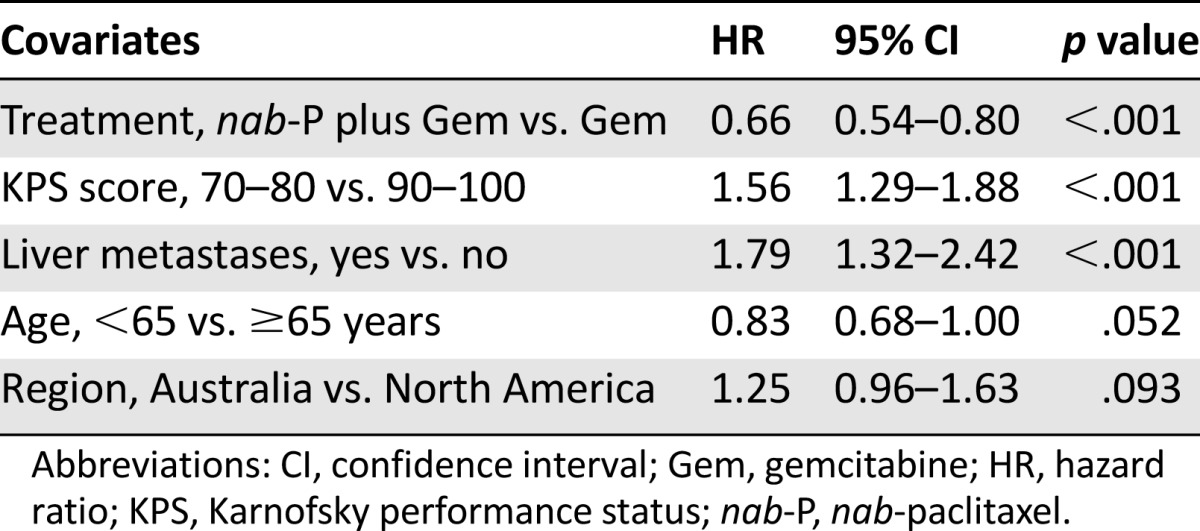
Median OS consistently favored nab-paclitaxel plus gemcitabine across most prespecified subgroups (Table 1). In a multivariate Cox model of OS, adjusting for the stratification factors, the treatment effect remained significant, with a similar magnitude of reduction in the risk of death compared with the primary analysis (HR: 0.71; 95% CI: 0.61–0.83; p < .001) (Table 2). In this model, poor KPS score and the presence of liver metastases were associated with an increased risk of death.
Table 1.
Overall survival in prespecified subgroups: intent-to-treat population
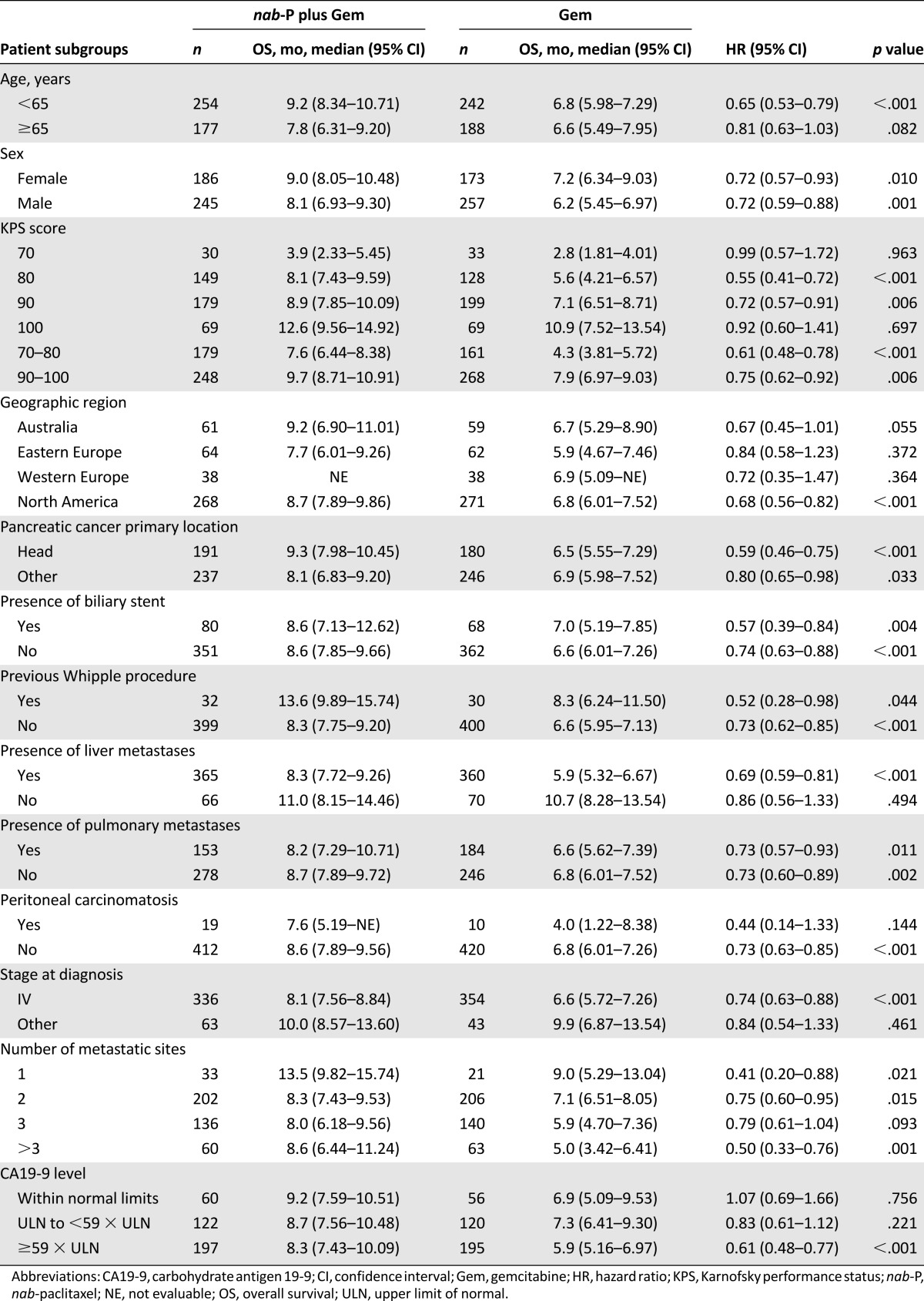
Table 2.
Multivariate Cox model of overall survival in the intent-to-treat population with stratification factors as covariates
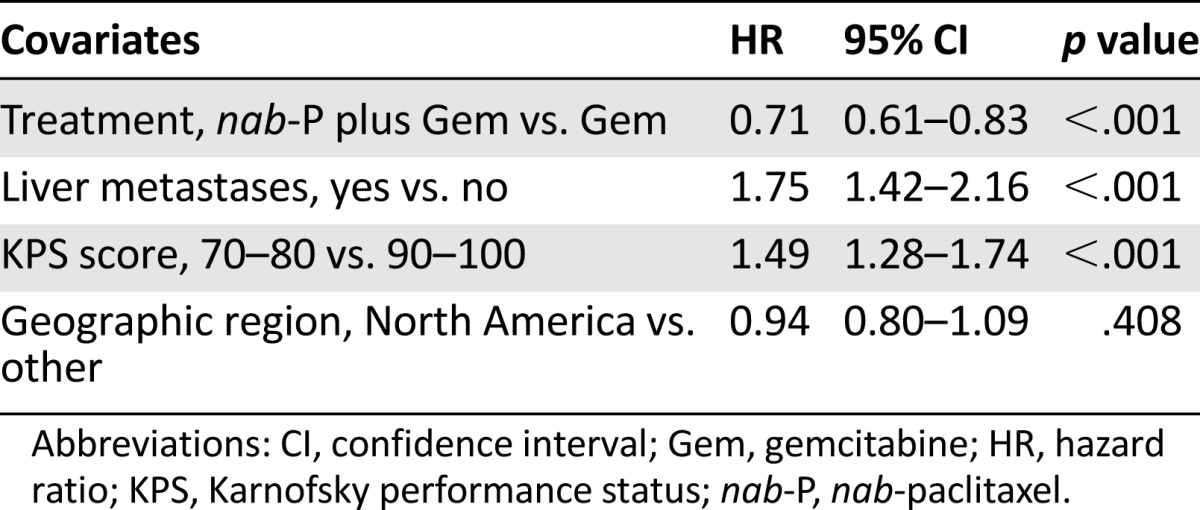
A stepwise multivariate analysis (with a significance level for entry of .20 and a significance level for stay of .10) was also performed to evaluate the treatment effect and to identify possible predictors for OS by including all the prognostic factors in the model (Table 3). The treatment effect for nab-paclitaxel plus gemcitabine versus gemcitabine remained statistically significant, with an HR of 0.72 (95% CI: 0.61–0.85; p < .001). A similar HR was observed in a nonstratified analysis (HR: 0.74; 95% CI: 0.63–0.86). In this analysis, age ≥65 years, poorer KPS score, presence of liver metastasis, and higher number of metastatic sites were associated with an increased risk of death; patients from Eastern Europe had a higher risk of death than those from North America, regardless of treatment. Baseline CA19-9 value was not an independent prognostic factor for OS in this model.
Table 3.
Stepwise multivariate analysis for predictors of overall survival in the intent-to-treat population
As observed with OS, the median PFS by treatment group consistently favored nab-paclitaxel plus gemcitabine across most prespecified subgroups (Table 4). In the multivariate analyses of PFS, treatment effect remained significant after adjustment for stratification factors and favored nab-paclitaxel plus gemcitabine (HR: 0.69; 95% CI: 0.59–0.83; p < .001).
Table 4.
Progression-free survival in prespecified subgroups: intent-to-treat population
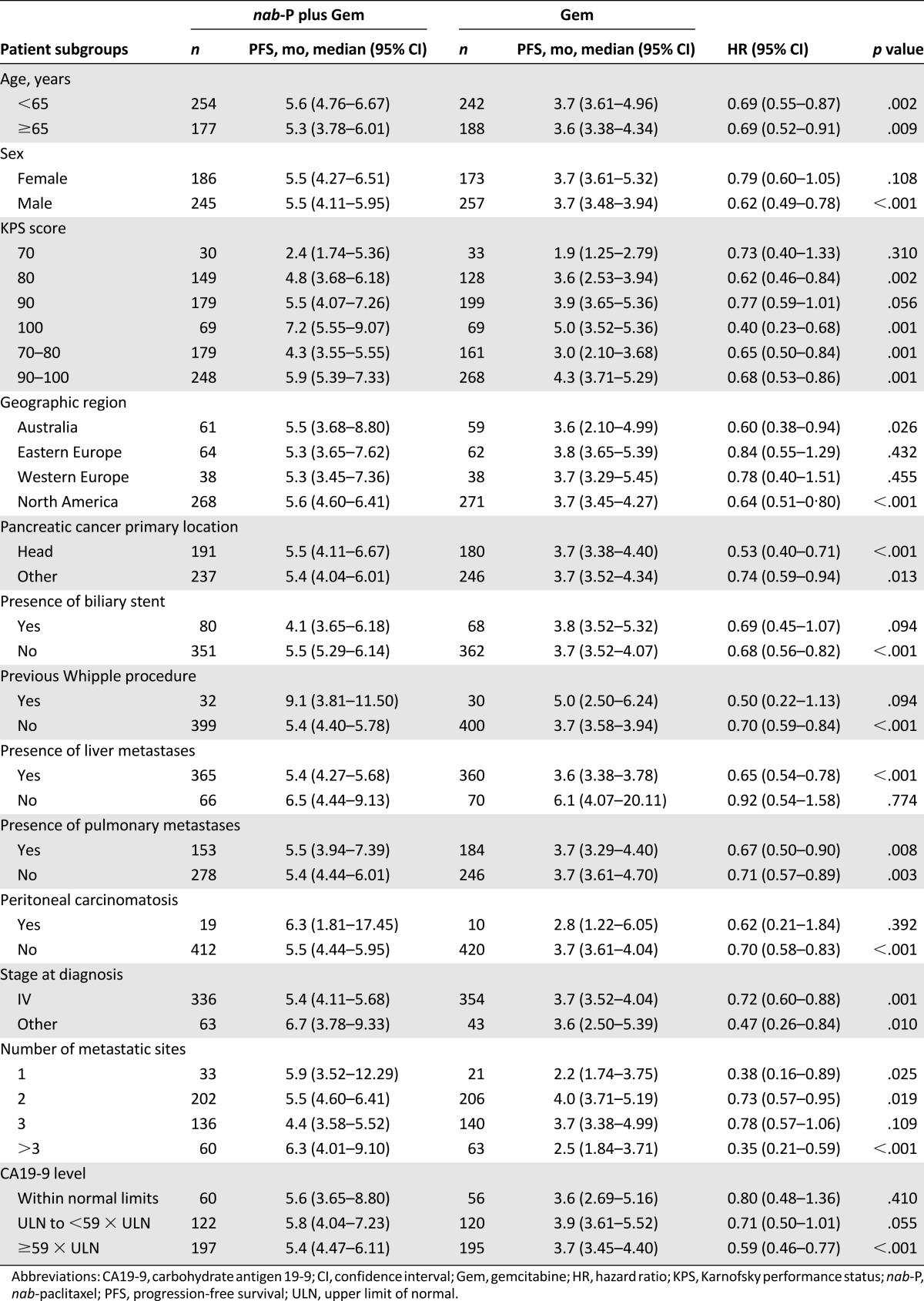
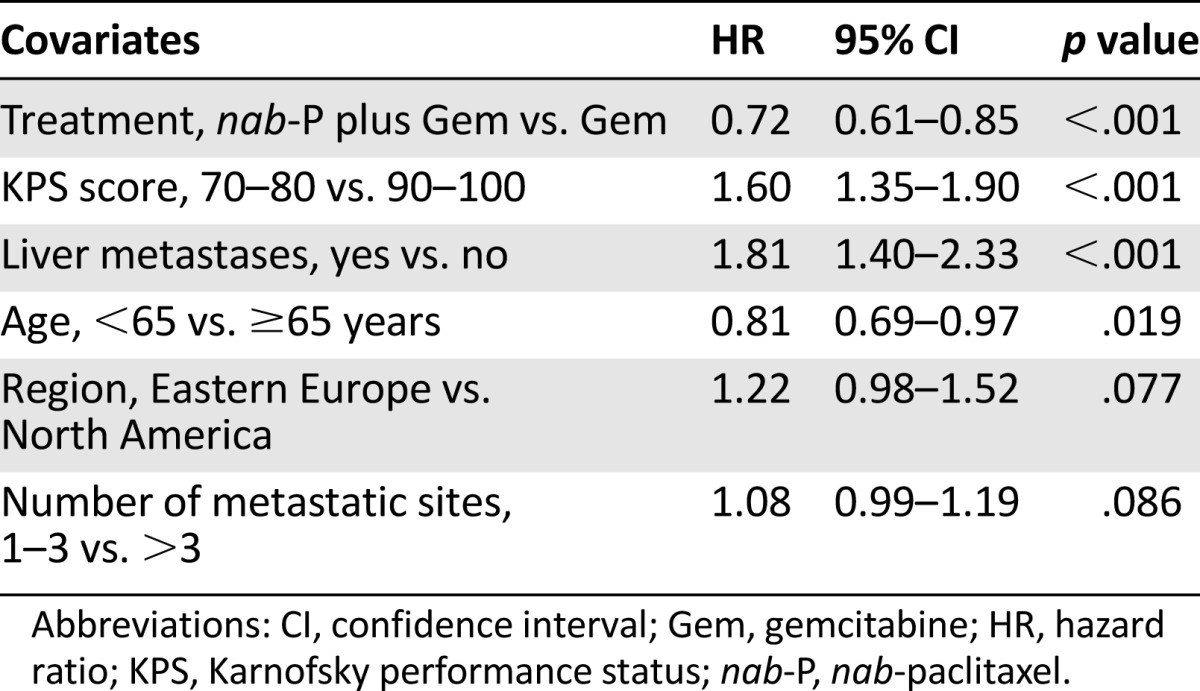
The multivariate analysis with the stepwise procedure also revealed a reduction in risk of progression favoring nab-paclitaxel plus gemcitabine (HR: 0.66; 95% CI: 0.54–0.80; p < .001), as did the multivariate analysis using factors selected from the stepwise procedure and CA19-9 (HR: 0.66; 95% CI: 0.55–0.79; p < .001). Certain prognostic factors affected PFS regardless of treatment (Table 5). Consistent with OS analyses, age ≥65 years, poorer KPS score, and the presence of liver metastasis were each associated with an increased risk of progression.
Table 5.
Stepwise multivariate analysis for predictors of progression-free survival
Discussion
MPACT is the largest published phase III trial to date to prospectively analyze known prognostic factors in patients with MPC [6–8, 10]. The results of this analysis confirm and extend the findings from the previous multivariate analysis of treatment effect adjusted for stratification factors, which identified treatment with nab-paclitaxel plus gemcitabine, KPS score, and presence or absence of liver metastases as independent predictors of survival. After adjusting for significant predictors of OS in the multivariate stepwise Cox proportional hazards model, the degree of the treatment effect on OS in favor of nab-paclitaxel plus gemcitabine was not significantly altered [5]. In addition, Cox modeling of prespecified prognostic factors revealed that age and number of metastatic sites were also independent prognostic factors of survival outcomes in the MPACT trial.
This analysis validates the stratification factors of KPS score and presence of liver metastases used for trial randomization because each was significantly predictive of OS in the multivariable stepwise Cox proportional hazards model analysis. An increased risk of death was observed for patients with poorer KPS score and patients with liver metastases compared with those without liver metastases. Interestingly, patients in Eastern Europe had a higher risk of death than those in North America. Treatment patterns differ by geographic region, and this may be reflected in the different outcomes observed. These findings suggest that the study was well designed and may help to inform future trial designs in the metastatic setting. In addition to confirming the prognostic value of KPS score and presence of liver metastases, the stepwise model revealed that age and number of metastatic sites were significant independent prognostic factors of increased risk of death in the MPACT trial. Patients with the most advanced disease characteristics generally had the greatest reduction in the risk of progression or death (i.e., those with poor performance status, presence of liver metastasis, or more than three metastatic sites). Accordingly, nab-paclitaxel plus gemcitabine resulted in a 3.8-month longer median OS for patients with more than three metastatic sites compared with gemcitabine alone (p < .001). In addition, patients aged ≥65 years receiving nab-paclitaxel plus gemcitabine had an ∼1-month longer median OS than those receiving gemcitabine alone (p = .082). No overall differences in effectiveness were observed between patients in the combination arm who were aged ≥65 and those aged <65 years. The differences in the elderly population between the two arms are intriguing and clinically relevant and thus warrant further evaluation. Although toxicity data in patients aged ≥65 years have not been reported for the MPACT study to date, a separate analysis of dosing in the study revealed that the percentage of dose reductions in the nab-paclitaxel plus gemcitabine arm was similar regardless of age (≥65 vs. <65 years) [11]. Taken together, the findings of this analysis are particularly relevant considering that patients with these characteristics (e.g., advanced age, poor performance status, presence of liver metastasis, or more than three metastatic sites) tend to have especially poor survival outcomes [12–15].
CA19-9 has been demonstrated to be a univariate predictor of OS in pancreatic cancer, with high values associated with poor survival [16]. Studies have shown correlations of CA19-9 levels with clinical outcomes [17, 18]. Baseline CA19-9 level has been demonstrated to be a prognostic factor for survival in patients with advanced pancreatic cancer treated with single-agent gemcitabine; however, CA19-9 response was not found to be independently predictive of survival with gemcitabine [18]. Levels of CA19-9 ≥59 × ULN have been associated with the poorest prognosis. In a large CA19-9 analysis (n = 247) of a randomized trial of patients with advanced pancreatic cancer receiving gemcitabine or gemcitabine plus capecitabine, a baseline level of CA19-9 greater than the study median of 59 × ULN was associated with a worse outcome than CA19-9 <59 × ULN [12]. Baseline CA 19-9 values were markedly elevated in this study but were balanced between arms [5]. Nearly half the patients (46% in the nab-paclitaxel plus gemcitabine arm and 45% in the gemcitabine-alone arm) had a CA19-9 level ≥59 × ULN. Patients with a baseline CA19-9 level ≥59 × ULN had a significantly longer median OS with nab-paclitaxel plus gemcitabine versus gemcitabine alone. In a separate analysis of the phase III MPACT trial, patients receiving nab-paclitaxel plus gemcitabine who had baseline CA19-9 at or above the median (2,469.75 U/mL) had a significantly longer median OS compared with those receiving gemcitabine alone (p < .0001); a similar nonsignificant trend was noted for patients with a CA19-9 below the median (p = .1126) [19]. Patients receiving nab-paclitaxel plus gemcitabine with a baseline CA19-9 level lower than the median had a similar median OS compared with those with a baseline CA19-9 level at or above the median (p = .470). Based on the results of this single phase III trial, whether CA19-9 should continue to be used as a stratification factor in trials of nab-paclitaxel plus gemcitabine remains unclear. Results of a prior phase I/II study demonstrated that CA19-9 level decline may be predictive of outcomes with nab-paclitaxel plus gemcitabine [20].
High stromal secreted protein acidic and rich in cysteine (SPARC) expression has also been associated with poor prognosis in resected pancreatic cancer, and high stromal and tumor SPARC expression has been associated with worse outcomes following adjuvant gemcitabine treatment [21, 22]. An exploratory analysis of OS and SPARC expression in the MPACT trial found neither stromal nor tumor SPARC levels to be prognostic or predictive of clinical outcomes in MPC [23]. Although KPS score and presence or absence of liver metastases were significant predictors of OS, no significant effect on OS was observed for baseline CA19-9 levels, and this finding is consistent with data from the current primary analysis of the ITT population. When taken together, the findings from the current analysis and additional exploratory analyses of the MPACT trial appear to suggest that CA19-9 level is not prognostic of survival in patients with MPC.
The relevance of the findings of the present analysis—which demonstrated the influence of KPS score, age, and region on OS—is also noteworthy with regard to the phase III trial of the FOLFIRINOX regimen versus gemcitabine for MPC [4]. That trial was conducted entirely in France and excluded patients aged ≥75 years or with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 2. MPACT was conducted in 11 countries, did not exclude patients based on age (10% of patients were aged ≥75 years), and included patients with a KPS score of ≥70 (8% of patients had a KPS score ≤70) [5]; a KPS score of 70 corresponds to an ECOG PS of 2 [24].
Although not significant with the models used in this analysis, the treatment effect trended in favor of nab-paclitaxel plus gemcitabine for OS and PFS in patients with other clinically relevant, prognostically poor baseline features. In patients with peritoneal carcinomatosis, an aggressive feature of pancreatic cancer that further compromises survival [25], a >3-month improvement in both median OS and PFS was observed for nab-paclitaxel plus gemcitabine versus gemcitabine. However, given the small number of patients and the fact that the two arms were not well balanced with respect to this variable, data must be interpreted with caution.
A limitation of this analysis is a lack of cross-validation. It should also be noted that some differences existed in the final multivariate models of OS and PFS. Any differences in care occurring after disease progression could have affected OS but not PFS. Consequently, it is not surprising that the final multivariate models of OS and PFS did not contain exactly the same variables.
Conclusion
In the phase III MPACT trial, KPS score, presence of liver metastases, age, and number of metastatic sites were found to be the most important predictors of survival, supporting the clinical utility of these factors when making treatment decisions and for future clinical trial design. Baseline CA19-9 level was not an independent predictor of OS in the multivariate analysis. After correcting for known prognostic factors, treatment with nab-paclitaxel plus gemcitabine remained an independent, highly significant predictor of improved OS and PFS in patients with MPC. The results of this analysis substantiate the overall finding of the MPACT trial that nab-paclitaxel plus gemcitabine is a more active treatment than gemcitabine alone across a broad spectrum of patients.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
We thank all participating sites and investigators for their support with the clinical study. The study was funded by Celgene Corporation. Statistical programming assistance was provided by Helen Liu, MS. Medical writing assistance was provided by Christopher Carter, MediTech Media, funded by Celgene Corporation. All authors had full access to the data and are fully responsible for content and editorial decisions for this manuscript. Josep Tabernero had final responsibility for the decision to submit for publication.
Author Contributions
Conception/Design: Josep Tabernero, E. Gabriela Chiorean, Jeffrey R. Infante, Sunil R. Hingorani, Vinod Ganju, Colin Weekes, Werner Scheithauer, Ramesh K. Ramanathan, David Goldstein, Alfredo Romano, Stefano Ferrara, Daniel D. Von Hoff
Provision of study material or patients: Josep Tabernero, E. Gabriela Chiorean, Jeffrey R. Infante, Sunil R. Hingorani, Vinod Ganju, Colin Weekes, Werner Scheithauer, Ramesh K. Ramanathan, David Goldstein, Daniel D. Von Hoff
Collection and/or assembly of data: Josep Tabernero, Darryl N. Penenberg, Alfredo Romano, Stefano Ferrara, Daniel D. Von Hoff
Data analysis and interpretation: Josep Tabernero, E. Gabriela Chiorean, Jeffrey R. Infante, Sunil R. Hingorani, Vinod Ganju, Colin Weekes, Werner Scheithauer, Ramesh K. Ramanathan, David Goldstein, Darryl N. Penenberg, Alfredo Romano, Stefano Ferrara, Daniel D. Von Hoff
Manuscript writing: Josep Tabernero, E. Gabriela Chiorean, Jeffrey R. Infante, Sunil R. Hingorani, Vinod Ganju, Colin Weekes, Werner Scheithauer, Ramesh K. Ramanathan, David Goldstein, Darryl N. Penenberg, Alfredo Romano, Stefano Ferrara, Daniel D. Von Hoff
Final approval of manuscript: Josep Tabernero, E. Gabriela Chiorean, Jeffrey R. Infante, Sunil R. Hingorani, Vinod Ganju, Colin Weekes, Werner Scheithauer, Ramesh K. Ramanathan, David Goldstein, Darryl N. Penenberg, Alfredo Romano, Stefano Ferrara, Daniel D. Von Hoff
Disclosures
Josep Tabernero: Celgene, Amgen, Inclone, Lilly, Merck KGaA, Millennium; Novartis, Roche, Sanofi, Chugai, Taiho (C/A); E. Gabriela Chiorean: Celgene (RF); Colin Weekes: Celgene (H, SAB); Werner Scheithauer: Celgene (C/A, RF, H); Ramesh K. Ramanathan: Celgene (C/A, RF); David Goldstein: Celgene (C/A, RF); Darryl N. Penenberg: Celgene (ET); Alfredo Romano: Celgene (ET); Stefano Ferrara: Celgene (ET, OI); Daniel D. Von Hoff: Celgene (C/A, RF, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.GLOBOCAN. 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available at http://globocan.iarc.fr/. Accessed September 30, 2014.
- 2.Howlader N, Noone AM, Krapcho M et al. SEER cancer statistics review, 1975–2010. Available at http://seer.cancer.gov/csr/1975_2010/. Accessed September 30, 2014.
- 3.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson B, Aho U, Pendse M, et al. Gemcitabine treatment in pancreatic cancer – prognostic factors and outcome. Ann Gastroenterol. 2007;20:130–137. [Google Scholar]
- 7.Bauer TM, El-Rayes BF, Li X, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: A pooled analysis of 6 prospective trials. Cancer. 2013;119:285–292. doi: 10.1002/cncr.27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inal A, Kos FT, Algin E, et al. Prognostic factors in patients with advanced pancreatic cancer treated with gemcitabine alone or gemcitabine plus cisplatin: Retrospective analysis of a multicenter study. J BUON. 2012;17:102–105. [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DX, Dai YD, Yuan SX, et al. Prognostic factors in patients with pancreatic cancer. Exp Ther Med. 2012;3:423–432. doi: 10.3892/etm.2011.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheithauer W, Von Hoff DD, Ramanathan RK et al. Dose delivery in a phase III trial (MPACT) of weekly nab-paclitaxel (nab-P) plus gemcitabine (Gem) vs Gem alone for patients with metastatic adenocarcinoma of the pancreas [abstract 2.586]. Presented at: European Cancer Congress; September 27–October 1, 2013; Amsterdam, The Netherlands. [Google Scholar]
- 12.Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132–138. doi: 10.1016/S1470-2045(08)70001-9. [DOI] [PubMed] [Google Scholar]
- 13.Eichbaum MH, Kaltwasser M, Bruckner T, et al. Prognostic factors for patients with liver metastases from breast cancer. Breast Cancer Res Treat. 2006;96:53–62. doi: 10.1007/s10549-005-9039-1. [DOI] [PubMed] [Google Scholar]
- 14.Maltoni M, Pirovano M, Scarpi E, et al. Prediction of survival of patients terminally ill with cancer. Results of an Italian prospective multicentric study. Cancer. 1995;75:2613–2622. doi: 10.1002/1097-0142(19950515)75:10<2613::aid-cncr2820751032>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Garrido-Laguna I, Janku F, Vaklavas C, et al. Validation of the Royal Marsden Hospital prognostic score in patients treated in the phase I clinical trials program at the MD Anderson Cancer Center. Cancer. 2012;118:1422–1428. doi: 10.1002/cncr.26413. [DOI] [PubMed] [Google Scholar]
- 16.Stocken DD, Hassan AB, Altman DG, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer. 2008;99:883–893. doi: 10.1038/sj.bjc.6604568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boeck S, Stieber P, Holdenrieder S, et al. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70:255–264. doi: 10.1159/000094888. [DOI] [PubMed] [Google Scholar]
- 18.Saad ED, Machado MC, Wajsbrot D, et al. Pretreatment CA 19-9 level as a prognostic factor in patients with advanced pancreatic cancer treated with gemcitabine. Int J Gastrointest Cancer. 2002;32:35–41. doi: 10.1385/IJGC:32:1:35. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein D, El Maraghi RH, Hammel P, et al. Updated overall survival from a randomized phase III trial (MPACT) of nab-paclitaxel plus gemcitabine versus gemcitabine alone for patients with metastatic adenocarcinoma of the pancreas. J Clin Oncol. 2014;32(suppl 3):178a. [Google Scholar]
- 20.Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 22.Sinn M, Sinn BV, Striefler JK, et al. SPARC expression in resected pancreatic cancer patients treated with gemcitabine: Results from the CONKO-001 study. Ann Oncol. 2014;25:1025–1032. doi: 10.1093/annonc/mdu084. [DOI] [PubMed] [Google Scholar]
- 23.Hidalgo M, Plaza C, Illei PB, et al. SPARC analysis in the phase III MPACT trial of nab-paclitaxel (nab-P) plus gemcitabine (Gem) vs Gem alone for patients with metastatic pancreatic cancer (PC) Ann Oncol. 2014;25(suppl 2):ii106. [Google Scholar]
- 24.Ma C, Bandukwala S, Burman D, et al. Interconversion of three measures of performance status: An empirical analysis. Eur J Cancer. 2010;46:3175–3183. doi: 10.1016/j.ejca.2010.06.126. [DOI] [PubMed] [Google Scholar]
- 25.Thomassen I, Lemmens VE, Nienhuijs SW, et al. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: A population-based study. Pancreas. 2013;42:72–75. doi: 10.1097/MPA.0b013e31825abf8c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.