Abstract
Introduction
The nuclear receptor pregnane X receptor (PXR) is a well-characterized hepatic xenobiotic sensor whose activation by chemically diverse compounds results in the induction of drug clearance pathways that rid the body of potentially toxic substances, thus conferring protection from foreign chemicals and endobiotics.
Area covered
PXR activities are implicated in drug-drug interactions and endocrine disruption. Recent evidence supports a hepatoprotective role for PXR in chronic liver injury, inhibiting liver inflammation through suppression of the NF-κB pathway. However, PXR-mediated induction of CYP3A enhances APAP-induced acute liver injury by generating toxic metabolites. While these observations implicate PXR as a therapeutic target for liver injury, they also caution against PXR activation by pharmaceutical drugs.
Expert opinion
While evidence of PXR involvement in acute and chronic liver injuries identifies it as a possible therapeutic target, it raises additional concerns for all drug candidates. The in vitro and in vivo tests for human PXR activation should be incorporated into the FDA regulations for therapeutic drug approval to identify potential liver toxicities. In addition, PXR pharmacogenetic studies will facilitate the prediction of patient-specific drug reactivities and associated liver disorders.
Keywords: PXR, liver injury, xenobiotic, nuclear receptor, drug target
1. Nuclear receptor superfamily
Nuclear receptors are members of a superfamily of ligand-inducible transcription factors mediating responses to endogenous steroids, retinoids and thyroid hormones. They regulate specific target genes involved in metabolism, development, reproduction and other physiological processes1,2,3. The cloning of the first nuclear receptor, the human glucocorticoid receptor (hGR) in 19854, facilitated the identification of multiple additional family members by low stringency hybridization screening of cDNA libraries such as the estrogen receptor (ER), progesterone receptor (PR), thyroid hormone receptor (TR), retinoic acid receptor (RAR), Vitamin D receptor (VDR), mineralocorticoid receptor (MR) and androgen receptor (AR) 4-9. The nuclear receptor family is comprised of 48 members in humans and includes 36 orphan nuclear receptors, receptors which lack identified physiological ligands 10.
The classical steroid hormone receptors such as GR, MR, PR, AR and ER form functional homodimers upon ligand binding that recognize hormone response elements (HREs) on target DNA to control gene expression. GR, MR, PR, and AR bind inverted repeats of the half site AGAACA (GRE) while ER binds an inverted repeat of the half site AGGTCA (ERE). The 36 orphan receptors bind to a half site either as a monomer, dimer, or heterodimer with the retinoid X receptor (RXR). Biological roles for the orphan receptors, particularly those which heterodimerize with RXR, have been elucidated through the identification of functional response elements and, ultimately, the discovery of relevant endogenous and synthetic ligands. Further understanding has been achieved by creating knock-out mouse models. It is now known that many orphan receptors act as low affinity sensors for abundant dietary lipids, contrasting with the high affinity steroid receptors have for low abundant hormones. For example, the liver X receptors (LXRs), peroxisome proliferator activated receptors (PPARs) and farnesoid X receptor (FXR) have been identified as sensors for cholesterol, fatty acids, and bile acids, respectively, and shown to cooperatively regulate lipid homeostasis (reviewed in 11).
2. PXR modulates hepatic drug metabolism
In the hepatic drug clearance system, Phase I enzymes, especially members of the cytochrome P450 (CYP) 1-4 families, play important roles in xenobiotic detoxification and survival of organisms 12. Among them, the human CYP3A and CYP2B isoenzymes are involved in the metabolism of a large portion of clinical drugs (reviewed in 13). One important characteristic of these CYP enzymes is their substrate inducibility, which allows production of these proteins to be tailored to meet requirements. For example, CYP3A is induced upon treatment with the antibiotic rifampicin14, while CYP2B production is increased by the treatment with the anti-epileptic drug phenobarbital (PB) in humans 15 and the planar hydrocarbon 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) in mice 16. In the early 1990s, researchers found that the Aryl hydrocarbon receptor (AhR) regulates transcriptional activation of the CYP1A genes 17, however, the mechanism of CYP gene induction by xenobiotics was not clear until pregnane X receptor (PXR) and constitutive androstane receptor (CAR) were defined as xenobiotic receptors.
2.1 PXR identified as a xenobiotic receptor
Pregnane X receptor (PXR), also known as the steroid and xenobiotic sensing nuclear receptor (SXR) and NR1I2, was originally identified in mice and found to be activated by naturally occurring steroids such as pregnenolone and progesterone, as well as by both synthetic glucocorticoid agonists and antagonists18-20. In addition, PXR was shown to bind to the CYP3A2 promoter and activate transcription in response to potent CYP3A2 inducers 18. This unusual response profile of PXR offered a possible explanation for the behavior of catatoxic compounds, compounds that induce their own catabolism, through the induction of CYP3A genes 2, 21, 22,23.
Evidence supporting PXR as a xenobiotic receptor regulating CYP3A's expression emerged rapidly. Both PXR and CYP3As are highly expressed in the liver and intestine, the major sites of drug clearance. PXR and its heterodimeric partner RXR, bind to a DR3 (direct repeats of AGGTCA or closely related sequences with a spacing of 3 nucleotides) or ER6 (everted repeats spaced by 6 nucleotides) sites in the CYP3A promoter. The binding of numerous structurally unrelated drugs to PXR, including those known to induce CYP3A expression, was found to dissociate co-repressor molecules such as the silencing mediator for retinoid and thyroid hormone receptor (SMRT) 24 and the nuclear receptor co-repressor (NCoR) from PXR. This is followed by simultaneous recruitment of co-activator molecules, including members of the p160 family (SRC-1, GRIP and ACTR), RIP140 and PBP (DRIP205 or TRAP220) 25. PXR null mice are both viable and fertile, indicating that in the absence of toxic insults the xenobiotic response is not required. However, PXR null mice completely lack inducibility of CYP3A by PCN or PCN-mediated induction of drug resistance 13, 26, 27. Together these observations clearly establish PXR as the central mediator of CYP3A induction.
2.2 Human ortholog of PXR
The human homologue of PXR (hPXR) was first isolated as the steroid and xenobiotic receptor (SXR) 19 and the pregnane-activated receptor (PAR) 20. Notably, the PXR ligand binding domain (LBD) from different species is considerably divergent. Within the LBD, the amino acid identity of human PXR with mouse PXR is only 76%, whereas the DNA-binding domain is highly conserved (96% identity). Thus the species-specificity of the induction of CYP3A enzymes 28 might be due to the pharmacological distinction of human and rodent PXRs. For example, corticosterone and rifampicin are potent hPXR but poor mPXR activators. In contrast, PCN and dexamethasone are strong mPXR but poor hPXR activators. Despite their differences in pharmacology, the two receptors appear to act through a common metabolic pathway. Thus, the structural and pharmacological differences between human and mouse PXRs may reflect differences in rodent and primate diets and the need to respond to a different set of xenobiotics (for review, see 2). For decades, rodent models have been standard components in the assessment of potential toxicities in the development of candidate human drugs. However, the reliability of rodents as predictors of the human xenobiotic response is compromised by the species variation. Xie et al. created a humanized PXR mouse model that responded only to human-specific inducers such as rifampicin but not PCN 26. The xenobiotic response in this mouse model offers a standardized in vivo system for predicting potential human drug-drug interactions and is beneficial for development of new therapeutic drugs. Cultured human primary hepatocytes are valuable alternative tools, but are compromised by inter-individual variability, limited and unpredictable availability as well as high cost. Thus, the generation of the humanized PXR mice represented a major step toward generating a standardized humanized toxicological model. For review, see 13, 29.
2.3 Diversity of PXR modulators
As a xenobiotic receptor, PXR binds a highly diverse range of structurally unrelated chemicals. In fact, X-ray crystal structures of the PXR LBD have revealed that its ligand-binding pocket is relatively large compared to most other nuclear receptors, and can even accommodate a single hydrophobic ligand in multiple configurations 30. Such molecular plasticity in ligand recognition is consistent with the low substrate specificity of xenobiotic enzymes. As a result, PXR activators include antibiotics such as rifampicin, cholesterol lowering drugs such as SR12813 and statins, antidepressants like the active component of St. John's wort hyperforin, the anti-neoplastic drug paclitaxel, the anti-mycotic clotrimazole (reviewed in 21), bisphenol A 31, organochlorine pesticides such as chlordane, Cafestol 32, dieldrin and endosulfan 33. Other environmental contaminants including endocrine disrupting chemicals such as nonylphenol and phthalic acid, nonplanar polychlorinated biphenyls (PCBs) and organochloride pesticides such as trans-nonachlor and chlordane have all been shown to activate mouse PXR 34. With relevance to endocrine disruption, human PXR is also activated by numerous endobiotics including bile acids, corticosterone and estradiol as well as other estrogenic chemicals including diethylstilbestrol, and the phytoestrogen coumestrol 19, 35. In addition to its activators, there are also a number of PXR antagonistic ligands. For example, ecteinascidin-734 blocks PXR-mediated induction of CYP3A 25, and arsenite inhibits both untreated and rifampicin induced CYP3A transcription in primary human hepatocytes by decreasing the activity of PXR, as well as expression of its heterodimeric nuclear receptor partner RXRα 36, 37. Thus, in principle, it should be possible to design specific drugs which selectively inhibit or promote the xenobiotic response.
3. PXR plays roles in chronic liver disease
Activation of PXR has been shown to have anti-fibrotic effects in a carbon tetrachloride-induced liver fibrogenesis model 38. In addition, the PXR activator rifampin has been shown to reduce pruritus associated with primary biliary cirrhosis in humans (PBC) 39, 40, although the mechanism of action was unclear. However, recent work has suggested that the anti-inflammatory actions of PXR agonists such as cyclosporine A are due to inhibition of NF-kB activity 41. Furthermore, the PXR activator clotrimazole was protective in an ischemia-reperfusion liver injury model in rats 42. As PXR activation promotes hepatocyte growth, is anti-fibrogenic 38, and anti-inflammatory, PXR activators may be better drugs for the treatment of chronic inflammatory liver fibrosis compared to other non-PXR activating drugs such as Tacrolimus (FK-506).
In addition to environmental toxins, our body is continuously exposed to a variety of endogenous chemicals. For example, the intrahepatic retention of cytotoxic bile acids (BAs) results in cholestasis, which is a very common type of liver injury. Farnesoid X receptor (FXR) plays a central role in regulating BA homeostasis and is a promising drug target for cholestatic liver injury 43-45. In addition to FXR, the xenobiotic nuclear receptors PXR and CAR also regulate BA homeostasis through sensing toxic by-products. For reviews, see 46,47. Lithocholic acid (LCA), a hydrophobic secondary bile acid is primarily formed in the intestine and known to cause chronic cholestatic liver disease 48. Staudinger et al. and Xie et al. first showed that PXR protects against liver toxicity by inducing the expression of CYP3A subfamily members in response to elevated LCA levels, resulting in hydroxylation of LCA that facilitates its excretion 49, 50. Three lines of evidence support this notion. First, LCA and its direct metabolite 3-keto LCA directly bind to and activate PXR. Second, in vivo activation of PXR by administration of PCN or by expression of a constitutively active form of PXR in the liver of transgenic mice results in marked resistance to LCA toxicity in rodents. Finally, as stated above, the potent CYP3A inducer and agonist for human PXR, rifampicin, has been reported to be effective in treating pruritus associated with chronic cholestasis. Detoxification of LCA by PXR appears to be mediated by the combined induction of CYP3A and the cytosolic sulfotransferase ST2A, both of which convert LCA to non-toxic metabolites. Thus, the drug clearance pathway regulated by PXR can be utilized to detoxify endogenously produced toxins. The reported ability of PXR to regulate inducible nitric oxide synthase (iNOS) gene expression has been proposed as a possible mechanism for the anti-inflammatory effects of steroids and xenobiotics 51.
PXR activation was also found to be protective in the bile duct ligation (BDL) model of cholestasis, through its regulation of cholesterol metabolism, bile acid synthesis, and multiple detoxification pathways 52. The marked increase in Cyp3a11 and Mrp3/4 hepatic expression levels in BDL mice suggest that PXR plays a protective role in cholestatic liver injury by increasing hydroxylation and efflux of toxic bile acids from hepatocytes into blood 53,54,55. Hepatic damage from bile acid accumulation was increased in PXR null mice, which suggests its potential role as a therapeutic target for the treatment of cholestasis and lipid disorders.
4. PXR – a double-edged sword in acute liver injury
Acute liver injury (ALI) refers to rapid degeneration of hepatic function in patients without known prior liver disease. When ALI becomes severe with impaired liver synthetic function, specifically coagulopathy and mental status changes (encephalopathy), it causes acute liver failure (ALF), which includes fulminant hepatic failure (FHF) and subfulminant hepatic failure (or late-onset hepatic failure). These ALF patients often present with the additional problems of confusion or coma (encephalopathy) and bruising or bleeding (coagulopathy). It was reported that up to 80% of people with fulminant hepatitis die within days to weeks. In the U.S., acetaminophen (Tylenol, or APAP) is the most common cause of ALF. Other causes of ALF include idiosyncratic reaction to medication (e.g. tetracycline, troglitazone), excessive alcohol intake, viral hepatitis, and acute fatty liver of pregnancy.
PXR activation and CYP3A induction have been shown to enhance APAP induced liver injury. APAP overdose triggers liver toxicities through the reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which is generated from APAP oxidized by hepatic CYP2E1, CYP1A2, and CYP3A. PCN treated mice 56, 57 or rifampin treated humanized mice 58 exhibit high sensitivity to APAP liver toxicity through the induction of the CYP3A subfamily. Furthermore, Polyinosinic-polycytidylic acid (polyI:C), which is known as a PXR suppressor /antagonist, suppresses APAP-induced hepatotoxicity 59, suggesting that PXR antagonists with less side-effects might help reduce APAP-induced liver toxicity.
Wang et al. recently showed that lipopolysaccharide (LPS)/D-galactosamine (GalN)-treated PXR-null mice had a greater degree of acute liver injury compared to wild type mice. The elevated alanine aminotransferase (ALT), hepatocyte apoptosis, necrosis, and hemorrhagic liver injury in PXR null mice may be due to deregulated MAP kinase activation and delayed Jak2/Stat3 activation, which lead to a compromise in defence mechanisms that involve Bcl-xL, HO-1, and autophagy-mediated pathways 60.
5. PXR gene polymorphism affects liver injury
The antibiotic flucloxacillin functions as a PXR agonist to induce the expression of CYP3A4 and CYP2C9 genes 61, and a PXR polymorphism (rs3814055; C-25385T) has been associated with an increased risk of drug induced liver injury 62. The cause of potentiation of the risk is unclear, however, the C-25385T homozygotes have significantly less basal PXR expression and thus less CYP3A induction, which may slow down the flucloxacillin disposition in these genotypes. In addition, it has been suggested that the PXR polymorphisms (rs7643645 and rs2461823) may contribute to disease severity in nonalcoholic fatty liver disease (NAFLD) by influencing the individual's susceptibility to progress to more severe stages of the disease 63.
6. Summary
PXR coordinately regulates a large number of genes involved in all stages of xenobiotic metabolism including cytochrome P450 subfamilies (CYP2C, CYP1A, CYP1B, CYP2A and CYP4F), and other Phase I reductases and hydrolases (carboxylesterase, monoamine oxidase, catalase and flavin-containing monooxygenases (FMO)), Phase II conjugating enzymes which solubilize hydrophobic compounds in preparation for clearance (UDP-glucuronosyltransferase (UGT), cytosolic sulfotransferase (SULT) and GST), and Phase III membrane-bound transporters which act as efflux pumps to clear drugs and drug conjugates (MDR1 and MRP2) (Figure 1) (reviewed in 21, also see25, 64, 65, 66). In combination with the constitutive androstane receptor (CAR, NR1I3A), PXR regulates the expression of the major hepatic Phase I enzymes responsible for clinical drug clearance, the CYP2Bs and 3As26. Indeed, analysis of the promoter regions and identification of receptor binding sites of PXR/CAR target genes reveal that both PXR and CAR can adaptively bind to common response elements51,67,68. In addition to CYP3A, CAR has also been shown to regulate a similar array of xenobiotic genes including several CYP enzymes, aldehyde dehydrogenase, esterase, FMO, methyl transferase, GST69, SULT70, UGT71, and MRP267 as well as iNOS51. The ability of the xenobiotic receptors to respond to a numerous yet overlapping set of drugs and regulate a sophisticated network of metabolic genes explains why in some cases CAR activation can have a similar protective role in liver injury. For example, Stedman et al. showed that both CAR and PXR influence cholesterol metabolism and bile acid synthesis by repressing and inducing the specific hepatic membrane transporters Oatp-c (organic anion transporting polypeptide C) and Oatp2 (Na+-dependent organic anion transporter 2), respectively 52. This suggests their complementary roles as therapeutic targets for the treatment of cholestasis and lipid disorders. For reviews, please see 46,47.
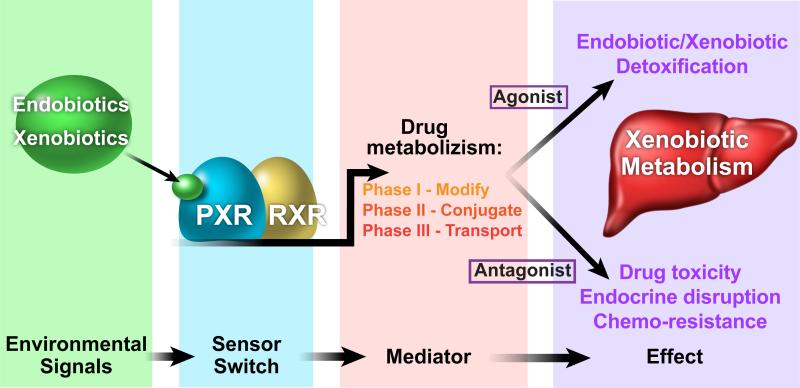
Figure 1.
Model depicting the central role of the nuclear receptor PXR in the liver response to endobiotic and xenobiotic compounds. Activation of PXR by structurally and functionally diverse ligands induces the transcription of drug metabolizing enzymes and transporters. Compound modifications facilitate their removal (detoxicification), or conversely, increase their toxicity, resulting in systemic disorders such as endocrine disruption and chemo-resistance.
While PXR regulates drug clearance related genes, its expression is in turn regulated, indicative that xenobiotic metabolism is involved in other physiological events. Activation of the glucocorticoid receptor (GR) has been shown to induce expression of PXR, CAR and their heterodimeric partner RXR in cultured cells72, 73. In addition, in the rodent liver PXR expression is auto-induced by PCN and PPARα specific drugs such as perfluorodecanoic acid and clofibrate74. Kamiya et al. also showed that HNF4α is the key transcription factor regulating responses to xenobiotics through activation of the PXR gene during fetal liver development75. In theory, induction of the xenobiotic receptors could potentiate the induction of downstream target genes. Therefore, some therapeutic drugs may be able to activate/induce PXR indirectly and influence liver injury. Further studies are expected to reveal the relevance of xenobiotic receptor regulation, and its impact on liver injury.
It is known that hPXR activators such as phenytoin and RU486 cause immunosuppressive side effects; on the other hand, inflammation and infection reduce hepatic CYP expression76-78. In addition, the levels of hepatic PXR and CAR mRNA have also been reported to be downregulated in response to inflammatory signals79,80. This broaches the question of whether xenobiotic receptors communicate with the immune system. Recently PXR has been reported to crosstalk with NF-κB signaling pathways, which regulate inflammation and the immune response81. The activation of hPXR inhibits NF-κB activity whereas NF-κB target genes are upregulated and small bowel inflammation is significantly increased in PXR null mice. On the other hand, NF-κB activation reciprocally inhibits hPXR and its target genes. Therefore, PXR activators may act as better drugs for the treatment of chronic inflammatory liver diseases comparing to other non-PXR activator drugs.
In addition to its xenobiotic metabolism function, increasingly PXR is being seen as a regulator of hepatic damage. Further advancements in our understanding of the complexities of PXR's xeno/endobiotic regulation will advance PXR as a potential pharmaceutical target for healing both chronic and acute liver injuries.
Expert Opinion
Recent findings implicate PXR as a potential therapeutic target in acute liver injury. Importantly, PXR targeted therapies offer the potential to be tailored to the specific liver injury; PXR antagonists for APAP-induced ALI and PXR agonists for LPS-induced ALI (section 4). However, given the central role of PXR in liver xenobiotic and endobiotic metabolism, potential side effects of such therapies need to be thoroughly investigated (section 2.3), as well as possible patient variability in drug efficacy due to PXR gene polymorphisms.
The activation of xeno-receptors (PXR, CAR and AhR) in liver induces hepatic drug clearance enzymes including CYP3A, CYP2B, CYP2C, CYP1A UGT1A and MDR1. Importantly, the CYP3A family of enzymes involved in the metabolism of a large number of therapeutic drugs are regulated by PXR. Many drug-drug interactions have been attributed to PXR activation including the reduced effectiveness of oral contraceptives by St. John's wort82, increased warfarin requirements when co-administered with rifampin83,84, reduced serum levels and pharmacological effects of Cyclosporine A when combined with troglitazone treatment85,86, and increased midazolam clearance after avasimibe administration87. The recent studies implicating PXR activation in both chronic and acute liver injuries further emphasize its central role in xenobiotic metabolism. While testing of candidate drugs for PXR activity is not mandatory, performing such tests during the development phases may identify serious drug-drug interactions or unexpected liver diseases. Such testing should involve a combination of in vitro and in vivo assays incorporating humanized liver drug metabolism genes such as hCYP3A88, hCYP2C89 and hUGT1A90,91 and humanized PXR to more accurately predict possible drug-drug interactions and toxicity.
Personalized medicine will play an increasingly important role in modern medicine. Already individual genetic information is used to predict disease susceptibility (e.g. BRCA1 and BRCA2 mutations and breast cancer92), and has been associated with empirically determined drug dosages (e.g. warfarin treatments based on VKOR gene SNPs93). The finding that particular PXR gene polymorphisms are associated with liver injury (section 5) suggests that, in the future, pharmacological dosing will also be determined based on the individual's genetic information. Already around 100 SNPs have been identified in the human PXR gene (UCSC Genome browser). The combination of increasingly lower costs for genome sequencing and an increase in pharmacogenetic studies on widely used drugs, will no doubt lead to additional PXR SNPs being associated with individual drug sensitivities and susceptibilities to a variety of liver diseases. We believe that the application of pharmacogenetics, in particular associating PXR gene polymorphisms with pharmaceutical drug pharmacokinetcs, will form a significant part of modern personalized medicine.
highlights.
- PXR belongs to the nuclear receptor superfamily
- Transcriptional regulation by PXR plays important roles in hepatic xeno/endobiotic metabolism
- PXR affects chronic liver diseases
- Transcriptional activities of PXR implicated in acute liver injury
- PXR gene polymorphisms are associated with susceptibility to liver injury
Acknowledgements
The authors thank E. Ong and S. Ganley for administrative assistance. This work was supported, in part, by grants from NIH (ES10337), Ipsen/Biomeasure, The Helmsley Charitable Trust, Howard Hughes Medical Institute, and National Health and Medical Research Council of Australia Project grant 512354.
Bibilography
Papers of special note have been highlighted as either of interest
• or of considerable interest
•• to readers.
- 1.McKenna NJ, O'Malley BW. Teaching resources. An interactive course in nuclear receptor signaling: concepts and models. Sci STKE. 2005;2005:tr22. doi: 10.1126/stke.2992005tr22. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg B, Evans RM. Orphan nuclear receptors--new ligands and new possibilities. Genes Dev. 1998;12:3149–55. doi: 10.1101/gad.12.20.3149. [DOI] [PubMed] [Google Scholar]
- 3.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Weinberger C, Hollenberg SM, Ong ES, et al. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985;228:740–2. doi: 10.1126/science.2581314. [This manuscript describes the cloning of the first nuclear receptor-Glucocorticoid receptor.] [DOI] [PubMed] [Google Scholar]
- 5.Green S, Walter P, Kumar V, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–9. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 6.Conneely OM, Sullivan WP, Toft DO, et al. Molecular cloning of the chicken progesterone receptor. Science. 1986;233:767–70. doi: 10.1126/science.2426779. [DOI] [PubMed] [Google Scholar]
- 7.Petkovich M, Brand NJ, Krust A, et al. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–50. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 8.McDonnell DP, Mangelsdorf DJ, Pike JW, et al. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987;235:1214–7. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- 9.Lubahn DB, Joseph DR, Sullivan PM, et al. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240:327–30. doi: 10.1126/science.3353727. [DOI] [PubMed] [Google Scholar]
- 10.Giguere V, Yang N, Segui P, et al. Identification of a new class of steroid hormone receptors. Nature. 1988;331:91–4. doi: 10.1038/331091a0. [DOI] [PubMed] [Google Scholar]
- 11.Chawla A, Repa JJ, Evans RM, et al. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–70. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 12.Waxman DJ. P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- 13.Xie W, Evans RM. Orphan nuclear receptors: the exotics of xenobiotics. The J Biol Chem. 2001;276:37739–42. doi: 10.1074/jbc.R100033200. [DOI] [PubMed] [Google Scholar]
- 14.Pichard L, Gillet G, Fabre I, et al. Identification of the rabbit and human cytochromes P-450IIIA as the major enzymes involved in the N-demethylation of diltiazem. Drug Metab Dispos. 1990;18:711–9. [PubMed] [Google Scholar]
- 15.Santisteban I, Povey S, Shephard EA, et al. The major phenobarbital-inducible cytochrome P-450 gene subfamily (P450IIB) mapped to the long arm of human chromosome 19. Ann Hum Genet. 1988;52:129–35. doi: 10.1111/j.1469-1809.1988.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 16.Smith G, Henderson CJ, Parker MG, et al. 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene, an extremely potent modulator of mouse hepatic cytochrome P-450 gene expression. Biochem J. 1993;289:807–13. doi: 10.1042/bj2890807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pendurthi UR, Okino ST, Tukey RH. Accumulation of the nuclear dioxin (Ah) receptor and transcriptional activation of the mouse Cyp1a-1 and Cyp1a-2 genes. Arch Biochem Biophys. 1993;306:65–9. doi: 10.1006/abbi.1993.1481. [DOI] [PubMed] [Google Scholar]
- 18.Kliewer SA, Moore JT, Wade L, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- 19••.Blumberg B, Sabbagh W, Jr., Juguilon H, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–205. doi: 10.1101/gad.12.20.3195. [Manuscripts 18 and 19 describe the first identification of the nuclear receptor pregnane X receptor.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertilsson G, Heidrich J, Svensson K, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95:12208–13. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane x receptor. Ann Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- 22.Sonoda J, Evans RM. Biological function and mode of action of nuclear xenobiotic receptors. Pure Appl Chem. 2003;75:1733–42. [Google Scholar]
- 23.Tao Li JS, Ronald M. Evans. Chapter. 2. Establishing Orphan Nuclear Receptors PXR and CAR as Xenobiotic Receptors. In: Xie W, editor. Nuclear Receptors in Drug Metabolism. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2008. [Google Scholar]
- 24.Johnson DR, Li CW, Chen LY, et al. Regulation and binding of pregnane X receptor by nuclear receptor corepressor silencing mediator of retinoid and thyroid hormone receptors (SMRT). Mol Pharmacol. 2006;69:99–108. doi: 10.1124/mol.105.013375. [DOI] [PubMed] [Google Scholar]
- 25.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–90. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 26••.Xie W, Barwick JL, Downes M, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–9. doi: 10.1038/35019116. [This manuscript describes the first in vivo demonstration of the diverse range of structurally different chemical compounds that bind and activate the human pregnane X receptor.] [DOI] [PubMed] [Google Scholar]
- 27.Staudinger J, Liu Y, Madan A, et al. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 2001;29:1467–72. [PubMed] [Google Scholar]
- 28.Gonzalez FJ, Gelboin HV. Human cytochromes P450: evolution, catalytic activities and interindividual variations in expression. Prog Clin Biol Res. 1991;372:11–20. [PubMed] [Google Scholar]
- 29.Sonoda J, Rosenfeld JM, Xu L, et al. A nuclear receptor-mediated xenobiotic response and its implication in drug metabolism and host protection. Curr Drug Metab. 2003;4:59–72. doi: 10.2174/1389200033336739. [DOI] [PubMed] [Google Scholar]
- 30.Watkins RE, Wisely GB, Moore LB, et al. The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science. 2001;292:2329–33. doi: 10.1126/science.1060762. [DOI] [PubMed] [Google Scholar]
- 31.Takeshita A, Koibuchi N, Oka J, et al. Bisphenol-A, an environmental estrogen, activates the human orphan nuclear receptor, steroid and xenobiotic receptor-mediated transcription. Eur J Endocrinol. 2001;145:513–7. doi: 10.1530/eje.0.1450513. [DOI] [PubMed] [Google Scholar]
- 32.Ricketts ML, Boekschoten MV, Kreeft AJ, et al. The cholesterol-raising factor from coffee beans, cafestol, as an agonist ligand for the farnesoid and pregnane X receptors. Mol Endocrinol. 2007;21:1603–16. doi: 10.1210/me.2007-0133. [DOI] [PubMed] [Google Scholar]
- 33.Coumoul X, Diry M, Barouki R. PXR-dependent induction of human CYP3A4 gene expression by organochlorine pesticides. Biochem Pharmacol. 2002;64:1513–9. doi: 10.1016/s0006-2952(02)01298-4. [DOI] [PubMed] [Google Scholar]
- 34.Schuetz EG, Brimer C, Schuetz JD. Environmental xenobiotics and the antihormones cyproterone acetate and spironolactone use the nuclear hormone pregnenolone X receptor to activate the CYP3A23 hormone response element. Mol Pharmacol. 1998;54:1113–7. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonoda J, Chong LW, Downes M, et al. Pregnane X receptor prevents hepatorenal toxicity from cholesterol metabolites. Proc Natl Acad Sci U S A. 2005;102:2198–203. doi: 10.1073/pnas.0409481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noreault TL, Jacobs JM, Nichols RC, et al. Arsenite decreases CYP3A23 induction in cultured rat hepatocytes by transcriptional and translational mechanisms. Toxicol Appl Pharmacol. 2005;209:174–82. doi: 10.1016/j.taap.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Noreault TL, Kostrubsky VE, Wood SG, et al. Arsenite decreases CYP3A4 and RXRalpha in primary human hepatocytes. Drug Metab Dispos. 2005;33:993–1003. doi: 10.1124/dmd.105.003954. [DOI] [PubMed] [Google Scholar]
- 38.Marek CJ, Tucker SJ, Konstantinou DK, et al. Pregnenolone-16alpha-carbonitrile inhibits rodent liver fibrogenesis via PXR (pregnane X receptor)-dependent and PXR-independent mechanisms. Biochem J. 2005;387:601–8. doi: 10.1042/BJ20041598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bachs L, Pares A, Elena M, et al. Effects of long-term rifampicin administration in primary biliary cirrhosis. Gastroenterology. 1992;102:2077–80. doi: 10.1016/0016-5085(92)90335-v. [DOI] [PubMed] [Google Scholar]
- 40•.Khurana S, Singh P. Rifampin is safe for treatment of pruritus due to chronic cholestasis: a meta-analysis of prospective randomized-controlled trials. Liver Int. 2006;26:943–8. doi: 10.1111/j.1478-3231.2006.01326.x. [Manuscripts 38 to 40 represent the early evidence that the activation of PXR's protective role in chronic liver diseases.] [DOI] [PubMed] [Google Scholar]
- 41.Wallace K, Cowie DE, Konstantinou DK, et al. The PXR is a drug target for chronic inflammatory liver disease. J Steroid Biochem Mol Biol. 2010;120:137–48. doi: 10.1016/j.jsbmb.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iannelli A, de Sousa G, Zucchini N, et al. Clotrimazole protects the liver against normothermic ischemia-reperfusion injury in rats. Transplant Proc. 2009;41:4099–104. doi: 10.1016/j.transproceed.2009.08.074. [DOI] [PubMed] [Google Scholar]
- 43.Repa JJ, Turley SD, Lobaccaro JA, et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–9. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 44.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–8. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 45.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–5. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 46.Kakizaki S, Takizawa D, Tojima H, et al. Nuclear receptors CAR and PXR; therapeutic targets for cholestatic liver disease. Front Biosci. 2012;17:2988–3005. doi: 10.2741/3893. [DOI] [PubMed] [Google Scholar]
- 47.Fiorucci S, Zampella A, Distrutti E. Development of FXR, PXR and CAR agonists and antagonists for treatment of liver disorders. Curr Top Med Chem. 2012;12:605–24. doi: 10.2174/156802612799436678. [DOI] [PubMed] [Google Scholar]
- 48.Fischer S, Beuers U, Spengler U, et al. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin Chim Acta. 1996;251:173–86. doi: 10.1016/0009-8981(96)06305-x. [DOI] [PubMed] [Google Scholar]
- 49.Staudinger JL, Goodwin B, Jones SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369–74. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie W, Radominska-Pandya A, Shi Y, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375–80. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toell A, Kroncke KD, Kleinert H, et al. Orphan nuclear receptor binding site in the human inducible nitric oxide synthase promoter mediates responsiveness to steroid and xenobiotic ligands. J Cell Biochem. 2002;85:72–82. [PubMed] [Google Scholar]
- 52••.Stedman CA, Liddle C, Coulter SA, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci USA. 2005;102:2063–8. doi: 10.1073/pnas.0409794102. [This manuscript demonstrates the protective role of PXR activation in a mouse cholestasis model.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Csanaky IL, Cheng X, et al. Organic Anion Transporting Polypeptide 1a1 Null Mice Are Sensitive to Cholestatic Liver Injury. Toxicol Sci. 2012;127:451–62. doi: 10.1093/toxsci/kfs123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chai J, Luo D, Wu X, et al. Changes of organic anion transporter MRP4 and related nuclear receptors in human obstructive cholestasis. J Gastrointest Surg. 2011;15:996–1004. doi: 10.1007/s11605-011-1473-2. [DOI] [PubMed] [Google Scholar]
- 55.Teng S, Piquette-Miller M. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 2007;151:367–76. doi: 10.1038/sj.bjp.0707235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo GL, Moffit JS, Nicol CJ, et al. Enhanced acetaminophen toxicity by activation of the pregnane X receptor. Toxicol Sci. 2004;82:374–80. doi: 10.1093/toxsci/kfh286. [DOI] [PubMed] [Google Scholar]
- 57.Wolf KK, Wood SG, Hunt JA, et al. Role of the nuclear receptor pregnane X receptor in acetaminophen hepatotoxicity. Drug Metab Dispos. 2005;33:1827–36. doi: 10.1124/dmd.105.005256. [DOI] [PubMed] [Google Scholar]
- 58•.Cheng J, Ma X, Krausz KW, et al. Rifampicin-activated human pregnane X receptor and CYP3A4 induction enhance acetaminophen-induced toxicity. Drug Metab Dispos. 2009;37:1611–21. doi: 10.1124/dmd.109.027565. [Manuscripts 56 to 58 demonstrate that PXR enhances the acetaminophen induced liver toxicity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghaffari AA, Chow EK, Iyer SS, et al. Polyinosinic-polycytidylic acid suppresses acetaminophen-induced hepatotoxicity independent of type I interferons and toll-like receptor 3. Hepatology. 2011;53:2042–52. doi: 10.1002/hep.24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang K, Damjanov I, Wan YJ. The protective role of pregnane X receptor in lipopolysaccharide/D-galactosamine-induced acute liver injury. Lab Invest. 2010;90:257–65. doi: 10.1038/labinvest.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huwyler J, Wright MB, Gutmann H, et al. Induction of cytochrome P450 3A4 and P-glycoprotein by the isoxazolyl-penicillin antibiotic flucloxacillin. Curr Drug Metab. 2006;7:119–26. doi: 10.2174/138920006775541534. [DOI] [PubMed] [Google Scholar]
- 62.Andrews E, Armstrong M, Tugwood J, et al. A role for the pregnane X receptor in flucloxacillin-induced liver injury. Hepatology. 2010;51:1656–64. doi: 10.1002/hep.23549. [DOI] [PubMed] [Google Scholar]
- 63••.Sookoian S, Castano GO, Burgueno AL, et al. The nuclear receptor PXR gene variants are associated with liver injury in nonalcoholic fatty liver disease. Pharmacogenet Genomics. 2010;20:1–8. doi: 10.1097/FPC.0b013e328333a1dd. [Manuscripts 62 to 63 describe the human genetic evidence that PXR is associated with liver injury response.] [DOI] [PubMed] [Google Scholar]
- 64.Rae JM, Johnson MD, Lippman ME, et al. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299:849–57. [PubMed] [Google Scholar]
- 65.Sonoda J, Xie W, Rosenfeld JM, et al. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR). Proc Natl Acad Sci USA. 2002;99:13801–6. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maglich JM, Stoltz CM, Goodwin B, et al. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–46. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- 67.Kast HR, Goodwin B, Tarr PT, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 68.Xie W, Yeuh MF, Radominska-Pandya A, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA. 2003;100:4150–5. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ueda A, Hamadeh HK, Webb HK, et al. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61(1):1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Allan C, Lord PG, Loughlin JM, et al. Identification of phenobarbitone-modulated genes in mouse liver by differential display. J Biochem Mol Toxicol. 2000;14(2):65–72. doi: 10.1002/(sici)1099-0461(2000)14:2<65::aid-jbt1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 71.Sugatani J, Kojima H, Ueda A, et al. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology. 2001;33:1232–8. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- 72.Pascussi JM, Gerbal-Chaloin S, Fabre JM, et al. Dexamethasone enhances constitutive androstane receptor expression in human hepatocytes: consequences on cytochrome P450 gene regulation. Mol Pharmacol. 2000;58:1441–50. doi: 10.1124/mol.58.6.1441. [DOI] [PubMed] [Google Scholar]
- 73.Pascussi JM, Drocourt L, Fabre JM, et al. Dexamethasone induces pregnane X receptor and retinoid X receptor-alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol. 2000;58:361–72. doi: 10.1124/mol.58.2.361. [DOI] [PubMed] [Google Scholar]
- 74.Zhang H, LeCulyse E, Liu L, et al. Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch Biochem Biophys. 1999;368:14–22. doi: 10.1006/abbi.1999.1307. [DOI] [PubMed] [Google Scholar]
- 75.Kamiya A, Inoue Y, Gonzalez FJ. Role of the hepatocyte nuclear factor 4alpha in control of the pregnane X receptor during fetal liver development. Hepatology. 2003;37:1375–84. doi: 10.1053/jhep.2003.50212. [DOI] [PubMed] [Google Scholar]
- 76.Antonakis N, Markogiannakis E, Theodoropoulou M, et al. The antiglucocorticoid RU486 downregulates the expression of interleukin-2 receptors in normal human lymphocytes. J Steroid Biochem Mol Biol. 1991;39:929–35. doi: 10.1016/0960-0760(91)90351-5. [DOI] [PubMed] [Google Scholar]
- 77.Riddick DS, Lee C, Bhathena A, et al. Transcriptional suppression of cytochrome P450 genes by endogenous and exogenous chemicals. Drug Metab Dispos. 2004;32:367–75. doi: 10.1124/dmd.32.4.367. [DOI] [PubMed] [Google Scholar]
- 78.Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997;29:1129–88. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 79.Pascussi JM, Gerbal-Chaloin S, Pichard-Garcia L, et al. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem Biophys Res Commun. 2000;274:707–13. doi: 10.1006/bbrc.2000.3219. [DOI] [PubMed] [Google Scholar]
- 80.Beigneux AP, Moser AH, Shigenaga JK, et al. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun. 2002;293:145–9. doi: 10.1016/S0006-291X(02)00196-1. [DOI] [PubMed] [Google Scholar]
- 81.Zhou C, Tabb MM, Nelson EL, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–89. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore LB, Goodwin B, Jones SA, et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA. 2000;97:7500–2. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romankiewicz JA, Ehrman M. Rifampin and warfarin: a drug interaction. Ann Intern Med. 1975;82:224–5. doi: 10.7326/0003-4819-82-2-224. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Ferguson SS, Negishi M, et al. Induction of human CYP2C9 by rifampicin, hyperforin, and phenobarbital is mediated by the pregnane X receptor. J Pharmacol Exp Ther. 2004;308:495–501. doi: 10.1124/jpet.103.058818. [DOI] [PubMed] [Google Scholar]
- 85.Kaplan B, Friedman G, Jacobs M, et al. Potential interaction of troglitazone and cyclosporine. Transplantation. 1998;65:1399–400. doi: 10.1097/00007890-199805270-00021. [DOI] [PubMed] [Google Scholar]
- 86.Sahi J, Hamilton G, Sinz M, et al. Effect of troglitazone on cytochrome P450 enzymes in primary cultures of human and rat hepatocytes. Xenobiotica. 2000;30:273–84. doi: 10.1080/004982500237668. [DOI] [PubMed] [Google Scholar]
- 87.Sahi J, Milad MA, Zheng X, et al. Avasimibe induces CYP3A4 and multiple drug resistance protein 1 gene expression through activation of the pregnane X receptor. J Pharmacol Exp Ther. 2003;306:1027–34. doi: 10.1124/jpet.103.050526. [DOI] [PubMed] [Google Scholar]
- 88.Katoh M, Watanabe M, Tabata T, et al. In vivo induction of human cytochrome P450 3A4 by rifabutin in chimeric mice with humanized liver. Xenobiotica. 2005;35:863–75. doi: 10.1080/00498250500296231. [DOI] [PubMed] [Google Scholar]
- 89.Inoue T, Nitta K, Sugihara K, et al. CYP2C9-catalyzed metabolism of S-warfarin to 7-hydroxywarfarin in vivo and in vitro in chimeric mice with humanized liver. Drug metab Dispos. 2008;36:2429–33. doi: 10.1124/dmd.108.022830. [DOI] [PubMed] [Google Scholar]
- 90.Chen S, Yueh MF, Evans RM, et al. The Pregnane-X-receptor controls hepatic glucuronidation during pregnancy and neonatal development in humanized UGT1 Mice. Hepatology. 2012 Feb 28; doi: 10.1002/hep.25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cai H, Nguyen N, Peterkin V, et al. A humanized UGT1 mouse model expressing the UGT1A1*28 allele for assessing drug clearance by UGT1A1-dependent glucuronidation. Drug Metab Dispos. 2010;38:879–86. doi: 10.1124/dmd.109.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang WW, Spurdle AB, Kolachana P, et al. A single nucleotide polymorphism in the 5' untranslated region of RAD51 and risk of cancer among BRCA1/2 mutation carriers. Cancer epidemiol Biomarkers Prev. 2001;10:955–60. [PubMed] [Google Scholar]
- 93.Li T, Lange LA, Li X, et al. Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. J Med Genet. 2006;43:740–4. doi: 10.1136/jmg.2005.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]