Abstract
Counterfactual feelings of regret occur when people make comparisons between an actual outcome and a better outcome that would have occurred under a different choice. We investigated the choices of individuals with damage to the ventral medial prefrontal cortex (VMPFC) and the lateral orbital frontal cortex (LOFC) to see whether their emotional responses were sensitive to regret. Participants made choices between gambles, each with monetary outcomes. After every choice, subjects learned the consequences of both gambles and rated their emotional response to the outcome. Normal subjects and lesion control subjects tended to make better choices and reported post-decision emotions that were sensitive to regret comparisons. VMPFC patients tended to make worse choices, and, contrary to our predictions, they reported emotions that were sensitive to regret comparisons. In contrast, LOFC patients made better choices, but reported emotional reactions that were insensitive to regret comparisons. We suggest the VMPFC is involved in the association between choices and anticipated emotions that guide future choices, while the LOFC is involved in experienced emotions that follow choices, emotions that may signal the need for behavioral change.
Keywords: VMPFC, OFC, regret, decision making, emotion, counterfactual comparison
“Let’s not forget that little emotions are the captains of our lives and we obey them without even realizing it.”
Vincent Van Gogh, 1889
Our emotional responses depend on the lives we live as well as the lives we could have lived. Counterfactual possibilities often serve as reference points against which we evaluate what actually occurred. Two counterfactual comparisons are particularly relevant to risky choice – disappointment and regret. Disappointment refers to the comparison between an actual outcome and a counterfactual one under a different state of the world (i.e., if a coin comes up heads instead of tails) (Bell, 1982: Loomes & Sugden, 1982). Negative comparisons are called disappointment, and positive ones are called elation. Regret refers to the comparison between an actual outcome and one that would have occurred if another option had been chosen (Bell, 1985; Loomes & Sugden, 1986). Negative comparisons are called regret, and positive ones are called rejoicing.
Research on emotions of pleasure and pain shows that regret comparisons typically have greater impact than disappointment comparisons (Mellers et al., 1999). Unlike disappointment comparisons, regret comparisons are under the control of the decision maker (i.e., who could have made the other choice) and are likely to be associated with a sense of personal responsibility and remorse. In this way, regret – even more than disappointment – may be beneficial for learning (Roese & Olsen, 1995; Zeelenberg & Pieters, 2007). In this paper, we investigate the unique contributions of the ventromedial prefrontal cortex (VMPFC) and lateral orbitofrontal cortex (LOFC) to risky choice and post-decision emotions indicative of regret comparisons.
The VMPFC has long been implicated in decision making and emotion (see Kingelbach, 2005 and Fellows, 2007 for review). Emerging and existing theories claim the VMPFC is involved in the integration of bodily signals that influence decisions (Bechara, Damasio & Damasio, 2000; Damasio, 1996). The VMPFC is also critical in the representation of stimulus value and the expected value of options (Fellows, 2007). Recent fMRI studies building on connections between VMPFC and decision making (Sommer et al., 2009; Lie et al., 2007; Chua et al., 2009; Ursu & Carter, 2005) have reported distinct activation patterns in the medial and lateral OFC during periods of regret. Coricelli et al., (2005) for example, found that medial OFC activity increased with both immediate regret and cumulative regret experienced throughout the task, whereas, lateral OFC activity increased only with immediate regret of the outcome. This pattern of neural activity suggests that the medial OFC may be involved in forming associations between an anticipated response and future behavior, whereas the lateral OFC may be involved in the counterfactual comparisons that follow choice.
Collectively the aforementioned results suggest unique roles for the VMPFC and LOFC in post-decision regret; however no human lesion research has compared the effects of VMPFC and LOFC damage on post-decision emotions. Existing work by Gomez, Beldarrain et al. (2005) showed that ventral prefrontal cortex patients reported fewer spontaneous counterfactual thoughts in response to questions. In addition, In addition, Camille et al. (2004) found that medial OFC patients reported emotions in a gambling task that were insensitive to regret. In neither study was it known whether lesions in the VMPFC extended to the LOFC.
To compare the functions of the VMPFC and LOFC regions, we administered a gambling task to patients with specific VMPFC and LOFC damage. On each trial, participants choose which of two gambles they preferred to play, each gamble having the possibility of a win or loss (Mellers et al, 1999). After making a choice, participants learned their outcome and that of the foregone gamble. Then they rated their pleasure with the outcome on a category rating scale from −50 (“Extremely Unhappy”) to 50 (“Extremely Happy”).
We expected that both the VMPFC and the LOFC group would report emotions that were less sensitive than other groups to regret comparisons. Our prediction was based on previous findings that VMPFC patients were less sensitive to regret, fMRI research linking LOFC to emotions involving, and the general tendency for negative emotions to signal behavioral change. We also predicted that the gamble choices made by the VMPFC patients would have lower expected values than those of the LOFC patients. This prediction is derived from past research showing that VMFPC patients made choices with lower chances of financial rewards, and damage to the VMPFC - not the LOFC - was linked to impairment in expected value calculations.
Experimental Procedures
Subjects
Neurological patients with focal brain lesions (n = 18) were participants in a gambling task. Lesion patients were recruited from the Patient Registry in the Department of Neurology at the University of Iowa. All patients had focal, stable, adult-onset lesions sustained at least 1 year prior to testing, and had previously undergone extensive screening and evaluation with background measures of neuropsychological function, reported previously in Bechara et al. (1998), Tranel et al. (2005) and Bar-On et al. (2003). A brief survey of the basic neuropsychological functions is presented in Table 1. Exclusion criteria were a history of mental retardation, a learning disability or a psychiatric illness including substance abuse. Patients were selected for eligibility on the basis of neuroanatomical status obtained from an MRI or a computed tomography (CT) scanning (see Neuroanatomical analysis section subsequently).
Table 1.
Demographic and Neuropsychological Characteristics of Participants
| Normal Comparisons | VMPFC | Lateral OFC | Lesion Comparisons | |
|---|---|---|---|---|
| Group size (N) | 26 | 7 | 6 | 5 |
| Gender M:F | 9M : 17F | 3M: 4F | 2M : 4F | 3M : 2F |
| Age | 41 (13) | 52 (11) | 45 (8) | 55 (9) |
| Education | 16 (18) | 14 (1.5) | 14 (2) | 13 (2) |
| VIQ | 101 | 100 | 97 | |
| PIQ | 101 | 107 | 95 | |
| FSIQ | 101 | 104 | 96 | |
| General Memory Index | 100 | 99 | 94 | |
| WCST | 5 | 5 | 5 | |
| Boston Naming Test | 57 | 55 | 42 | |
| COWA | 38 | 39 | 37 | |
| Facial Recognition Test | 47 | 47 | 43 | |
| BDI | 9 | 9 | 8 |
Note: Mean is presented with standard deviation shown in parentheses. VIQ, Verbal IQ; PIQ, performance IQ; FSIQ, full scale IQ (including the General Memory Index are all from the WAIS-III); WCST, Wisconsin Card Sorting Test (data value is average number of categories completed); COWA, Controlled Oral Word Association Test from the Multilingual Aphasia Exam; Beck, Beck Depression Inventory.
Patients in the VMPFC group (n=7) had bilateral damage in portions of the mesial orbital/ventromedial sector of the prefrontal cortex and/or the frontal pole (Figure. 1). Lesion aetiology in the VMPFC group was hemorrhage due to ruptured aneurysm of the anterior communicating artery or tumor resections. Inclusion in the LOFC lesion group (n = 6) was based on unilateral damage (left n= 3, right n= 3) to any part of the ventrolateral sector (including lateral orbital) of the prefrontal cortex, but spared bilateral damage to the mesial orbital/ventromedial prefrontal cortex and frontal pole, albeit in cases the damage extended to the mesial region, but only on one unilateral side (Figure 2). All lesions were due to either tumor resection or strokes in the overlapping territories of the middle and anterior cerebral arteries.
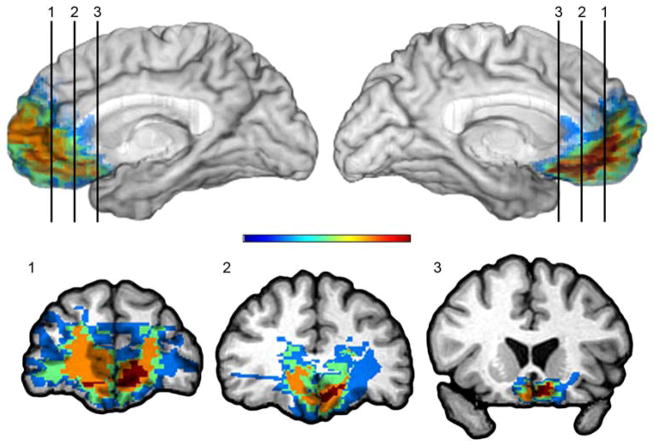
Figure 1.
VMPFC lesion group
Overlap of lesions in the VMPFC patients. Red indicates a maximal overlap of lesions from 5 patients, whereas blue reflects regions in which damage is unique to one patient. The color bar indicates that warmer colors represent greater degree of overlap across subjects; cooler colors indicate less overlap across subjects with blue representing only one lesion. As can be seen from the color-coding, the area in red is restricted to the ventral medial PFC. The red area seems larger on the left side, but note that the orange color (reflecting overlap of 4 lesions) is almost symmetrical on both sides. Two of the patients in this group had CT scans and are not shown in the figure, but their scans confirm bilateral lesions within the same VMPFC territories.
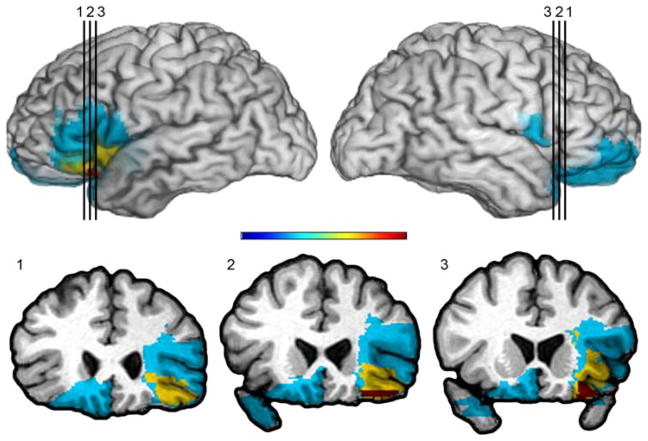
Figure 2.
Lateral OFC lesion group
Overlap of lesions in the LOFC patients. The color bar indicates that warmer colors represent greater degree of overlap across subjects, whereas cooler colors represent less overlap across subjects, with blue reflecting regions in which damage is unique to a participant. On the left side of the brain, red indicates a maximal overlap of lesions from 3 patients with left LOFC lesions, whereas the blue reflects regions in which damage is unique to one patient. The yellow color indicates overlap from 2 lesions. On the right side of the brain, there is only one lesion represented in the figure, which includes LOFC area, but extends to the medial side as well. Two of the lesion patients had only CT scans with smaller lesions that include the ventrolateral prefrontal and lateral orbitofrontal cortex, but spare the mesial orbitofrontal cortex. As can be seen from the color-coding, the area in red is restricted to the lateral OFC, specifically the posterior lateral OFC. By comparing slice 3 from this figure to slice 3 from Figure 1, it is clear that the areas in red are in distinct anatomical regions, namely the VMPFC (Figure 1) and LOFC (this figure).
Although some overlap in the damaged areas cannot be ruled out (i.e., individual lesions from a LOFC group may overlap with a lesion from a VMPFC group or vice versa), the VMPFC and LOFC groups are distinct in terms of lesion location. The area of maximal lesion overlap in the VMPFC group (i.e., the area coded in red color in Figure 1, slice 3) has no overlap with the area of maximal lesion overlap in the LOFC group (i.e., the area coded in red color in Figure 2, slice 3).
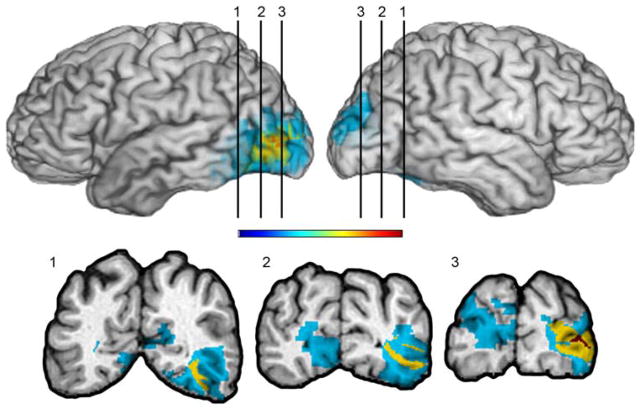
The non-frontal lesion comparison group (n=5) had damage in any part of the occipital and or temporal lobes that did not include the hippocampus, entorhinal cortex or amygdala (Figure. 3). These participants had left unilateral (n=3) or bilateral (n=2) damage due to strokes or tumor resections.
Figure 3.
Lesion comparison subject group
Overlap of lesions in the comparison group. The color bar indicates that warmer colors indicate greater degree of overlap across subjects; area in red is the region of maximal overlap indicating overlap from all 5 subjects. Cooler colors indicate less overlap across subjects; areas in blue reflect regions in which damage is unique to a participant. As can be seen from the color-coding, damage is primarily restricted to the occipital cortex with few areas of commonality across participants in this region.
The three lesion groups were compared to 26 normal age-matched comparison subjects who were recruited through community advertising. Demographic characteristics for all groups are displayed in Table 1. Subjects were paid for their participation and tested in quiet laboratory conditions with task responses recorded via a touch-sensitive monitor. The study was approved by the human subjects committee at the University of Iowa. Before enrollment in the study, written informed consent was acquired in accordance with the Declaration of Helsinki.
Lesion analysis
Lesion location was confirmed with either an MRI scan or a CT scan if MRI scanning was not possible or available. Two of the seven VMPFC patients had CT scans because of clipped aneurysms (tilt angle was optimized per subject to avoid clip-related artifact (zoom 2.4, field of view 51 cm, fovea 212.5 mm, slice thickness 2–4 mm)). Two of the six LOFC patients had only CT scans, and no MRI scans were available. All patients form the lesion control group had MRI scans. Lesions of individual patients who had MRI scans were transferred manually onto a normal reference brain using the MAP-3 technique (Damasio & Frank, 1992; Damasio, 1995; Frank et al., 1997) that involved (i) slicing a normal 3D brain in such a way that the slices match those of the MRI scan of the subject with the brain lesion; (ii) transposing the lesion onto the slices of the normal brain, taking into consideration the relation of the lesion and the identified pertinent anatomical landmarks; (iii) rendering each transposed lesion as an ‘object’ that can intersect in space, and thus yield a maximal overlap relative to both surface and depth extension of damage. The few patients with only CT scans were inspected visually and assigned, based on the neuro-radiologist report, as belonging to the VMPFC or LOFC group.
Stimuli and Design
Participants were told that the experiment involved choices between gambles with real monetary wins and losses. Their payments would be the total of their 84 outcomes, making it unlikely that participants would be able to keep track of their payment total during the study. Stimuli were two-outcome gambles, presented on a computer screen, as shown in Figure 4. Each gamble appeared as a pie chart with colored regions representing the probabilities of different outcomes. Monetary outcomes were specified in or near the region. On each trial, participants selected the gamble they preferred to play. A box appeared around the chosen gamble. Then spinners appeared in the centers of both gambles and rotated independently. Eventually, the spinners stopped, and participants learned the outcomes. They rated their pleasure or displeasure with their outcome on a category rating scale from −50 (“Extremely Unhappy”) to 50 (“Extremely Happy”).
Figure 4.
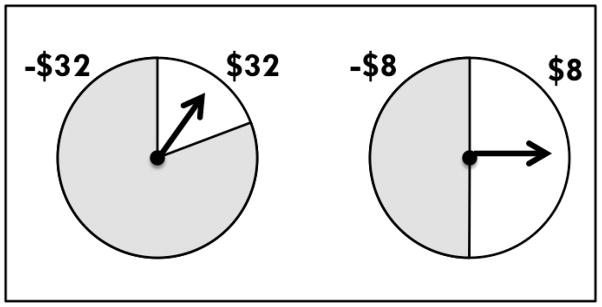
Individual trial stimuli were pairs of two-outcome gambles, as illustrated above. Each gamble appeared as a pie chart with colored regions representing the probabilities of different outcomes. On each trial, participants selected the gamble they preferred to play. A box appeared around the chosen gamble, and then spinners appeared in the center of both gambles and rotated independently. Eventually, the spinners stopped, and participants saw the outcome of their choice and the outcome of the foregone gamble.
The experiment consisted of the 21 gamble pairs listed in Table 2. Each pair was presented 4 times. Since the goal of the experiment was to understand post-decision emotions, we slightly adjusted the probabilities on some trials to obtain emotional reactions to more combinations of actual and foregone outcomes. This change was intentional since our goal was to understand emotions that follow from decisions. In previous experiments, Mellers, et al. (1999) interviewed subjects after the experiment and learned that no participants were aware of, suspicious of, or concerned about the stated versus the actual probabilities of outcomes. That is, none were aware that, on some occasions, probabilities were slightly adjusted. For these reasons, we believe the design was ideal for inferences about emotions, though not for learning.
Table 2.
Gamble Pairs Used in the Experimental Design
| Gamble 1 | Gamble 2 | Gamble Type | |||||
|---|---|---|---|---|---|---|---|
| Pair | Out A | Prob A | Out B | Out A | Prob A | Out B | |
| 1 | $8 | 0.2 | −$8 | $32 | 0.2 | −$32 | B/W |
| 2 | −$8 | 0.5 | −$32 | $32 | 0.2 | −$32 | B/W |
| 3 | $8 | 0.5 | −$32 | $32 | 0.2 | −$32 | B/W |
| 4 | $8 | 0.5 | −$8 | $32 | 0.2 | −$32 | B/W |
| 5 | −$8 | 0.8 | −$32 | $32 | 0.2 | −$32 | B/W |
| 6 | $8 | 0.8 | −$32 | $32 | 0.2 | −$32 | B/W |
| 7 | $8 | 0.8 | −$8 | $32 | 0.2 | −$32 | B/W |
| 8 | $8 | 0.2 | −$8 | $32 | 0.5 | −$32 | SS/RR |
| 9 | $32 | 0.2 | −$8 | $32 | 0.5 | −$32 | RA/RS |
| 10 | $32 | 0.2 | $8 | $32 | 0.5 | −$32 | B/W |
| 11 | $8 | 0.5 | −$8 | $32 | 0.5 | −$32 | RA/RS |
| 12 | −$8 | 0.8 | −$32 | $32 | 0.5 | −$32 | SS/RR |
| 13 | $8 | 0.8 | −$32 | $32 | 0.5 | −$32 | RA/RS |
| 14 | $8 | 0.8 | −$8 | $32 | 0.5 | −$32 | B/W |
| 15 | $8 | 0.2 | −$8 | $32 | 0.8 | −$32 | SS/RR |
| 16 | $32 | 0.2 | −$8 | $32 | 0.8 | −$32 | SS/RR |
| 17 | $32 | 0.2 | $8 | $32 | 0.8 | −$32 | SS/RR |
| 18 | $8 | 0.5 | −$8 | $32 | 0.8 | −$32 | SS/RR |
| 19 | $32 | 0.5 | −$8 | $32 | 0.8 | −$32 | SS/RR |
| 20 | $32 | 0.5 | $8 | $32 | 0.8 | −$32 | RA/RS |
| 21 | $8 | 0.8 | −$8 | $32 | 0.8 | −$32 | SS/RR |
Note: B/W = Better Gamble A (higher expected return and lower risk) vs Worse Gamble B (lower expected return and greater risk); SS/RR = Small-and-safe Gamble A (lower expected return and lower risk) vs Risky-and rewarding Gamble B (higher expected return and higher risk); RA/RS = Risk averse Gamble A (identical expected return and lower risk) vs Risk seeking Gamble B (identical expected return and more risk).
Statistical Analyses
We used regressions to investigate whether reported emotions were predictable from outcomes, disappointment comparisons, and regret comparisons. These predictor variables were weighted according to decision affect theory (Mellers et al., 1999). Outcome was the monetary value (in dollars) of the amount won or lost and ranged from −32 to +32. Disappointment comparisons were differences in monetary amounts of the realized and unrealized outcomes of the chosen gamble multiplied by the surprise associated with the realized outcome (i.e., one minus the probability of the obtained outcome). Regret comparisons were differences in the monetary values of the realized and foregone outcomes multiplied by the surprise of the joint event (i.e., one minus the product of the probability of the actual and foregone outcomes).
To assess whether the emotional responses of normal controls were influenced by disappointment and regret comparisons, we conducted statistical tests of relevant coefficients. Each of the 26 normal controls made 84 judgments, but since these judgments were not independent, we used the number of subjects (26) rather than the product of responses (26 × 84) as our degrees of freedom. In this case, there were three estimated parameters, leaving 23 degrees of freedom.
Comparisons between normal controls and the other three groups (lesion, ventral medial, and ventral lateral) were also conducted using regression analyses. These regressions included the same three variables – outcome, disappointment, and regret - and four new variables. Those were group membership (coded as 0 for normal controls and 1 for the target group) and interactions between group membership and the three previously mentioned variables. A significant group effect implies that emotional responses of the target group differ from those of normal controls in terms of an additive shift. A significant interaction between group and outcome means that, on average, emotional reactions are stronger or weaker for the target group when the coefficient is positive or negative, respectively. A significant interaction between group and disappointment comparisons means that the degree of disappointment/elation reflected in reported emotions differs between the target group and normal controls. Similarly, a significant interaction between group and regret comparisons means the degree of regret/rejoicing reflected in reported emotional reactions differs between the target group and normal controls.
Our hypothesis was that VMPFC and LOFC patients would report emotions with less sensitivity to regret comparisons than the emotions of normal controls. This hypothesis implies significant interactions between the target group and regret comparisons (with negative coefficients). Statistical tests of these hypotheses were based on the total number of subjects (normal controls plus target subjects) minus the number of estimated parameters. We estimated 7 coefficients, and our lesions groups had 5, 7, and 6 for lesion controls, VMPFC patients, and LOFC patients, respectively. That left us with 24, 26, and 25 degrees of freedom for tests with lesion controls, VMPFC patients, and LOFC patients, respectively. Our second hypothesis about the LOPC patients making more financially worse choices is tested by comparing the percentage of their worse choices to those of other groups.
Results
We present our findings in three sections. The first shows emotional responses of normal comparison subjects. The second presents emotional responses of patients (lesion comparisons, VMPFC and LOFC) relative to those of normal subjects. We also compare the relative frequencies of counterfactual comparisons (either disappointment/elation or regret/rejoicing) in patient groups relative to normal subjects. The third section compares the choices of patient groups relative to those of normal comparisons.
Emotions of Normal Comparison Subjects
Previous work by Mellers et al. (1997, 1999) demonstrated that emotional reactions to the consequences of risky choice depended on outcomes, disappointment (vs. elation) comparisons, regret (vs. rejoicing) comparisons, and surprise. We used this framework to evaluate the emotional experiences in this study. According to decision affect theory, counterfactual comparisons of disappointment and regret are weighted by the surprise of the outcome (or outcomes) that occurred. Disappointment is weighted by the surprise of the obtained outcome (one minus the probability of the obtained outcome), and regret is weighted by the surprise of the actual and foregone outcomes (one minus the product of the probabilities of the outcomes).
Results of regression analyses for normal controls are shown in Table 3. The effect of outcome was significant, and disappointment weighted by surprise was a significant trend (p = .06). Participants reported greater negative emotional reactions when their outcome was inferior to the other possible outcome of the chosen gamble. Finally, regret weighted by surprise was significant. Participants reported greater negative emotional reactions when their outcome was inferior to the foregone outcome of the gamble not selected. This model gave a reasonable description of the emotions with a multiple correlation of 0.71 (F(3,23) = 723.5, p <.001). Results were generally similar to those obtained by Mellers et al. (1999) using normal undergraduates.
Table 3.
Regression Results for Normal Comparison Subjects and Comparisons of Patient Groups with Normal Comparisons
| Normal Comparisons | Lesion Comparisons | Medial OFC | Lateral OFC | Medial vs Lateral OFC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Coef | SE | t Stat | Coeffs | SE | t Stat | Coeffs | SE | t Stat | Coeffs | SE | t Stat | Coeffs | SE | t Stat | |
| Intercept | 4.05 | 0.39 | 10.37** | 4.05 | 0.39 | 10.35** | 4.05 | 0.40 | 10.1** | 4.05 | 0.40 | 10.22** | 4.80 | 0.82 | 5.84** |
| Outcome | 0.47 | 0.03 | 14.8** | 0.48 | 0.03 | 14.76** | 0.47 | 0.03 | 14.4** | 0.47 | 0.03 | 14.6** | 0.71 | 0.07 | |
| Disappoint | 0.05 | 0.03 | 1.66 | 0.05 | 0.03 | 1.66 | 0.05 | 0.03 | 1.62 | 0.05 | 0.03 | 1.64 | 0.14 | 0.06 | 2.14* |
| Regret | 0.14 | 0.02 | 7.5** | 0.14 | 0.02 | 7.5** | 0.14 | 0.02 | 7.31** | 0.14 | 0.02 | 7.4** | 0.16 | 0.04 | 3.74** |
| Group | −0.78 | 0.98 | −0.79 | 0.75 | 0.86 | 0.87 | −1.71 | 0.92 | −1.86 | −2.46 | 1.22 | −2.02* | |||
| G x Out | 0.10 | 0.08 | 1.20 | 0.24 | 0.07 | 3.42** | 0.37 | 0.08 | 4.91** | 0.13 | 0.10 | 1.33 | |||
| G x Dis | 0.03 | 0.08 | 0.41 | 0.09 | 0.07 | 1.27 | 0.01 | 0.07 | 0.08 | −0.08 | 0.10 | −0.83 | |||
| G x Reg | −0.07 | 0.05 | −1.46 | 0.01 | 0.04 | 0.33 | −0.09 | 0.04 | −2.09* | −0.11 | 0.06 | −1.77* | |||
Note:
p<.01;
p<.05 The first regression shows normal comparison subjects, and regressions two through four show patient groups relative to normal comparisons. The final regression compares the medial and lateral OFC lesion groups.
Outcome, disappointment, and regret effects are shown graphically in Figures 5, 6, and 7, respectively. Normal comparison subjects appear on the left of each figure. Figure 5 shows outcome effects; gains are reported as more pleasurable than losses. Figure 6 shows effects due to disappointment comparisons. Emotional reactions are plotted against outcomes of −$8 and $8 with separate curves for the other outcome of the chosen gamble (−$32 and $32). Normal controls generally reported greater positive emotional reactions to their outcome when the other outcome was worse (−$32). Similarly, normal controls tended to report greater negative emotional reactions to their outcome when the other outcome was better ($32). Disappointment comparisons appear in the graph as the spacing between the curves; the greater the distance, the more the disappointment comparison influenced reported emotions.
Figure 5.
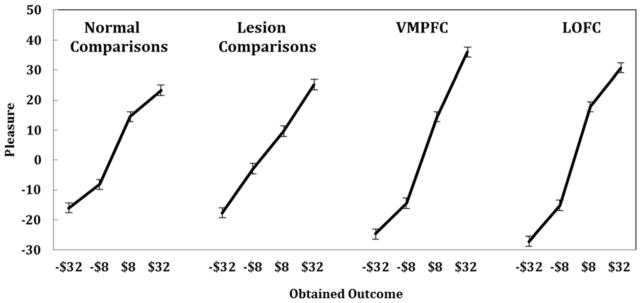
This figure illustrates outcome effects for the four groups of subjects. Emotional reactions are plotted against outcomes of −$32, −$8, $8, and $32. Slopes of the lines represent the impact of the realized outcome on feelings. Normal controls and lesion controls do not differ. But outcome effects are significantly greater for the medial and lateral OFC patients.
Figure 6.
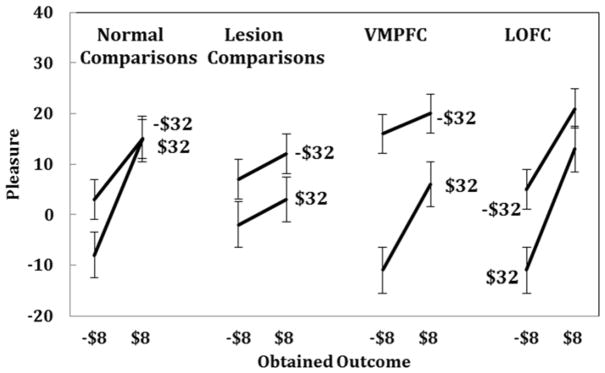
This figure illustrates effects of disappointment comparisons for the four groups. Emotional reactions are plotted against outcomes of −$8 and $8. Slopes of the lines represent effects of outcomes, and the spacing between the lines shows effects of disappointment comparisons. The upper line shows instances where the other possible outcome was worse (−$32) and the lower line shows cases when the other possible outcome was better ($32). All groups showed effects of disappointment comparisons.
Figure 7.
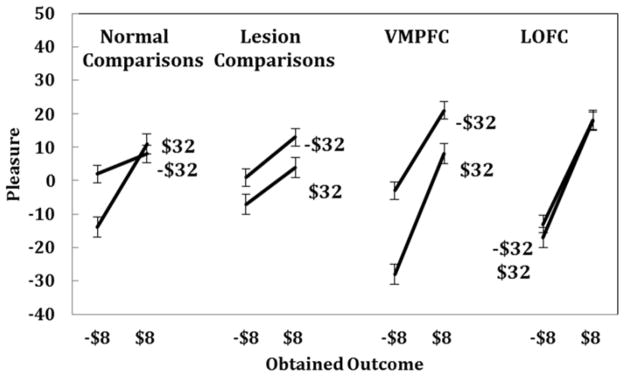
This figure illustrates the effects of regret comparisons for the four groups. Emotional reactions are plotted against outcomes of −$8 and $8. Slopes of the lines represent outcome effects, and the spacing between the lines shows effects of regret comparisons. The upper line represents instances in which the foregone outcome was worse (−$32) and the lower line shows instances in which the foregone outcome was better ($32). Normal controls, lesion controls, and the medial OFC lesion group experienced emotions that were sensitive to regret comparisons, and neither lesion group differed significantly from normal controls. However, the lateral OFC lesion participants showed less sensitivity to regret comparisons than normal participants.
Figure 7 shows regret effects, plotted as in Figure 6, except curves now refer to the foregone outcome of the unselected gamble (−$32 or $32) rather than the other outcome of the selected gamble. Regret is seen in terms of the spacing between the lines. Normal controls generally reported greater positive emotional reactions when their outcome was better and greater negative emotional reactions when their outcome was worse.
Emotions of Patient Groups Relative to Normal Comparison Subjects
We compared each lesion group to normal comparison subjects using linear regressions. Table 3 shows the results of these comparison regressions. First, we examine the comparison between normal controls and lesion controls. These subjects did not differ from normal controls; there were no effect of group (p >.1) and no group interactions (ps >.1). Emotional responses of the lesion controls appear in Figures 5, 6, and 7. These emotional reactions were sensitive to outcomes (Figure 5) disappointment comparisons (Figure 6) and regret comparisons (Figure 7). Despite the brain damage, these subjects reported emotions that resembled those of normal comparisons.
Next, we examined the relative frequencies of positive or negative counterfactual comparisons in the actual and foregone combinations that each group experienced. Since choices were under the control of participants, the relative frequency of disappointment and regret comparisons might influence emotional ratings. We compared the percentages of disappointing, elating, rejoicing, and regretful outcomes of normal control and lesion control subjects, but found no differences (ps>.1).
Regression comparisons between normal controls and VMPFC patients appear in Table 3. Contrary to our prediction, VMPFC patients reported emotional reactions that were sensitive to regret comparisons. In fact, VMPFC patients reported stronger emotional reactions to outcomes than normal controls, but there were no differences between in sensitivity to disappointment or regret comparisons.
Mean ratings of the reported emotions for VMPFC patients appear in Figures 5, 6, and 7. The steeper line in Figure 5 for VMPFC patients relative to controls shows the greater effect of outcomes, as found in the regression. Greater outcome effects can also be seen in Figures 6 and 7 with steeper slopes for VMPFC lesion subjects than normal comparison subjects. Reported emotions of VMPFC patients were also sensitive to disappointment and regret comparisons. The spacing between the lines in Figures 6 and 7 reflects disappointment and regret effects, respectively. VMPFC patients did not differ from normal controls in either of these respects.
Reported emotions of the LOFC patients can also be seen in Figures 5, 6, and 7. LOFC patients reported heightened emotional reactions to outcomes, as found with VMPFC lesion patients (see Table 3). Critically, however, LOFC patients reported emotions that were less sensitive to regret comparisons than those of normal controls, as seen in the decrease in the vertical spacing between the lines in Figure 7. A separate regression analysis of the LOFC patients alone revealed no effects of disappointment or regret comparisons (ps>.1). An additional regression analysis comparing VMPFC and LOFC patients (shown in Table 3) indicated that the emotional reactions of VMPFC and LOFC patients differed only in terms of regret comparisons. Lateral OFC lesion patients reported emotional reactions that were less sensitive than VMPFC lesion patients to regret comparisons at the level of a trend (t(6) = −1.77, p<.07). Our hypothesis about the LOFC group reporting emotions that were less sensitive to regret comparisons was supported.
We wondered whether these differences in disappointment and regret comparisons were due to the relative frequency of experiencing disappointing or regretful outcomes. To find out, we compared the relative frequency of these disappointment and regret comparisons in patients to those of normal controls. These rates were indistinguishable (ps>.1 and ps>.1, respectively). Finally to determine whether differences in task payoffs may have influenced the emotional responses of participants, we compared task payoffs (money earned) across groups. Payoff rates were statistically indistinguishable (ps >.1).
To summarize, the emotional responses of lesion comparison subjects and normal comparison subjects did not differ. Contrary to our prediction, the emotional sensitivity of the VMPFC group to regret comparisons did not differ from that of normal controls. However, the emotional sensitivity of the LOFC patients and the normal controls did differ; LOFC patients were less sensitive to regret comparisons1.
Choices of Patient Groups Relative to Normal Comparison Subjects
Next we turn to the choices of patient groups relative to normal controls. We separated gamble pairs into: 1) financially better versus financially worse gambles, 2) safer-and-smaller versus risky-and-rewarding gambles, and 3) risk averse versus risk seeking gambles. In the better vs. worse gamble pairs (B/W), better gambles had higher expected returns and less risk. With these pairs, we could examine whether subjects selected made good risk/return tradeoffs. With the safer-and-smaller vs. risky-and-rewarding pairs (SS/RR), we could evaluate preferences for lower expected values and less variance versus greater return and more variance. With the risk averse vs. risk seeking pairs (RA/RS), we could observe preferences for pure risk holding return (i.e., expected value) constant. There were 9 B/W pairs, and each pair was presented 4 times, so counts were divided by 36 pair presentations. There were 8 (SS/RR) pairs each presented 4 times, or 32 such presentations, and there were 4 (RA/RS) pairs each presented 4 times, for a total of 16 presentations.
Table 4 shows the percentages of better choices, risk-and-rewarding choices, and risk seeking choices for each lesion group relative to normal comparison subjects. Mann-Whitney U tests indicated no differences between normal controls and lesion controls. In addition, there were no differences in preferences for risk (either risk aversion vs. risk seeking preferences or risky-and-rewarding vs. safe-and-smaller preferences) between any patient group and normal comparison subjects.
Table 4.
Percentage of Financially Better (vs. Financially Worse) choices, Risk Seeking (vs. Risk Averse) choices, and Risky-Rewarding (vs. Safer-Smaller) gamble choices independent of and dependent on prior gamble outcome for all participant groups.
| Choice | Normal Comp. Group | Lesion Comp. Group | VMPFC Group | Lateral OFC Group |
|---|---|---|---|---|
| Better/Worse | 77% | 64% | 48%* | 66% |
| Risk Seeking/Risk Averse | 62% | 69% | 74% | 56% |
| Risky-Rewarding/Small-Safe | 79% | 81% | 83% | 82% |
Note: Comp. = Comparison. Comparison between lesion groups and Normal comparison subjects,
p <.05
We predicted that the choices of VMPFC patients would be worse than those of LOFC patients, indicative of VMPFC patients’ lower sensitivity to differences in expected value. As expected, VMPFC patients were less able to identify financially better gambles and selected more financially worse gambles than normal comparison subjects (z=2.252, p < .05). LOFC patients made better gamble choices at a rate that was similar to those of normal comparison subjects. Finally, we compared the percentage of better gamble choices, risk-and-rewarding choices and risk seeking choices of VMPFC and LOFC lesion subjects. VMPFC subjects choose more gambles that were financially worse than normal comparison subjects and (though not predicted), VMPFC patients were also more risk seeking than LOFC patients (z=1.963, p < .05).
To further investigate the choices of VMFPC patients, we plotted the frequency of choosing gamble A as a function of the differences in expected value between option A and B. Figure 8 shows estimated choice probabilities based on logistic regressions for each group. Probabilities of selecting the better gamble are similar for normal comparison subjects, lesion comparison subjects, and LOFC subjects. VMPFC subjects however exhibit a shallower logistic curve, reflecting a choice pattern that indicated less sensitivity to differences in expected value between option A and option B.
Figure 8.
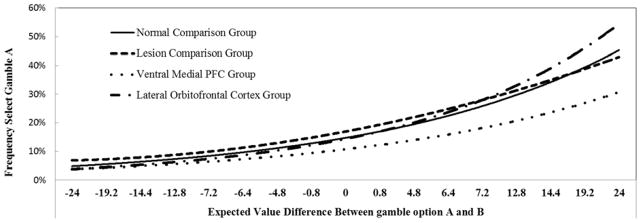
This figure illustrates the percentage of time Gamble A was chosen as a function of the value of Gamble A minus the value of Gamble B. The logistic curves of normal and lesion comparison subjects, and LOFC subjects are similar; VMPFC subjects however have a shallower logistic curve reflecting a choice pattern indicative of decreased sensitivity to differences in expected value between option A and option B.
Discussion
This study examined the role of the medial and lateral OFC in the experience of regret. Participants made choices between risky options with monetary outcomes. After each choice, gambles were resolved and revealed; participants learned both outcomes and rated their pleasure or displeasure with the outcome. This process continued for 84 trials. LOFC patients reported emotional reactions that were less sensitive than normal controls to regret comparisons, as predicted. But contrary to our predictions, VMPFC patients and normal comparison subjects did not differ in their reported sensitivity to regret comparisons. Our prediction that the VMPFC group would select more gambles that were financially worse was supported.
Taken together, the results were surprising. The VMPFC group selected more gambles that were financially worse, but they appeared to experience regret. In contrast, the LOFC group selected more gambles that were financially better, but they appeared to be less sensitive to regret. Less regret did not impair the choices of LOFC patients; their selections resembled those of normal comparison subjects.
This study is the first to compare the effects of VMPFC and LOFC damage on choice and subsequent emotions. The LOFC region of maximal overlap represents an anatomical impairment that is specific to the LOFC group (see slice 3 in Figures 1 and 2). The VMPFC group region of maximal overlap includes bilateral damage to the medial portions of the PFC. The relatively distinct anatomical damage (albeit some individual lesion overlap) leads us to believe that the VMPFC and LOFC may play distinct roles in choice and emotional reactivity. What might those roles be?
Emotions are critical for learning. Experienced emotions (i.e., those occurring after a choice) are a consequence of choice. They presumably inform anticipated emotions (i.e., those imagined prior to making a choice), and anticipated emotions, in turn, guide future decisions. The VMPFC (as well as the anterior and posterior cingulate) are important to both the formation of associations between choices and outcomes and the encoding of choice values (Rangel et al., 2008; Rushworth & Behrens, 2008; Seo & Lee, 2008). Awareness of the psychological value of a realized outcome is essential for adaptive decision making. There may also be advantages to awareness of the value of options not taken and the consequences that follow, otherwise known as counterfactual outcomes.
Based on our findings that VMPFC lesion participants made financially worse choices, but reported emotions that were sensitive to regret, we propose that the VMPFC is not necessary for the post-decision experience of regret. Rather we support the long held theory that the VMPFC is necessary for representing the anticipated value of choice options. The poor choices of VMPFC lesion subjects, as well as the exaggerated emotional response to gains and losses suggest that VMPFC patients may have been less able to anticipate the value of options, and possibly as a consequence, were surprised by the resulting gains and losses, heightening their emotional responses.
The LOFC however, appears to have a distinct role in experienced emotions. Lateral OFC patients reported emotional reactions not indicative of regret. Regret is a counterfactual comparison between an actual outcome and a foregone outcome under a different choice, so we propose that the LOFC is critical for representing the reward of forgone outcomes that could signal adaptive changes in behavior. A representation of the reward potential of forgone outcome would enable individuals to exploit valuable, otherwise discarded, information in complex environments and allow for more efficient transitions in behavior (i.e. reward potential of forgone option exceeds reward potential of chosen option, presumably changing behavior, Boorman, Behrens & Rushworth, 2011).
The claim that the LOFC is necessary for representing the reward potential of forgone choices is consistent with evidence that the lateral frontal polar cortex (lFPC), a neighboring anterior region that overlaps slightly with the lateral OFC, contributes to the representation of the forgone outcome. For example, Boorman et al. (2009) showed that, during a binary choice fMRI task, lFPC activity increased with the reward associated with the option not taken. In addition, a primate lesion study conducted by Noonan et al. (2010) that compared effects of medial and lateral OFC damage in monkeys performing a value-learning task found results that suggested the LOFC codes the value of choices. Noonan and colleagues found that monkeys with medial OFC damage were less able to assess differences in expected values of choice options, whereas monkeys with lateral OFC damage were impaired at assigning post outcome ‘reward credit’ to a choice option.
Individuals with lateral OFC damage may not experience regret because they are unable to conduct a counterfactual comparison between the reward value of the choice taken and the reward value of the forgone choice. The LOFC may guide choices via the comparison of choice options, the integration of context (Beer, Knight and D’Esposito, 2006), and other changes or shifts in behavior (Kingelbach & Rolls, 2004; Dias, Robbins & Roberts, 1996), which occur due to sustained representation of the reward potential of alternate choices. In conjunction with the literature about the lateral prefrontal cortex, we propose that the lateral OFC represents the reward potential of alternate choice outcomes (either explicitly or implicitly) to signal behavioral change and aid learning.
Our findings may at first glance appear inconsistent with the finding of Camille et al (2004), who used a similar task and reported that VMPFC patients did not experience regret. A direct comparison of the two studies is difficult because Camille et al., only present the area of maximal overlap in their VMPFC lesion participants. If there was substantial lesion overlap in the lateral regions of the PFC as well, Camille et al.’s VMPFC patients may have resembled the present LOFC group in which LOFC damage may have contributed to the lack of regret reported by Camille et al.’s patients and VMPFC damage contributed to their poor choices. A more extensive comparison of the lesion groups from the two studies is needed to discover whether our results are, in fact, contradictory, and if so, why.
Our results that LOFC damage subjects made financially better choices, yet still reported emotions indicative of less regret, might suggest that representation of the forgone outcome is not necessary for good decisions. We are arguing nothing of the sort. Our paradigm was not designed to measure anticipated emotions or learning. Second, individuals with lateral OFC had an intact VMPFC, and it is damage to the medial OFC, not damage to the lateral OFC, that has been linked to impairment in expected value calculations (Fellows, 2007). Since the VMPFC of the LOFC patients was intact, LOFC patients were able to assess differences in expected value and choose adaptively despite exhibiting less regret. However, in a task designed to measure the impact of anticipated regret on learning, we postulate that individuals with LOFC damage would exhibit impaired maintenance of the reward value of relevant alternate choices. Future research should clarify the unique roles of the VMPFC and LOFC in anticipated emotions, counterfactual comparisons, and learning from regret.
In conclusion, we offer a new framework. The LOFC represents the value of forgone/alternate outcomes to signal changes in behavior, and the VMPFC strengthens the association between anticipated emotions and choices, an association that is necessary to facilitate learning and guide future choices. Anticipating regret involves the pre-choice comparison of choice and forgone-choice alternatives. Mellers et al. (1999) have shown that, in this gambling paradigm, experienced regret aligns with anticipated regret in normal comparison subjects. Further, previous fMRI research has shown that LOFC activity increases with anticipated negative outcomes while VMPFC activity increases with anticipated positive outcomes (Ursu & Carter, 2005). Both the LOFC and VMPFC may be critical for differential components of anticipated emotions and learning from emotional outcomes over time. Future research should therefore examine whether these regions also have unique roles in the emotional reactions that people imagine or anticipate prior to choice as well as the application of knowledge learned when similar choice are encountered in the future.
Highlights.
LOFC damage patients made financially better choices, but reported emotions that were less sensitive to regret comparisons than normal controls.
VMPFC damage patients made financially worse choices, but reported heightened emotions relative to controls. These emotions were also sensitive to regret comparisons.
Acknowledgments
We would like to thank Reid Stevens and Raghuram Iyengar for their assistance with analyses. We thank the Haas School of Business for their support. This work was supported by the National Institute of Neurological Disorders and Stroke [P50 NS19632], and by the National Institute on Drug Abuse [R01 DA023051, R01 DA022549].
Footnotes
The LOFC group had 3 individuals with right and 3 individuals with left side lesions. To investigate laterality, we conducted a post hoc analysis of differences in the emotional reactions of patients with left vs right lateralized LOFC lesions. One regression had predictors of outcome, disappointment comparisons, regret comparisons, and group. Due to limitations in the degrees of freedom, we did not include interactions. Results revealed a significant effect of outcome (t(2)= 6.581, p<.05) (i.e., LPFC patients were sensitive to gains versus losses), but there were no significant main-effects of disappointment comparisons, regret comparisons, or group (all ps >.1).
Next, to test whether the reduced regret in LOFC patients was a function of lesion laterality, we conducted a focused post hoc regression in which the group by regret interaction was examined. Results revealed no differences in reported sensitivity to regret comparisons between patients with left and right lateralized LOFC lesions (p >.1). Finally, Mann-Whitney U tests comparing the percentage of better gamble choices, risk-and-rewarding gamble choices and risk seeking gamble choices revealed no discernible differences between LOFC patients with damage on the left versus right (all ps >.1). Therefore all data for the LOFC patients were treated as a single group. We note that all of the VMPFC group participants had bilateral damage, and therefore comparing left to right side lesions was not possible.
References
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain; 2003;126:1790–800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, Decision Making and the Orbitofrontal Cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. J Nuerosci. 1998;18:428–37. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6(2):215–25. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Beer JS, Knight RT, D’Esposito M. Controlling the integration of emotion and cognition: the role of frontal cortex in distinguishing helpful from hurtful emotional information. Psychol Sci. 2006;17(5):448–53. doi: 10.1111/j.1467-9280.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- Gomez Beldarrain M, Garcia-Monco JC, Astigarraga E, Gonzalez A, Grafman J. Only spontaneous counterfactual thinking is impaired in patients with prefrontal cortex lesions. Brain Res Cogn Brain Res. 2005;24(3):723–6. doi: 10.1016/j.cogbrainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Bell DE. Regret in decision making under uncertainty. Oper Res. 1982;30:961–981. [Google Scholar]
- Bell DE. Disappointment in decision making under uncertainty. Oper Res. 1985;33:1–27. [Google Scholar]
- Boles TL, Messick DM. Reverse outcome bias: The influence of multiple reference points on the evaluations of outcomes and decisions. Organ Behav Hum Dec Processes. 1995;61:262–275. [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Rushworth MF. Counterfactual choice and learning in a neural network centered on human lateral frontopolar cortex. PLOS Biology. 2011;9(6):e1001093. doi: 10.1371/journal.pbio.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camille N, Coricelli G, Sallet J, Pradat-Diehl P, Duhamel JR, Sirigu A. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304:1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- Chua HF, Gonzalez R, Taylor SF, Welsh RC, Liberzon I. Decision-related loss: regret and disappointment. Neuroimage. 2009;47:2031–2040. doi: 10.1016/j.neuroimage.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Coricelli G, Critchley HD, Joffily M, O’Doherty JP, Sirigu A, Dolan RJ. Regret and its avoidance: a neuroimaging study of choice behavior. Nat Neurosci. 2005;8(9):1255–62. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond [Biol] 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Damasio H. Human brain anatomy in computerized images. New York: Oxford University Press; 1995. [Google Scholar]
- Damasio H, Frank R. Three-dimensional in vivo mapping of brain lesions in humans. Arch Neurol. 1992;49:137–43. doi: 10.1001/archneur.1992.00530260037016. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins T, Roberts A. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380:69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JF. Hedonic and informational functions of the human orbitofrontal cortex. Cereb Cortex. 2010;20(1):198–204. doi: 10.1093/cercor/bhp092. Epub. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Fellows LK. The role of orbitofrontal cortex in decision making: a component process account. Ann N Y Acad Sci. 2007;1121:421–30. doi: 10.1196/annals.1401.023. Review. [DOI] [PubMed] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, Polkey CE. Reward-related reversal learning after surgical excisions in orbitofrontal and dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbital frontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Landman J. Regret and elation following action and inaction: Affective responses to positive versus negative outcomes. Pers Soc Psychol B. 1987;13:524–536. [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27:4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes G, Sugden R. Regret theory: An alternative theory of rational choice under uncertainty. Econ J. 1982;92:805–824. [Google Scholar]
- Loomes G, Sugden R. Disappointment and dynamic consistency in choice under uncertainty. Rev Econ Stud. 1986;53:271–282. [Google Scholar]
- Mellers BA. Choice and the relative pleasure of consequences. Psychol Bull. 2000;126:910–924. doi: 10.1037/0033-2909.126.6.910. [DOI] [PubMed] [Google Scholar]
- Mellers BA, Schwartz A, Ho K, Ritov I. Elation and disappointment: Emotional responses to risky options. Psych Sci. 1997;8:423–429. [Google Scholar]
- Mellers BA, Schwartz A, Ritov I. Emotion-based choice. J Exp Psychol Gen. 1999;128:332–345. [Google Scholar]
- Noonan MP, Walton ME, Behrens TEJ, Sallet J, Buckley MJ, Rushworth MFS. Separate value comparison and learning mechanisms in macaque medial and lateral orbital frontal cortex. PNAS. 2010;107(47):20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roese NJ, Olson JM, editors. What might have been: The social psychology of counterfactual thinking. Mahwah, NJ: Erlbaum; 1995. [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–1524. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Seo H, Lee D. Cortical mechanisms for reinforcement learning in competitive games. Philos Trans R Soc Lond B Biol Sci. 2008;363:3845–3857. doi: 10.1098/rstb.2008.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T, Peters J, Gläscher J, Büchel C. Structure-function relationships in the processing of regret in the orbitofrontal cortex. Brain Struct Funct. 2009;213(6):535–51. doi: 10.1007/s00429-009-0222-8. Review. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–81. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Ursu S, Carter CS. Outcome representations, counterfactual comparisons and the human orbitofrontal cortex: implications for neuroimaging studies of decision-making. Brain Res Cogn Brain Res. 2005;23:51–60. doi: 10.1016/j.cogbrainres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Zeelenberg M. Anticipated regret, expected feedback and behavioral decision making. J Behav Dec Making. 1999;12:93–106. [Google Scholar]
- Zeelenberg M, Pieters R. A theory of regret regulation 1.0. J Consum Psychol. 2007;17:3–18. doi: 10.1207/s15327663jcp1701_5. [DOI] [PMC free article] [PubMed] [Google Scholar]