Abstract
OBJECTIVES
To determine whether lymphovascular invasion (LVI) in radical prostatectomy (RP) specimens has prognostic significance.
The study examined whether LVI is associated with clinicopathological characteristics and biochemical recurrence (BCR).
PATIENTS AND METHODS
LVI was evaluated based on routine pathology reports on 1298 patients treated with RP for clinically localized prostate cancer between 2004 and 2007.
LVI was defined as the unequivocal presence of tumour cells within an endothelium-lined space.
The association between LVI and clinicopathological features was assessed with univariate logistic regression. Cox regression was used to test the association between LVI and BCR.
RESULTS
LVI was identified in 10% (129/1298) of patients.
The presence of LVI increased with advancing pathological stage: 2% (20/820) in pT2N0 patients, 16% (58/363) in pT3N0 patients and 17% (2/12) in pT4N0 patients; and was highest in patients with pN1 disease (52%; 49/94).
Univariate analysis showed an association between LVI and higher preoperative prostate-specific antigen levels and Gleason scores, and a greater likelihood of extraprostatic extension, seminal vesicle invasion, lymph node metastasis and positive surgical margins (all P < 0.001).
With a median follow-up of 27 months, LVI was significantly associated with an increased risk of BCR after RP on univariate (P < 0.001) and multivariate analysis (hazard ratio, 1.77; 95% confidence interval, 1.11–2.82; P = 0.017).
As a result of the relatively short follow-up, the predictive accuracy of the standard clinicopathological features was high (concordance index, 0.880), and inclusion of LVI only marginally improved the predictive accuracy (0.884).
CONCLUSIONS
Although associated with features of aggressive disease and BCR, LVI added minimally to established predictors on short follow-up.
Further study of cohorts with longer follow-up is warranted to help determine its prognostic significance.
Keywords: prostate, prostatic neoplasms, prostatectomy, disease progression, lymphovascular invasion
INTRODUCTION
Predictive models have been developed to identify patients at increased risk for disease recurrence after radical prostatectomy (RP). Accepted clinicopathological factors associated with disease recurrence after RP include serum PSA, Gleason score, pathological stage, lymph node metastasis and status of the surgical margins [1]. Lymphovascular invasion (LVI) is an additional pathological feature that has been receiving attention as a potential risk factor for cancer recurrence, and the College of American Pathologists has recommended reporting whether or not LVI was identified in the RP specimen [2]. It is hypothesized that the presence of LVI could indicate micrometastases as seen in other malignancies, such as urothelial carcinoma [3–5].
Previous retrospective studies report not only differing incidence rates of LVI, but also differing conclusions with respect to its prognostic significance [6–20]. In the present study, data from a large contemporary cohort of patients treated with RP for clinically localized prostate cancer were analyzed to determine whether LVI was associated with either clinicopathological characteristics or biochemical recurrence (BCR), and whether the presence of LVI was more important in patients with organ-confined disease. In addition, the study examined whether a postoperative model (i.e. a nomogram) predicting BCR after RP could be improved by including LVI as a factor.
PATIENTS AND METHODS
PATIENT POPULATION
Between August 2004 and July 2007, 2150 consecutive patients with clinically localized prostate cancer were treated with RP at Memorial Sloan-Kettering Cancer Center. Patients who received neoadjuvant therapy (n = 145) were excluded. The pathology reports of the remaining patients were reviewed. Pathology reports that did not include LVI status (n = 379) or other pathological features (Gleason score, extent of extraprostatic extension, seminal vesicle invasion or lymph node metastasis; n = 328) were also excluded, leaving a total of 1298 patient reports available for univariate analyses of pathological features. Of these, 74 were missing PSA measurements and 75 had no data on BCR, leaving 1149 patient reports available for multivariate analyses for BCR. Patient data were collected prospectively and entered into an electronic database.
Patients were followed at 3-month intervals for the first year, at 6-month intervals for the next 4 years, and annually thereafter with DRE and serum PSA measurements. BCR was defined as a serum PSA >0.1 ng/mL at least 6 weeks after surgery with a confirmatory rise. Patients who received adjuvant therapy (n = 24) before BCR were not considered to have disease recurrence until they met the same criteria.
PATHOLOGICAL EVALUATION
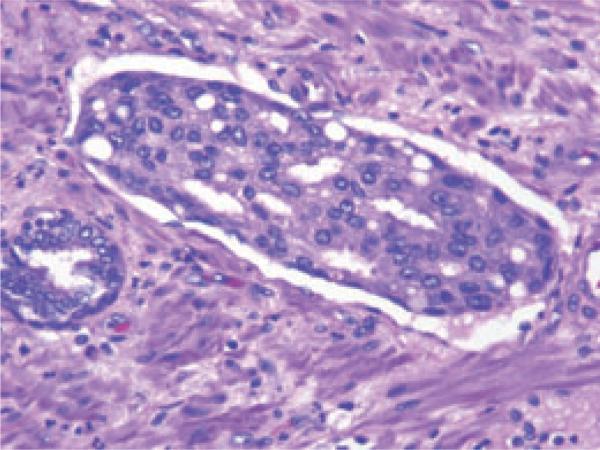
All RP specimens were uniformly processed and submitted in their entirety. The prostate and seminal vesicles were fixed in 10% neutral formalin overnight after inking the outer surface. The superficial fragments of muscular tissue surrounding the proximal urethra were shaved and the most apical 3 mm was embedded on end after radial sectioning in cone-like fashion, to allow assessment of both the bladder neck and inked apical margins. The seminal vesicles were amputated at their junction with the prostate and submitted separately. Finally, the remaining prostate was serially sectioned from apex to base at 3- to 5-mm intervals and submitted as whole-mount sections for examination. Whole-mount sections of 5 μm thickness were stained with haematoxylin and eosin. Specimens were assigned a Gleason grade and staged according to the 2002 TNM clinical staging system developed by the American Joint Committee on Cancer and the International Union Against Cancer. LVI was defined as the unequivocal presence of tumour cells within an endothelium-lined space (Fig. 1). Because of the difficulty and lack of reproducibility when using routine light microscopy, no attempt was made to differentiate between lymphatic and vascular vessels [17]. LVI was identified based on routine pathology reports and, beginning in August 2004, was a parameter that required a yes or no response on our institutional online synoptic sign-out sheet. A positive surgical margin was defined as presence of tumour cells at the inked margin of the specimen.
FIG. 1.
Lymphovascular invasion in prostate cancer. Magnification ×200.
STATISTICAL ANALYSIS
Univariate logistic regression was used to evaluate the association between LVI and clinicopathological features (preoperative PSA level and Gleason score, postoperative extraprostatic extension, seminal vesicle invasion, lymph node metastasis and surgical margin status). The probability of freedom from BCR following RP was estimated using Kaplan–Meier methods. Multivariate Cox regression analysis was used to test for the association between LVI and BCR, adjusting for the effects of preoperative PSA and standard pathological features (Gleason score, extraprostatic extension, seminal vesicle invasion, lymph node metastasis and margin status). The present study also explored whether the association between LVI and BCR was different according to pathological stage (≤ pT2 vs > pT2) by including an interaction term between LVI and pathological stage in the multivariate model.
To determine whether the addition of LVI improved the predictive accuracy of a base postoperative nomogram that included preoperative PSA and standard pathological features, the concordance index of the model was calculated with and without the addition of LVI [21] and bootstrap methods were used to reduce overfit bias, which may inflate the predictive accuracy [22]. P < 0.05 (two-sided) was considered statistically significant. Statistical analyses were performed using the Stata 10 software package (StataCorp, College Station, TX, USA).
RESULTS
Table 1 lists the clinical and pathological features that were included in the present evaluation and their associations with LVI in 1298 patients treated with RP for clinically localized prostate cancer. The median patient age was 59 years and the median preoperative serum PSA level was 5.3 ng/mL.
TABLE 1.
Association between lymphovascular invasion and clinicopathological features in 1298 patients treated with radical prostatectomy for clinically localized disease
| Lymphovascular invasion |
||||
|---|---|---|---|---|
| Characteristics | Patients (n) | Absent (n = 1169) | Present (n = 129) | P |
| Age (years), median (IQR) | 1298 | 59 (55–64) | 61 (56–66) | NA |
| Preoperative PSA (ng/mL), median (IQR)* | 1224 | 5.1 (3.7–7.1) | 7.3 (4.9–11.3) | <0.001 |
| Gleason score, n (%) | <0.001 | |||
| 2–6 | 320 (25) | 314 (27) | 6 (5) | |
| 7 | 874 (67) | 806 (69) | 68 (53) | |
| 8–10 | 104 (8) | 49 (4) | 55 (42) | |
| Extraprostatic extension, n (%) | <0.001 | |||
| Absent | 853 (66) | 821 (70) | 32 (25) | |
| Present | 445 (34) | 348 (30) | 97 (75) | |
| Seminal vesicle invasion, n (%) | <0.001 | |||
| Absent | 1208 (93) | 1128 (96) | 80 (62) | |
| Present | 90 (7) | 41 (4) | 49 (38) | |
| Lymph node metastasis, n (%) | <0.001 | |||
| Absent | 1204 (93) | 1124 (96) | 80 (62) | |
| Present | 94 (7) | 45 (4) | 49 (38) | |
| Surgical margins, n (%) | <0.001 | |||
| Negative | 1127 (87) | 1034 (88) | 93 (72) | |
| Positive | 171 (13) | 135 (12) | 36 (28) | |
Missing in 74 patients. IQR, interquartile range; NA, not applicable.
Overall, LVI was identified in 10% (129/1298) of patients. The presence of LVI increased with advancing disease stage: it was found in 2% (20/820) of patients with pT2N0 disease, 16% (58/363) with pT3N0 and 17% (2/12) with pT4N0, and was highest in patients with pN1 disease (52%, 49/94). Patients with LVI were more likely to have pT3 or higher stage disease than those without LVI (81% vs 30%, respectively). On univariate analyses, LVI was associated with higher preoperative PSA, higher pathological Gleason score, extraprostatic extension, seminal vesicle invasion, lymph node metastasis and positive surgical margins (all P < 0.001). Some 43% of men with LVI had a Gleason score of ≥8 vs only 4% of men without LVI.
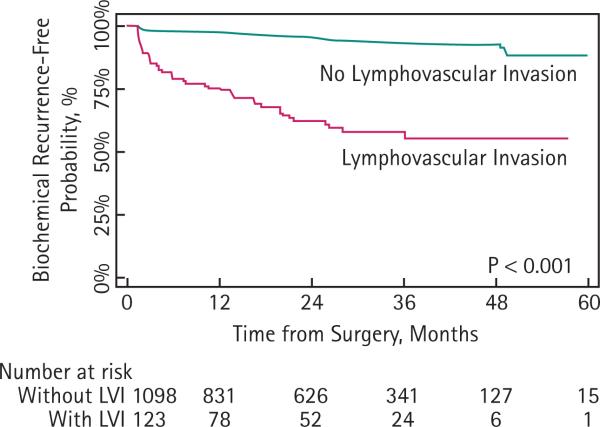
In total, 99 of the 1149 patients included in multivariate analyses experienced BCR, including 41 patients with LVI and 58 without. The median follow-up for patients without BCR was 2.3 years. The 2-year BCR-free probability was 95% (95% CI, 94–96) for men without LVI and 62% (95% CI, 52–71) for those with LVI (Fig. 2).
FIG. 2.
Probability of freedom from biochemical recurrence according to the presence or absence of lymphovascular invasion.
On univariate analysis, LVI was significantly associated with an increased risk of BCR after RP (P < 0.001) and remained significantly associated after adjusting for the effects of preoperative PSA and standard pathological features in multivariate analysis (hazard ratio, HR, 1.77; 95% CI, 1.11–2.82; P = 0.017) (Table 2).
TABLE 2.
Multivariate Cox regression analysis of clinicopathological features for the prediction of biochemical recurrence in 1149 patients treated with radical prostatectomy for clinically localized disease
| Hazard ratio | 95% CI | P | |
|---|---|---|---|
| Preoperative PSA | 1.02 | 1.01–1.04 | 0.004 |
| Lymphovascular invasion | 1.77 | 1.11–2.82 | 0.017 |
| Gleason score | <0.001 | ||
| ≤6 | 1.00 | Reference | – |
| 7 | 4.64 | 1.10–19.55 | |
| ≥8 | 12.35 | 2.75–55.43 | |
| Extraprostatic extension | 3.45 | 1.91–6.22 | <0.001 |
| Seminal vesicle invasion | 2.33 | 1.44–3.75 | <0.001 |
| Lymph node metastasis | 1.38 | 0.85–2.23 | 0.19 |
| Surgical margin positivity | 1.98 | 1.28–3.06 | 0.002 |
In evaluating whether the addition of LVI improved the predictive accuracy of the standard clinicopathological features, it was noted that the standard model's concordance index (0.880) was higher than previously reported [23]. This is likely a result of the short length of follow-up; it is much easier to predict those patients who will experience BCR in the short term (<2 years) than over the long term (>5 years). Given the high predictive accuracy of the base model, the concordance index would not be expected to be substantially improved by the inclusion of any additional variable. The addition of LVI improved the predictive accuracy of the postoperative nomogram only marginally (from 0.880 to 0.884).
There was no evidence to suggest that organ-confined disease status modified the association between LVI and BCR (P = 0.5 for interaction term). In a model that included only LVI, lymph node involvement, and an interaction term, the effect of LVI appeared to be stronger without lymph node involvement (HR, 2.75; 95% CI, 1.17–6.43; P = 0.020 for interaction term); however, after adjustment for the effects of standard prognostic variables, this association was no longer statistically significant (P = 0.9). This is probably a result of patients with LVI in the subgroup with negative lymph nodes being more likely to have extraprostatic extension or seminal vesicle invasion compared to those without LVI (72% vs 29%).
DISCUSSION
The present study identified LVI in 10% of patients undergoing RP for clinically localized prostate cancer. The reported incidence of LVI is in the range 5–53% (Table 3) [6–20]. Among the 363 patients in our series with pT3N0 disease, the LVI rate was 16%, below the reported rates of 22–35% in previous studies [12,15]. These discrepancies may be attributable to various factors, including specimen handling, differences in patient populations, inter-observer variability and the lack of uniform definitions of LVI. LVI was defined in the present study as the unequivocal presence of tumour cells within an endothelium-lined space. However, to ultimately assess the true prognostic value of LVI, standardized histological criteria and consistent pathological examination are essential.
TABLE 3.
Literature review of lymphovascular invasion incidence and prognostic significance for biochemical recurrence in patients treated with radical prostatectomy
| References | Surgery (years) | Patients (n) | Pathological stage | % LVI | Mean follow-up (months) | BCR predictor |
|---|---|---|---|---|---|---|
| Bahnson et al. [7] | 1975–1982 | 55 | pT2-3, pN0-1 | 51 | 84 | No |
| Salomao et al. [17] | 1991–1992 | 210 | pT2-3, pN0-1 | 53 | NA | No |
| McNeal and Yemoto [16] | 1984–1991 | 357 | pT2-3, pN0-1 | 14 | NA | Yes |
| van den Ouden et al. [19] | 1977–1994 | 273 | pT2-4, pN0-1 | 12 | 49 (median) | Yes |
| Herman et al. [12] | 1983–1997 | 263 | pT3, pN0 | 35 | 36 (median) | Yes |
| Epstein et al. [10] | 1984–1994 | 60 | pT3b, pNna | 22 | NA | Yes |
| de la Taille et al. [9] | 1993–1998 | 241 | pT2-4, pNna | 12 | 23 | Yes |
| Babaian et al. [6] | 1987–1993 | 265 | pT2-3, pN0 | NA | 48 (minimum) | Yes |
| Ito et al. [13] | 1989–1998 | 82 | pT2-3, pN0 | 46 | 22 | Yes |
| Ferrari et al. [11] | 1990–1998 | 620 | pT2-3, pN0-1 | 18 | 90 | Yes |
| Shariat et al. [18] | 1994–2002 | 630 | pT2-3, pN0-1 | 5 | 21 (median) | No |
| Cheng et al. [8] | 1990–1998 | 504 | pT2-3, pN0-1 | 21 | 44 | Yes |
| Loeb et al. [14] | 1989–2004 | 1709 | pT2-3, pN0-1 | 7 | 74 | No |
| May et al. [15] | 1996–2003 | 412 | pT2-3, pN0 | 10 | 53 | Yes |
| Yamamoto et al. [20] | 1994–2005 | 94 | pT3aN0 | 28 | 47 (median) | Yes |
BCR, biochemical recurrence; LVI, lymphovascular invasion; NA, not available.
In the present study, the strong and significant association of LVI with established adverse clinicopathological features was confirmed, including higher preoperative PSA, higher Gleason score, extraprostatic extension, seminal vesicle invasion, lymph node metastasis and positive surgical margins [8,11,14,18]. Although an association with pathological features is interesting, an association with BCR after RP is more important in the management of patients with prostate cancer. LVI was found to be an independent predictor of BCR after RP in a multivariate analysis that controlled for the effects of standard predictors. Infiltration of the vascular and/or lymphatic structures by tumour cells is an important step in tumour dissemination because these pathways enable them to access distant organs. The initial entry of neoplastic cells into the circulation occurs through the local microvascular network, including the lymphatic and/or blood vessels. This premise, together with the strong association of LVI with lymph node metastasis and BCR after apparently effective local treatment, suggests that LVI plays a role in the metastatic process.
The addition of LVI, however, only marginally improved the concordance index (from 0.880 to 0.884) of our postoperative nomogram, and therefore was not clinically meaningful. Because LVI was significantly associated with all other standard prognostic features, its limited clinical usefulness is not unexpected once these features are taken into account. On the other hand, although the small incidence of LVI may limit its statistical value, its real value may be in helping clinical decision-making regarding receiving adjuvant therapy for patients treated with RP.
Previous studies have investigated the predictive value of LVI with conflicting results (Table 3) [6–20]. Although several studies found LVI to be an independent predictor of BCR [6,8–13,15,16,19,20], others did not [7,14,17,18]. Unlike the present study, these studies found that LVI was associated with BCR after RP on univariate, but not multivariate, analysis [14,18]. Loeb et al. [14] concluded that the effect of LVI on BCR was mediated through its strong association with other adverse pathological features. Shariat et al. [18] also failed to show an independent association between LVI and BCR, but found that LVI was associated with established features of biologically aggressive prostate cancer (e.g. shorter PSA doubling times after biochemical failure, rapid failure to respond to salvage local radiation therapy and, most remarkably, increased likelihood of early distant metastases and death). According to Shariat et al. [18], the association of LVI with early biochemical and clinical disease progression suggests that LVI is a feature of the metastatic process, putting patients at the highest risk for early metastasis and death.
Conversely, May et al. [15] found LVI in 10% of 412 patients undergoing RP, and reported an association between LVI and higher preoperative PSA, PSA density, percentage of positive biopsy cores, Gleason score and seminal vesicle invasion. A statistically significant difference in BCR-free probability between patients with and without LVI was also shown [15].
Given these divergent results, the prognostic significance of LVI remains unclear. Although some studies find LVI to be a statistically significant factor in predicting BCR, it may not be useful in improving existing prognostic models. In their 1999 consensus statement, the College of American Pathologists considered LVI a category 3 prognostic factor, meaning that there was insufficient evidence to support its prognostic value [24]. Currently, they consider the assessment of LVI to be clinically important, at the same time recognizing that it has not yet been validated and is not regularly used in patient management [2].
LVI may have differing prognostic significance for specific subgroups of patients with prostate cancer. Patients with pT3N0 tumours have an intermediate risk of disease recurrence after RP compared to patients with organ-confined or lymph node-positive disease. In a cohort of 263 patients with pT3N0 tumours, Herman et al. [12] found LVI to be an independent predictor of disease recurrence on multivariate analysis. Similarly, studies by Epstein et al. [10] and Yamamoto et al. [20] found LVI to be an independent predictor of BCR in patients with pT3aN0 and pT3b tumours. In a study by Babaian et al. [6], LVI was associated with BCR in the overall population but not in the pT2 (margin negative or positive) disease subgroups. In the present study, there was no evidence to suggest that the association between LVI and BCR was stronger in either pT2N0 or pT3N0 disease subgroups.
Several limitations of the present study should be acknowledged. We did not quantitate the extent of LVI or determine the location of LVI foci because other investigators have found adverse outcomes even among cases with a limited number of foci [11,12]. As with previous studies, LVI was identified based on routine pathology reports rather than a detailed slide review by a single uropathologist. Another limitation is that the inherent difficulty in determining the presence of LVI at the morphological level is exacerbated when there are significant differences between local pathologists and central pathology review [8,25]. For example, retraction artefacts present in the surrounding stromal tissue can mimic vascular invasion [26,27]. In addition, we did not perform immunohistochemical staining for endothelial markers (e.g. CD31) because this is not recommended for the routine evaluation of LVI. We also did not perform immunohistochemical staining to identify the vessels because heterogeneity in the expression of immunohistochemical markers by different capillary structures renders it impractical for routine clinical use [28,29].
Other potential shortcomings of the present study include not addressing the impact of LVI location (i.e. peritumoural vs intratumoural invasion) [30] or the differential impact of lymphatic vs blood vasculatures on outcomes. In most cases, only capillary structures are recognizable, making it very difficult to distinguish lymphatics from blood vessels. Although fairly reliable vessel identification is possible with haematoxylin and eosin staining, it is of utmost importance that strict morphological criteria are established to standardize and make the diagnosis of LVI reproducible, and consequently allow its recommendation in a clinical setting.
Finally, the present study was performed at a tertiary referral centre and may not be applicable to the general population of patients with prostate cancer. Despite these limitations, we found that LVI was a significant independent predictor of BCR. Its inclusion as an additional pathological feature, however, did not meaningfully improve the accuracy of a postoperative nomogram predicting BCR after RP.
LVI is present in ≈10% of RP specimens taken from patients with clinically localized prostate cancer. Although the inclusion of LVI only marginally improved the predictive accuracy of the concordance index of established clinicopathological variables, it is associated with features of aggressive disease and is an independent predictor of BCR. Longer follow-up is needed to corroborate these results and to determine whether LVI is a useful prognostic indicator in prostate cancer.
What's known on the subject? and What does the study add?
The reported incidence of lymphovascular invasion (LVI) in radical prostatectomy specimens ranges from 5% to 53%. Although LVI has a strong and significant association with adverse clinicopathologic features, it has almost uniformly not been found to be a predictor of biochemical recurrence (BR) on multivariate analysis.
This study confirms that LVI is associated with features of aggressive disease and is an independent predictor of BCR. Given that LVI may play a role in the metastatic process, it may be useful in clinical decision-making regarding adjuvant therapy for patients treated with RP.
ACKNOWLEDGEMENTS
This study was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers.
Abbreviations
- BCR
biochemical recurrence
- HR
hazard ratio
- LVI
lymphovascular invasion
- RP
radical prostatectomy
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Diblasio CJ, Kattan MW. Use of nomograms to predict the risk of disease recurrence after definitive local therapy for prostate cancer. Urology. 2003;62(Suppl. 1):9–18. doi: 10.1016/j.urology.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 2.Srigley JR, Amin MB, Epstein JI, et al. Updated protocol for the examination of specimens from patients with carcinomas of the prostate gland. Arch Pathol Lab Med. 2006;130:936–46. doi: 10.5858/2006-130-936-UPFTEO. [DOI] [PubMed] [Google Scholar]
- 3.Kikuchi E, Margulis V, Karakiewicz PI, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol. 2009;27:612–8. doi: 10.1200/JCO.2008.17.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shariat SF, Svatek RS, Tilki D, et al. International validation of the prognostic value of lymphovascular invasion in patients treated with radical cystectomy. BJU Int. 2010;105:1402–12. doi: 10.1111/j.1464-410X.2010.09217.x. [DOI] [PubMed] [Google Scholar]
- 5.Lotan Y, Gupta A, Shariat SF, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol. 2005;23:6533–9. doi: 10.1200/JCO.2005.05.516. [DOI] [PubMed] [Google Scholar]
- 6.Babaian RJ, Troncoso P, Bhadkamkar VA, et al. Analysis of clinicopathologic factors predicting outcome after radical prostatectomy. Cancer. 2001;91:1414–22. [PubMed] [Google Scholar]
- 7.Bahnson RR, Dresner SM, Gooding W, et al. Incidence and prognostic significance of lymphatic and vascular invasion in radical prostatectomy specimens. Prostate. 1989;15:149–55. doi: 10.1002/pros.2990150208. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Jones TD, Lin H, et al. Lymphovascular invasion is an independent prognostic factor in prostatic adenocarcinoma. J Urol. 2005;174:2181–5. doi: 10.1097/01.ju.0000181215.41607.c3. [DOI] [PubMed] [Google Scholar]
- 9.De la Taille A, Rubin MA, Buttyan R, et al. Is microvascular invasion on radical prostatectomy specimens a useful predictor of PSA recurrence for prostate cancer patients? Eur Urol. 2000;38:79–84. doi: 10.1159/000020256. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI, Partin AW, Potter SR, et al. Adenocarcinoma of the prostate invading the seminal vesicle: prognostic stratification based on pathologic parameters. Urology. 2000;56:283–8. doi: 10.1016/s0090-4295(00)00640-3. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari MK, McNeal JE, Malhotra SM, et al. Vascular invasion predicts recurrence after radical prostatectomy: stratification of risk based on pathologic variables. Urology. 2004;64:749–53. doi: 10.1016/j.urology.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 12.Herman CM, Wilcox GE, Kattan MW, et al. Lymphovascular invasion as a predictor of disease progression in prostate cancer. Am J Surg Pathol. 2000;24:859–63. doi: 10.1097/00000478-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Ito K, Nakashima J, Mukai M, et al. Prognostic implication of microvascular invasion in biochemical failure in patients treated with radical prostatectomy. Urol Int. 2003;70:297–302. doi: 10.1159/000070139. [DOI] [PubMed] [Google Scholar]
- 14.Loeb S, Roehl KA, Yu X, et al. Lymphovascular invasion in radical prostatectomy specimens: prediction of adverse pathologic features and biochemical progression. Urology. 2006;68:99–103. doi: 10.1016/j.urology.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 15.May M, Kaufmann O, Hammerman F, et al. Prognostic impact of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2007;99:539–44. doi: 10.1111/j.1464-410X.2006.06650.x. [DOI] [PubMed] [Google Scholar]
- 16.McNeal JE, Yemoto CE. Significance of demonstrable vascular space invasion for the progression of prostatic adenocarcinoma. Am J Surg Pathol. 1996;20:1351–60. doi: 10.1097/00000478-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Salomao DR, Graham SD, Bostwick DG. Microvascular invasion in prostate cancer correlates with pathologic stage. Arch Pathol Lab Med. 1995;119:1050–4. [PubMed] [Google Scholar]
- 18.Shariat SF, Khoddami SM, Saboorian H, et al. Lymphovascular invasion is a pathological feature of biologically aggressive disease in patients treated with radical prostatectomy. J Urol. 2004;171:1122–7. doi: 10.1097/01.ju.0000113249.82533.28. [DOI] [PubMed] [Google Scholar]
- 19.Van den Ouden D, Kranse R, Hop WC, et al. Microvascular invasion in prostate cancer: prognostic significance in patients treated by radical prostatectomy for clinically localized carcinoma. Urol Int. 1998;60:17–24. doi: 10.1159/000030197. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto S, Kawakami S, Yonese J, et al. Lymphovascular invasion is an independent predictor of prostate-specific antigen failure after radical prostatectomy in patients with pT3aN0 prostate cancer. Int J Urol. 2008;10:895–9. doi: 10.1111/j.1442-2042.2008.02140.x. [DOI] [PubMed] [Google Scholar]
- 21.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson AJ, Scardino PT, Eastham JA, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005–12. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostwick DG, Grignon DJ, Hammond ME, et al. Prognostic factors in prostate cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000. 124:995–1000. doi: 10.5858/2000-124-0995-PFIPC. [DOI] [PubMed] [Google Scholar]
- 25.Sesterhenn IA, Weiss RB, Mostofi FK, et al. Prognosis and other clinical correlates of pathologic review in stage I and II testicular carcinoma: a report from the Testicular Cancer Intergroup Study. J Clin Oncol. 1992;10:69–78. doi: 10.1200/JCO.1992.10.1.69. [DOI] [PubMed] [Google Scholar]
- 26.Lapham RL, Grignon D, Ro JY. Pathologic prognostic parameters in bladder urothelial biopsy, transurethral resection, and cystectomy specimens. Semin Diagn Pathol. 1997;14:109–22. [PubMed] [Google Scholar]
- 27.Larsen MP, Steinberg GD, Brendler CB, et al. Use of Ulex europaeus agglutinin I (UEAI) to distinguish vascular and ‘pseudovascular’ invasion in transitional cell carcinoma of bladder with lamina propria invasion. Mod Pathol. 1990;3:83–8. [PubMed] [Google Scholar]
- 28.Miyata Y, Kanda S, Ohba K, et al. Tumor lymphangiogenesis in transitional cell carcinoma of the upper urinary tract: association with clinicopathological features and prognosis. J Urol. 2006;176:348–53. doi: 10.1016/S0022-5347(06)00520-9. [DOI] [PubMed] [Google Scholar]
- 29.Straume O, Jackson DG, Akslen LA. Independent prognostic impact of lymphatic vessel density and presence of low-grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res. 2003;9:250–6. [PubMed] [Google Scholar]
- 30.Roma AA, Magi-Galluzzi C, Kral MA, et al. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate carcinoma. Mod Pathol. 2006;19:392–8. doi: 10.1038/modpathol.3800546. [DOI] [PubMed] [Google Scholar]