Abstract
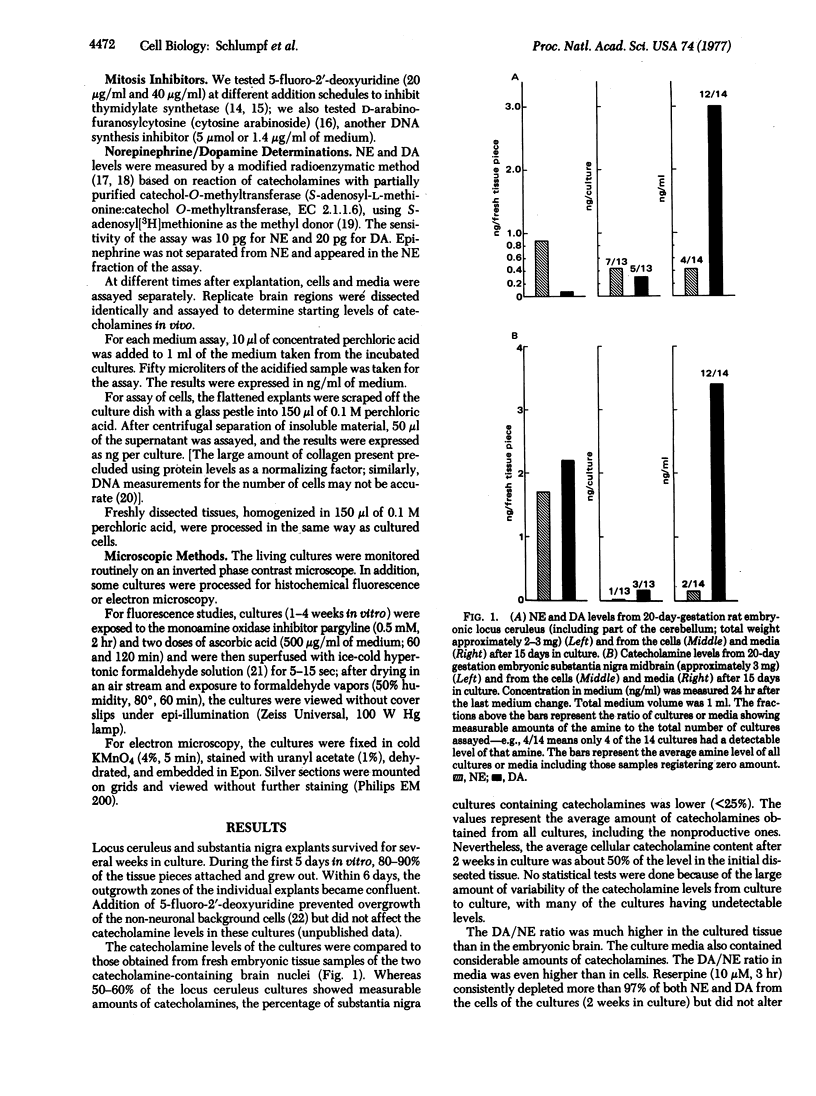
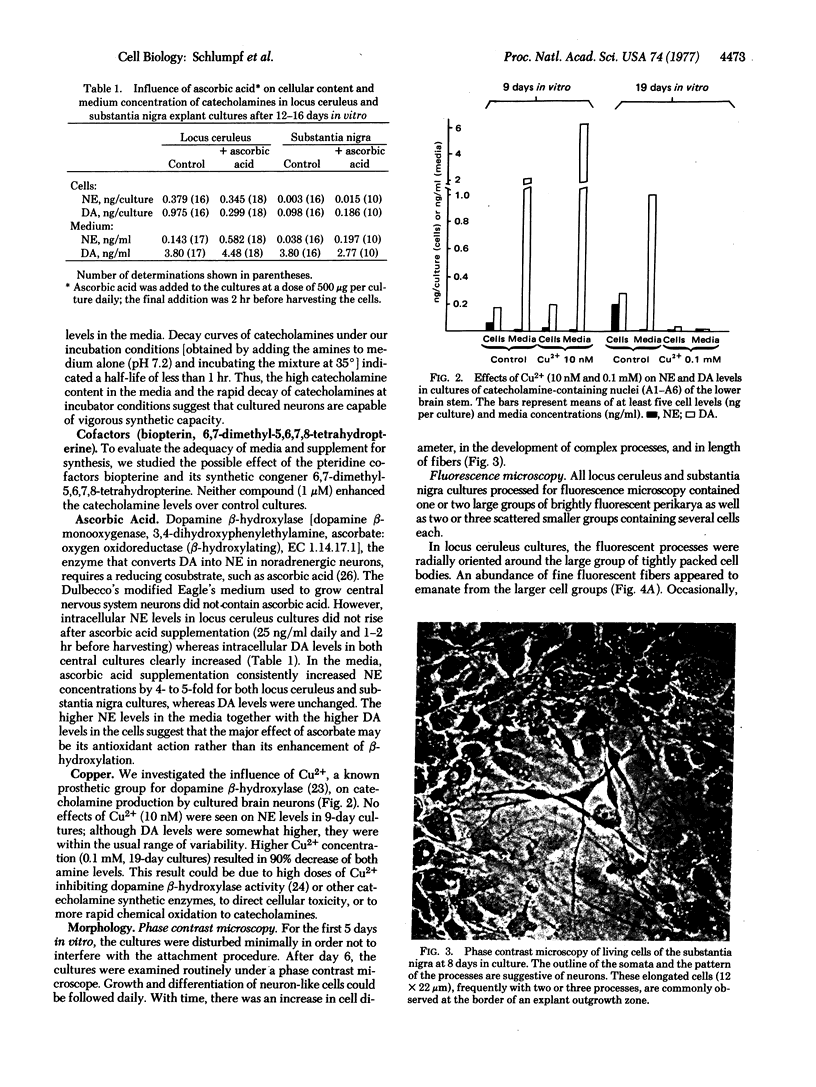
Norepinephrine (NE)-producing cells of the nucleus locus ceruleus and dopamine (DA)-producing cells of the substantia nigra were dissected microscopically from embryonic rat brain, explanted, and maintained in culture for up to 5 weeks. The cultured neurons of both brain regions showed normal maturation of axons and dendrites and formed ultrastructurally defined synaptic contacts. Fluorescence microscopy of cultured neurons from both brain regions showed typical in situ cytological features: long axonal processes with multiple varicosities for locus ceruleus cultures, and smooth, wispy nonvaricose processes in the substantia nigra cultures. All cultures processed for fluorescence microscopy contained specific catecholamine-fluorescent cells. By radioenzyme assay for catecholamines, more than half of the locus ceruleus cultures contained measurable (>10 pg) quantities of NE and DA, but, unlike results on intact brains, DA content exceeded NE content. Cultures of substantia nigra neurons retained no NE and very little DA. Media from substantia nigra and locus ceruleus cultures contained substantial quantities of DA. Addition of reserpine (10 μM) to the medium depleted locus ceruleus neurons of both amines.
The long survival time in culture of locus ceruleus cells, the normal appearance of fluorescent cell bodies and processes, the apparent development of morphologically specialized interneuronal connections, and the ability to synthesize and store NE make these cultures ideally suited for neurophysiological recording as well as morphological, biochemical, and pharmacological experiments.
Keywords: locus ceruleus, substantia nigra, tissue culture, norepinephrine, dopamine
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J. Enzymatic formations of adrenaline and other catechols from monophenols. Science. 1963 May 3;140(3566):499–500. doi: 10.1126/science.140.3566.499. [DOI] [PubMed] [Google Scholar]
- Azmitia E. C., Henriksen S. J. A modification of the Falck-Hillarp technique for 5-HT fluorescence employing hypertonic formaldehyde perfusion. J Histochem Cytochem. 1976 Dec;24(12):1286–1288. doi: 10.1177/24.12.1002978. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Axelrod J. Development of the uptake and storage of L-( 3 H)norepinephrine in the rat brain. J Neurochem. 1971 Nov;18(11):2061–2075. doi: 10.1111/j.1471-4159.1971.tb05065.x. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Henry D. Catecholamines in fetal and newborn rat brain. J Neurochem. 1973 Jul;21(1):61–67. doi: 10.1111/j.1471-4159.1973.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Jacobowitz D., Klein D., Axelrod J. Dopaminergic neurons in explants of substantia nigra in culture. J Neurobiol. 1973;4(5):461–470. doi: 10.1002/neu.480040507. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D. Synapse formation between dissociated nerve and muscle cells in low density cell cultures. Dev Biol. 1972 Jun;28(2):407–429. doi: 10.1016/0012-1606(72)90023-1. [DOI] [PubMed] [Google Scholar]
- Geller H. M., Cechner R. L., Fleming D. G. Effect of goldthioglucose on long-term cultures of mouse hypothalamus. Neurobiology. 1972;2(4):154–161. [PubMed] [Google Scholar]
- Godfrey E. W., Nelson P. G., Schrier B. K., Breuer A. C., Ransom B. R. Neurons from fetal rat brain in a new cell culture system: a multidisciplinary analysis. Brain Res. 1975 Jun 6;90(1):1–21. doi: 10.1016/0006-8993(75)90679-4. [DOI] [PubMed] [Google Scholar]
- Hökfelt T. In vitro studies on central and peripheral monoamine neurons at the ultrastructural level. Z Zellforsch Mikrosk Anat. 1968;91(1):1–74. doi: 10.1007/BF00336984. [DOI] [PubMed] [Google Scholar]
- Hökfelt T. On the ultrastructural localization of noradrenaline in the central nervous system of the rat. Z Zellforsch Mikrosk Anat. 1967;79(1):110–117. doi: 10.1007/BF00335247. [DOI] [PubMed] [Google Scholar]
- Hösli E., Meier-Ruge W., Hösli L. Monoamine-containing neurones in cultures of rat brain stem. Experientia. 1971 Mar 15;27(3):310–311. doi: 10.1007/BF02138165. [DOI] [PubMed] [Google Scholar]
- KAUFMAN S., FRIEDMAN S. DOPAMINE-BETA-HYDROXYLASE. Pharmacol Rev. 1965 Jun;17:71–100. [PubMed] [Google Scholar]
- Kim S. U. Light and electron microscope study of mouse cerebral neocortex in tissue culture. Exp Neurol. 1972 May;35(2):305–321. doi: 10.1016/0014-4886(72)90157-4. [DOI] [PubMed] [Google Scholar]
- Lasher R. S., Zagon I. S. The effect of potassium on neuronal differentiation in cultures of dissociated newborn rat cerebellum. Brain Res. 1972 Jun 22;41(2):482–488. doi: 10.1016/0006-8993(72)90521-5. [DOI] [PubMed] [Google Scholar]
- Levitt P., Moore R. Y., Garber B. B. Selective cell association of catecholamine neurons in brain aggregates in vitro. Brain Res. 1976 Jul 30;111(2):311–320. doi: 10.1016/0006-8993(76)90776-9. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Patterson P. H. Primary cultures of dissociated sympathetic neurons. I. Establishment of long-term growth in culture and studies of differentiated properties. J Cell Biol. 1973 Nov;59(2 Pt 1):329–345. doi: 10.1083/jcb.59.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurovsky E. B., Benitez H. H., Murray M. R. Synaptic development in long-term organized cultures of murine hypothalamus. J Comp Neurol. 1971 Nov;143(3):263–278. doi: 10.1002/cne.901430302. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Brownstein M., Saavedra J. M., Axelrod J. Norepinephrine and dopamine content of hypothalamic nuclei of the rat. Brain Res. 1974 Aug 30;77(1):137–149. doi: 10.1016/0006-8993(74)90810-5. [DOI] [PubMed] [Google Scholar]
- Pollack R. E., Green H., Todaro G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc Natl Acad Sci U S A. 1968 May;60(1):126–133. doi: 10.1073/pnas.60.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUECKERT R. R., MUELLER G. C. Studies on unbalanced growth in tissue culture. I. Induction and consequences of thymidine deficiency. Cancer Res. 1960 Dec;20:1584–1591. [PubMed] [Google Scholar]
- Schrier B. K., Shapiro D. L. Effects of fluorodeoxyuridine on growth and choline acetyltransferase activity in fetal rat brain cells in surface culture. J Neurobiol. 1974;5(2):151–159. doi: 10.1002/neu.480050206. [DOI] [PubMed] [Google Scholar]
- Schrier B. K. Surface culture of fetal mammalian brain cells: effect of subculture on morphology and choline acetyltransferase activity. J Neurobiol. 1973;4(2):117–124. doi: 10.1002/neu.480040204. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Seil F. J., Kelly J. M., 3rd, Leiman A. L. Anatomical organization of cerebral neocortex in tissue culture. Exp Neurol. 1974 Dec;45(3):435–450. doi: 10.1016/0014-4886(74)90150-2. [DOI] [PubMed] [Google Scholar]
- Sensenbrenner M., Booher J., Mandel P. Cultivation and growth of dissociated neurons from chick embryo cerebral cortex in the presence of different substrates. Z Zellforsch Mikrosk Anat. 1971;117(4):559–569. doi: 10.1007/BF00330715. [DOI] [PubMed] [Google Scholar]
- Shashoua V. E., Wolf M. K. CNS tissue cultures: changes in the ratio of incorporation of leucine and valine into proteins during myelinogenesis. J Neurochem. 1971 Jun;18(6):1149–1152. doi: 10.1111/j.1471-4159.1971.tb12043.x. [DOI] [PubMed] [Google Scholar]
- Sobkowicz H. M., Bleier R., Monzain R. Cell survival and architectonic differentiation of the hypothalamic mamillary region of the newborn mouse in culture. J Comp Neurol. 1974 Jun 1;155(3):355–375. doi: 10.1002/cne.901550306. [DOI] [PubMed] [Google Scholar]
- Wolf M. K., Dubois-Dalcq M. Anatomy of cultured mouse cerebellum. I. Golgi and electron microscopic demonstrations of granule cells, their afferent and efferent synapses. J Comp Neurol. 1970 Nov;140(3):261–280. doi: 10.1002/cne.901400303. [DOI] [PubMed] [Google Scholar]
- Yavin E., Menkes J. H. The culture of dissociated cells from rat cerebral cortex. J Cell Biol. 1973 Apr;57(1):232–237. doi: 10.1083/jcb.57.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]