Abstract
A baroreflex equilibrium diagram describes the relation between input pressure and sympathetic nerve activity (SNA) and that between SNA and arterial pressure (AP). To calibrate the SNA axis (abscissa) of the baroreflex equilibrium diagram, the AP response to intravenous bolus injections of phenylephrine (0.2–50 μg/kg) or norepinephrine (NE, 0.02–5 μg/kg) was examined in normal control rats (NC, n = 9) and rats with chronic heart failure (CHF, n = 6). The maximum slope of the dose–effect curve was significantly smaller in the CHF group than in the NC group (57.3 ± 5.2 vs 80.9 ± 6.3 mmHg/decade for phenylephrine, 60.2 ± 7.8 vs 80.4 ± 5.9 mmHg/decade for NE; P < 0.01). The CHF/NC ratio of the maximum slope was used to calibrate SNA. While the calibrated baroreflex equilibrium diagram showed increased maximum SNA and operating-point SNA in CHF rats compared with NC rats, the magnitude of increase was smaller than that expected from the excess plasma NE concentration in CHF rats. Plasma NE concentration in the CHF group could be disproportionally high relative to SNA.
Keywords: carotid sinus baroreflex, open-loop analysis, arterial pressure, phenylephrine, norepinephrine
Introduction
The arterial baroreflex is a major negative feedback system that stabilizes arterial pressure (AP) during daily activities. From an analytical point of view, this system may be divided into two principal subsystems: a neural arc subsystem that defines the input–output relation between baroreceptor pressure and efferent sympathetic nerve activity (SNA), and a peripheral arc subsystem that defines the input–output relation between efferent SNA and AP.1,2 Under normal physiological conditions, changes in AP alter SNA via the neural arc, while changes in SNA affect AP via the peripheral arc (Fig. 1A). This closed-loop negative feedback operation makes it difficult to separately identify the characteristics of the neural and peripheral arcs. For instance, if pharmacological intervention is used to assess the SNA response to changes in AP, it usually becomes impossible to observe the AP response to SNA independently of the drug’s effect on AP. To circumvent this problem, we have employed a method where the carotid sinus baroreceptor regions are isolated from the systemic circulation3,4 so that we can identify the characteristics of the neural and peripheral arcs under open-loop conditions (Fig. 1B).2,5–7 Once the characteristics of the neural and peripheral arcs are identified, a baroreflex equilibrium diagram can be constructed by drawing the two arcs on a pressure–SNA plane (Fig. 1C). The baroreflex equilibrium diagram provides information on how the operating-point SNA and AP are determined through the interaction of the neural and peripheral arcs.8–11
Figure 1.
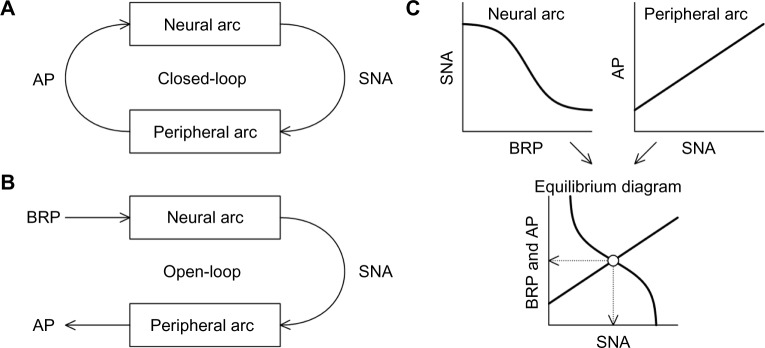
(A) Schematic diagram of the closed-loop negative feedback operation between neural and peripheral arcs of the arterial baroreflex. (B) Schematic diagram of the open-loop analysis of the baroreflex system. (C) Input–output relationships of the neural and peripheral arcs.
Notes: The baroreflex equilibrium diagram is obtained by plotting the neural and peripheral arcs on a pressure–SNA plane. The intersection (open circle) indicates the operating point of the baroreflex control. The dotted arrows indicate the operating-point SNA and AP.
Abbreviations: AP, arterial pressure; SNA, sympathetic nerve activity; BRP, baroreceptor pressure.
A number of studies have indicated that arterial baroreflex function is depressed in chronic heart failure (CHF).12–15 We have identified the open-loop static characteristics of the neural and peripheral arcs in rats with CHF following myocardial infarction and compared them with those in normal control (NC) rats.6,16 These studies indicate that the neural arc characteristics approximate an inverse sigmoid curve, and the response range of the neural arc is reduced in CHF rats compared with NC rats. The inability of the impaired neural arc to suppress SNA could contribute to an increase in SNA and the resultant aggravation of cardiac function typically observed in CHF individuals.17,18 Peripheral arc characteristics normally approximate a straight line. The slope of the peripheral arc is smaller in CHF rats than in NC rats, in accordance with the notion of a blunted target-organ response in CHF.14,15 One of the limitations in our open-loop baroreflex studies is that SNA was expressed in percentage units in each animal because the absolute amplitude of multifiber SNA varied largely among animals depending on the recording conditions. As a result, a comparison of the absolute levels of SNA between CHF and NC groups was impossible.6,16 The response range of the neural arc was smaller in the CHF group than in the NC group, but it could have been underestimated and might have been larger if the SNA was expressed in absolute units. Conversely, the slope of the peripheral arc could have been overestimated in CHF rats, and the AP response to SNA might have been much smaller in CHF rats than in NC rats if the SNA was expressed in absolute units.
If we know the difference in the AP response to adrenergic activation between NC and CHF rats, we may be able to calibrate the SNA axis (abscissa) of the baroreflex equilibrium diagram because the baroreflex peripheral arc characterizes sympathetic AP control. Feng et al.19 examined the AP response to electrical preganglionic sympathetic nerve stimulation in pithed CHF rats after myocardial infarction. Their data indicated that the AP response to the sympathetic stimulation was significantly attenuated in CHF rats compared with a sham-operated control group. They also demonstrated that the AP response to pharmacological adrenergic activation was decreased in CHF rats.19 Although these data may be utilized to calibrate the SNA axis of the baroreflex equilibrium diagram, the severity of CHF could be different between their study19 and our previous studies.6,16 In addition, differences in the experimental setting (eg, pithed rats vs rats anesthetized with α-chloralose and urethane) may affect the AP response to adrenergic activation. Accordingly, the aim of the present study was to compare the AP response to adrenergic activation between NC and CHF rats in our own experimental setting and to calibrate the SNA axis of the baroreflex equilibrium diagram based on the AP response to adrenergic activation.
Materials and Methods
Animal care was provided in strict accordance with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences, approved by the Physiological Society of Japan. All protocols were reviewed and approved by the Animal Subject Committee of the National Cerebral and Cardiovascular Center.
Surgical preparation
Myocardial infarction was produced in eight-week-old, male Sprague–Dawley rats according to a previously established procedure.6,16,20,21 Eight weeks after myocardial infarction, the surviving rats underwent an acute experiment as follows. Rats in the NC (n = 9) and the CHF (n = 6) groups were anesthetized with an intraperitoneal injection (2 mL/kg) of a mixture of urethane (250 mg/mL) and α-chloralose (40 mg/mL) and were mechanically ventilated with oxygen-supplied room air. Anesthesia was maintained by a continuous intravenous infusion of a diluted solution of the above anesthetic mixture from a catheter inserted into the right femoral vein. Another venous catheter was inserted into the left femoral vein for measuring baseline central venous pressure and for administering pharmacological agents. An arterial catheter was inserted into the right femoral artery to measure AP. Body temperature of the animal was maintained at approximately 38 °C using a heating pad and a lamp.
Protocols
Hexamethonium bromide (60 mg/kg) was given as an intravenous bolus injection to block sympathetic and parasympathetic ganglionic transmissions. Ten minutes later, 0.5 mL of saline was given as a control injection; thereafter, an α1-adrenergic agonist, phenylephrine (PE), was given as intravenous bolus injections (0.2 mL of drug solution followed by 0.3 mL of saline to flush the solution) in an increasing order at 0.2, 0.5, 1, 2, 5, 10, 20, and 50 μg/kg. After finishing the PE protocol, a 30-minute recovery period was interposed, and another injection of hexamethonium bromide (60 mg/kg) was added. Ten minutes later, 0.5 mL of saline was given as a control injection; thereafter, norepinephrine (NE) was given as intravenous bolus injections (0.2 mL of drug solution, followed by 0.3 mL of saline to flush the solution) in an increasing order at 0.02, 0.05, 0.1, 0.2, 0.5, 1, 2, and 5 μg/kg. An interval of a minimum of three minutes and a maximum of 15 minutes was allowed between successive injections. In three of the NC rats, splanchnic SNA was recorded as previously reported.5–7 At the end of the PE protocol, a 10-second averaged value of SNA was less than 5% of a 10-second averaged baseline activity measured before the first administration of hexamethonium bromide, indicating a sufficient ganglionic blockade throughout the protocol.
Data analysis
Data were continuously recorded at a sampling frequency of 1,000 Hz using a 16-bit analog-to-digital converter. The peak AP response corresponding to each injection was determined from a 10-second moving averaged signal. The relation between the logarithmic dose of PE or NE and the peak AP response was analyzed by linear regression as follows.
| (1) |
where x and y denote the dose of PE or NE and the peak AP response, respectively. Pa represents the slope (in mmHg/decade), and Pb represents the intercept (in mmHg) of the regression line.
The dose–effect curve was also analyzed using a four-parameter logistic function as follows.
| (2) |
where x and y represent the dose of PE or NE and the peak AP response, respectively. P1 is the response range of AP (in mmHg); P2 is the slope coefficient; P3 is the logarithmic dose corresponding to the midpoint of the logistic function; and P4 is the lower plateau of AP (in mmHg). The parameter values were determined by nonlinear iterative least-square fitting. The maximum slope (in mmHg/decade) was calculated from P1 × P2/4. The dose corresponding to the midpoint (in μg/kg) was calculated from 10 to the power of P3.
Statistical analysis
All data are presented in mean ± standard error values. Body weight and baseline hemodynamics were compared between the NC and CHF groups by unpaired t-test. The slope of the linear regression and the parameters of the fitted logistic function were compared by two-way analysis of variance (ANOVA) in which the first factor was a disease effect (NC vs CHF groups) and the second factor was a drug effect (PE vs NE).22 The difference was considered significant at P < 0.05. Because of the logarithmic transformation of the dose of PE or NE, the intercept of the linear regression merely indicates the estimation of an AP value corresponding to a specific dose of 1 μg/kg without general meaning. Therefore, the intercept is not reported.
One NC rat died after the PE protocol, possibly due to an acute disappearance of the adrenergic pressor effect, and one CHF rat died in the midst of the NE protocol from a gradual deterioration in hemodynamics. Accordingly, the number of animals in the NE protocol became eight and five for the NC and CHF groups, respectively, and ANOVA was performed as nonrepeated comparisons.
Results
As shown in Table 1, body weight did not differ statistically between the NC and CHF groups, though the mean value was slightly heavier in the NC group. Biventricular weight was significantly heavier in CHF rats than in NC rats, both for the absolute value and for the normalized value relative to the body weight. Central venous pressure, measured before administering hexamethonium bromide under baseline conditions, was significantly higher in CHF rats than in NC rats. Mean AP under baseline conditions did not differ between the two groups. Although the mean value of heart rate was higher in the NC group than in the CHF group, the heart rate showed a large variance among animals and the difference between the two groups was not statistically significant.
Table 1.
Body weight, biventricular weight, and baseline hemodynamics.
| NC (N = 9) | CHF (N = 6) | P VALUE | |
|---|---|---|---|
| Body weight, g | 479 ± 24 | 445 ± 13 | 0.320 |
| Biventricular weight, g | 1.079 ± 0.056 | 1.334 ± 0.053 | 0.009 |
| Normalized biventricular weight, g/kg | 2.099 ± 0.058 | 3.013 ± 0.145 | <0.001 |
| Central venous pressure, mmHg | 1.72 ± 0.30 | 4.20 ± 1.09 | 0.029 |
| Mean arterial pressure, mmHg | 116.4 ± 1.8 | 111.7 ± 9.0 | 0.545 |
| Heart rate, beats/min | 422.3 ± 17.0 | 379.3 ± 17.9 | 0.116 |
Notes: Values are means ± standard error. P value was obtained by unpaired t-test.
Abbreviations: NC, normal control; CHF, chronic heart failure.
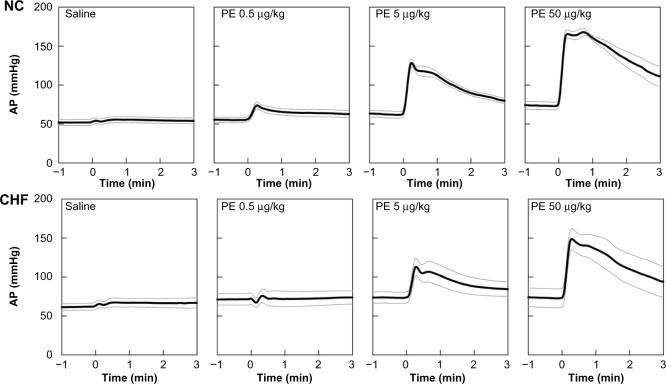
Figure 2 depicts group-averaged time series obtained from the PE protocol. Time series only for saline and 0.5-, 5-, and 50-μg/kg injections of the drug are shown for the sake of simplicity. Black and gray lines indicate mean values and mean ± standard error values, respectively. Intravenous saline showed only a slight fluctuation in AP. Intravenous PE elevated AP in a dose-dependent manner in the NC group. Peak AP responses were observed at 17 ± 1, 18 ± 3, and 37 ± 8 seconds in response to 0.5-, 5-, and 50-μg/kg injections, respectively. Although PE also increased AP dose-dependently in the CHF group, the magnitude of the AP response was smaller compared with that in the NC group. Peak AP responses were observed at 23 ± 9, 21 ± 5, and 23 ± 6 seconds in response to 0.5-, 5-, and 50-μg/kg injections, respectively.
Figure 2.
Time series of 10-second moving averaged signals of arterial pressure (AP) in response to intravenous injections of saline and 0.5-, 5-, and 50-μg/kg phenylephrine (PE) injections averaged for the normal control group (NC, n = 9, top panel) and for the chronic heart failure group (CHF, n = 6, bottom panel).
Note: Black and gray lines indicate mean values and mean ± standard error values.
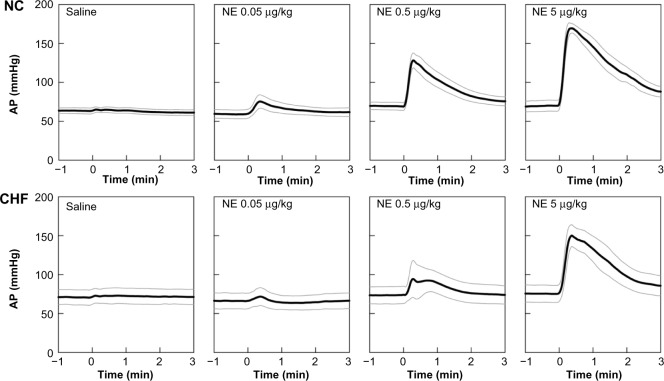
Figure 3 depicts group-averaged time series obtained from the NE protocol. Time series only for saline and 0.05-, 0.5-, and 5-μg/kg injections are shown. Intravenous saline did not change AP appreciably; intravenous NE elevated AP in a dose-dependent manner in the NC group. The peak AP responses were observed at 28 ± 7, 18 ± 1, and 16 ± 2 seconds in response to 0.05-, 0.5-, and 5-μg/kg injections, respectively. Although NE also increased AP dose-dependently in the CHF group, the magnitude of the AP response was smaller compared with that in the NC group. The peak AP responses were observed at 16 ± 4, 26 ± 8, 20 ± 2 seconds in response to 0.05-, 0.5-, and 5-μg/kg injections, respectively.
Figure 3.
Time series of 10-second moving averaged signals of arterial pressure (AP) in response to intravenous injections of saline and 0.05-, 0.5-, and 5-μg/kg norepinephrine (NE) injections averaged for the normal control group (NC, n = 8, top panel) and for the chronic heart failure group (CHF, n = 5, bottom panel).
Note: Black and gray lines indicate mean values and mean ± standard error values.
Figure 4 summarizes the peak AP responses versus the dose of PE (Fig. 4A) and NE (Fig. 4B). Open circles and filled circles represent the NC and CHF data, respectively. As shown in Table 2, the slope of the linear regression was significantly smaller in the CHF group than in the NC group (P = 0.002). When a four-parameter logistic function was fitted to the data for each animal, the maximum slope was significantly smaller in CHF rats than in NC rats (P = 0.003). The lower plateau of AP was not significantly different (P = 0.902), but the response range of AP was significantly narrower in the CHF rats than in the NC rats (P = 0.004). The dose corresponding to the midpoint of the logistic function tended to be higher in CHF rats than in NC rats (P = 0.077) and was significantly lower for NE than for PE (P < 0.001). No interaction effects were observed for any parameters. As an alternative analysis, four-parameter logistic functions were fitted to mean data points in each group (Fig. 4, smooth curves). In the PE protocol, the maximum slopes were estimated to be 78.9 and 50.6 mmHg/decade in the NC and CHF groups, respectively. In the NE protocol, the maximum slopes were estimated to be 78.2 and 44.9 mmHg/decade in the NC and CHF groups, respectively.
Figure 4.
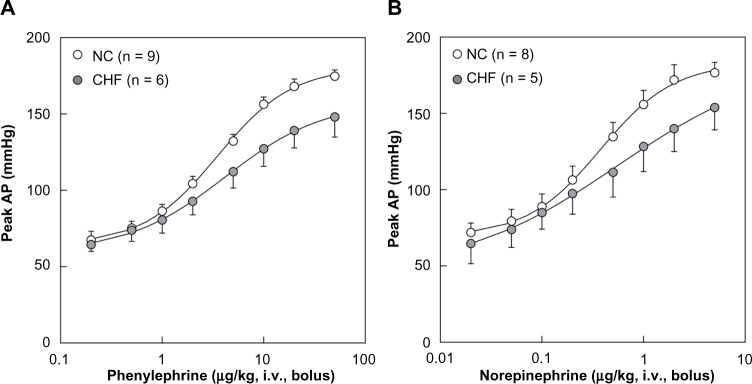
(A) Relation between the dose of phenylephrine and the peak arterial pressure (AP) response. (B) Relation between the dose of norepinephrine and the peak AP response. Data points represent means and standard errors. Smooth curves represent four-parameter logistic functions derived from the mean data points in each group.
Abbreviations: NC, normal control; CHF, chronic heart failure; iv, intravenous administration.
Table 2.
Relation of peak arterial pressure response to a dose of phenylephrine (PE) or norepinephrine (NE).
| PE | NE | NC VS CHF | PE VS NE | INTERACTION | |||
|---|---|---|---|---|---|---|---|
| NC (N = 9) | CHF (N = 6) | NC (N = 8) | CHF (N = 5) | ||||
| Slope of linear regression, mmHg/decade | 54.6 ± 4.7 | 37.4 ± 3.9 | 52.5 ± 3.7 | 36.9 ± 5.0 | 0.002 | 0.780 | 0.871 |
| Maximum slope, mmHg/decade | 80.9 ± 6.3 | 57.3 ± 5.2 | 80.4 ± 5.9 | 60.2 ± 7.8 | 0.003 | 0.851 | 0.796 |
| Response range of AP, mmHg | 125.1 ± 8.8 | 89.7 ± 10.1 | 116.0 ± 5.7 | 90.9 ± 14.0 | 0.004 | 0.683 | 0.590 |
| Lower plateau of AP, mmHg | 61.2 ± 4.5 | 67.5 ± 7.8 | 74.3 ± 9.1 | 69.9 ± 9.8 | 0.902 | 0.333 | 0.501 |
| Dose at midpoint, μg/kg | 3.87 ± 0.59 | 6.15 ± 1.26 | 0.51 ± 0.10 | 0.75 ± 0.24 | 0.077 | <0.001 | 0.150 |
Notes: Values are means ± standard error. P value was obtained by two-way analysis of variance.
Abbreviations: NC, normal control; CHF, chronic heart failure; AP, arterial pressure.
Discussion
AP response to pharmacological adrenergic stimulation
The slope of the linear regression and the maximum slope of the AP response to the intravenous PE were lower in the CHF group compared with the same in the NC group, which is consistent with the results reported by Feng et al.19 using pithed rats. The decreased AP response to PE in CHF rats is in opposition to the enhanced α1-adrenergic receptor-mediated vascular response in CHF rats observed under in vitro settings.23,24 An increased vasoconstrictor response has also been reported in response to renal nerve stimulation in CHF rats.25 This change accompanies reduced autoregulation of renal blood flow in CHF rats. In contrast, Stassen et al.26 reported that maximal vasoconstrictor responses to PE in the presence of calcium and the sensitivity to calcium in the presence of PE were reduced in mesenteric arteries isolated from rats five weeks after myocardial infarction. In their study, the contractile responses in the absence of extracellular calcium were not modified, indicating that the agonist-induced calcium influx may be impaired in resistance arteries after myocardial infarction. Adverse milieu such as hypoperfusion, hypoxia,27 and acidosis28 might have also restricted the in vivo vascular response to PE in CHF rats compared with NC rats. In the study by Feng et al.19, the pressor response to electrical preganglionic sympathetic nerve stimulation was decreased in CHF rats compared to that in sham-operated rats. Because percentage changes in cardiac index in response to the preganglionic sympathetic stimulation were not significantly different between the CHF and sham-operated groups, they assumed that the difference in the AP response in the pithed rats mainly represented the difference in the vascular response. However, when cardiac output is reduced in CHF rats, the same amount of changes in the peripheral vascular resistance would yield a smaller AP response compared with the response in NC rats.
PE is a selective α1-adrenergic agonist, and therefore any effects on the responsiveness to β-adrenergic activation cannot be assessed in the PE protocol. The sympathetic AP response is mediated by the neurotransmitter NE, which acts on both α- and β-adrenergic receptors. While high plasma NE levels may not necessarily be the mechanism responsible for the following observation,29 selective β1-adrenergic receptor down-regulation occurs in the ventricular myocardium in CHF.30 To explore whether the AP response is different depending on the type of adrenergic activation, the AP response to exogenous NE was also examined. While the dose necessary to increase AP was an order of magnitude less for NE than for PE, none of the parameters, such as slope of the regression, maximum slope, lower plateau of AP, and the response range of AP, was significantly different between the PE and NE protocols (Table 2). Therefore, the attenuation of the AP response to adrenergic activation in CHF rats compared with NC rats was observed to a comparable degree regardless of whether the AP response was examined by using PE or NE in the present experimental setting.
Calibration of baroreflex equilibrium diagram
The ultimate goal of the arterial baroreflex is to maintain perfusion for vital organs via regulation of AP. While the SNA response to pharmacological pressure perturbation is frequently used to evaluate arterial baroreflex function,31,32 it does not allow the direct assessment of arterial baroreflex regulation of AP. The AP response to SNA needs to be combined with the SNA response to pressure perturbation to understand the total picture of arterial baroreflex function. The baroreflex equilibrium diagram is useful for describing changes in the baroreflex control of SNA and AP such as those induced by muscle mechanoreflex,9 electroacupuncture,11 head-up tilt,10,33 vagal nerve stimulation,34,35 and pharmacologic interventions.5,36 However, when we want to compare the baroreflex equilibrium diagram among different groups of subjects, quantification of SNA becomes problematic. In a previous study, open-loop characteristics of the carotid sinus baroreflex were examined in NC rats and rats with CHF eight weeks postmyocardial infarction; the duration after myocardial infarction is the same as that in the present study.16 Figure 5A presents the baroreflex equilibrium diagram constructed using mean parameter values of the neural and peripheral arcs obtained from that study.16 The slope of the peripheral arc in the CHF group was reduced to approximately 0.76 times that in the NC group. The operating-point SNA and AP in the NC group were 89.8% and 121.8 mmHg, respectively (solid arrows). The operating-point SNA and AP in CHF rats were 94.2% and 111.0 mmHg, respectively (dashed arrows). Although the SNA level at the operating point was higher in CHF rats than in NC rats, the difference was very small, casting doubt on the utility of the baroreflex equilibrium diagram for describing sympathetic hyperactivity in CHF rats compared with NC rats when SNA is expressed in percentage units.
Figure 5.
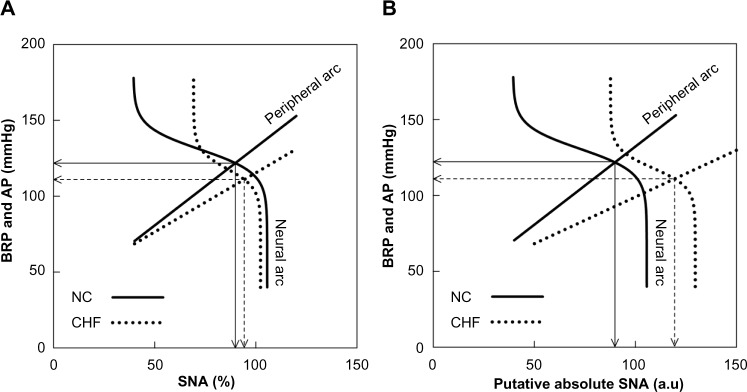
(A) Baroreflex equilibrium diagrams of normal control (NC) and chronic heart failure (CHF) constructed using mean parameter values of the neural and peripheral arcs in our previous study.16 In the neural arc of NC, the response range of SNA is 66.2%, the slope coefficient is 0.13 mmHg−1, the midpoint input pressure is 130.8 mmHg, and the lower plateau of SNA is 39.4%. In the peripheral arc of NC, the slope is 1.03 mmHg/% and the intercept is 29.3 mmHg. In the neural arc of CHF, the response range is 33.1%, the slope coefficient is 0.16 mmHg−1, the midpoint input pressure is 118.1 mmHg, and the lower plateau of SNA is 69.2%. In the peripheral arc of CHF, the slope is 0.78 mmHg/% and the intercept is 37.5 mmHg. (B) Baroreflex equilibrium diagrams of NC and CHF when the SNA axis for the CHF group was scaled so that the slope of the peripheral arc in CHF relative to that in NC became 0.6.
Notes: Solid arrows indicate the operating point in NC. Dashed arrows indicate the operating point in CHF.
Abbreviations: AP, arterial pressure; au, arbitrary units; BRP, baroreceptor pressure; SNA, sympathetic nerve activity.
In our previous studies regarding baroreflex function in CHF,6,16 the noise level of SNA measured after ganglionic blockade was assigned to be 0%, which may also be treated as the absolute zero level of SNA. Therefore, using zero as a transform origin, the SNA axis for CHF can be scaled to reflect the difference in the absolute SNA. In our previous study,6 a putative baroreflex equilibrium diagram was drawn by scaling the SNA axis so that the maximum absolute SNA in the CHF group became two times higher than that in the NC group. The putative baroreflex equilibrium diagram predicted the slope of the peripheral arc in CHF rats to be less than half that in NC rats.6
In the present study, we aimed to estimate the relative slope of the peripheral arc in CHF rats compared with NC rats, based on the AP response to pharmacological adrenergic activation. The CHF/NC ratio of the maximum slope of the AP response to PE was 0.71 (57.3/80.9) (Table 2). If the four-parameter logistic function was determined using the mean data points of the PE protocol, the ratio was 0.64 (50.6/78.9). The CHF/NC ratio of the maximum slope of the AP response to NE was 0.75 (60.2/80.4) (Table 2). If the four-parameter logistic function was determined using the mean data points of the NE protocol, the ratio was 0.57 (44.9/78.2). These calculations suggest that the CHF/NC ratio of the slope of the peripheral arc may be in the range from 0.6 to 0.7 in our experimental settings. On the other hand, the result of the AP response to PE under pithed conditions indicates that the slope of the AP response in the CHF group relative to the sham-operated group may be as low as 0.5.19
When we scale the SNA axis so that the slope of the peripheral arc in the CHF group became 0.6 times that in the NC group, the resulting equilibrium diagram yielded a maximum SNA in CHF rats higher than that in NC rats (Fig. 5B). The operating-point SNA in CHF group was 119.4 au (arbitrary units), while that in the NC group was unchanged at 89.8 au. When we assume the CHF/NC ratio of the slope to be 0.5, the operating-point SNA in the CHF group becomes 143.3 au. Because plasma NE concentration becomes two to three times higher or more in CHF rats compared with NC rats,37,38 the putative SNA value at the operating point in the CHF group still seems to be lower than that expected from the excess plasma NE concentration in CHF rats. One possible explanation may be the dissociation of plasma NE concentration from SNA due to impaired NE disposition. In the study by Feng et al.19, plasma NE concentration remained 2.4 times higher in CHF rats than in sham-operated rats 40 minutes after pithing, but AP values were not different between the two groups. Impairment of neuronal NE uptake may contribute to the pathophysiology of sympathetic abnormality in CHF.39 Because plasma NE concentration is significantly increased after the blockade of neuronal NE uptake,40 the impaired neuronal NE uptake function in CHF would elevate plasma NE concentration disproportionally to SNA. Other mechanisms such as an increased amount of NE release at the same level of SNA may also account for the dissociation between SNA and plasma NE concentration. For instance, presynaptic β2-adrenergic stimulation by epinephrine facilitates NE release from the sympathetic nerve terminals.41,42 Peripheral presynaptic inhibition via α2-adrenergic receptors is functionally important in inhibiting NE release in healthy humans, but this function is lost in heart failure.43 It may be worth noting that circulating NE levels do not necessarily correlate with the hemodynamic abnormality in CHF44 despite its prognostic value of mortality in CHF patients.45
Limitations
Several limitations in our study need to be addressed. First, the NE protocol was always performed after the PE protocol. Although the response range of AP was not significantly different between the PE and NE protocols, there might have been some aftereffect of α1-adrenergic activation induced by high dose of PE on the AP response to NE. Second, AP did not completely return to the baseline levels at higher doses of PE or NE. Accordingly, the drug effect needs to be interpreted as cumulative to a certain extent. However, because the maximum slope of the dose–effect curve occurred in the midrange concentration (1–10 μg/kg for PE and 0.1–1 μg/kg for NE), the cumulative effect on the estimation of the logistic function-derived maximum slope is probably limited. Third, the experiment was performed under anesthetic conditions. Although this was necessary to observe the AP response to adrenergic activation under the same anesthetic conditions as in our previous studies,6,16 the results may not directly be extrapolated to interpret the baroreflex control under conscious conditions. Finally, systemic administration of pressor agents might have induced vasoconstriction in a relatively homogenous manner throughout the body. In contrast to the pharmacological pressor response, there exist regional differences in sympathetic tone to regulate AP.46 Further studies are required to elucidate how the regional difference in SNA affects AP regulation in CHF.
Conclusion
The AP responses to both PE and NE were reduced in CHF rats when compared with the same in NC rats. On the basis of the relative slope of CHF to NC, the SNA axis of the baroreflex equilibrium diagram was calibrated so that the slope of the peripheral arc in CHF rats became 0.6 times that in NC rats. While the calibrated equilibrium diagram explained increased maximum SNA and operating-point SNA in CHF rats compared with NC rats, the magnitude of the difference in SNA between CHF and NC groups seemed smaller than that expected from the difference in plasma NE concentration between CHF and NC rats. Further studies are warranted to solve the discrepancy and to obtain a comprehensive picture of the arterial baroreflex regulation in CHF rats compared with NC rats.
Footnotes
Author Contributions
Conceived and designed the experiments: TK, YS, MS. Performed the experiments: TK, ML, CZ. Analyzed the data: TK, SS. Wrote the first draft of the manuscript: TK. Contributed to the writing of the manuscript: TK, MJT, SS, MS. Agree with manuscript results and conclusions: TK, ML, YS, CZ, MJT, SS, MS. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
FUNDING: This study was partly supported by the Support Program to break the bottlenecks at R&D System for accelerating the practical use of Health Research Outcome from the Japan Science and Technology Agency, and by the Grant-in-Aid for Scientific Research (JSPS KAKENHI grants 26750153, 26461099, 26430103, 13J05473). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Mohrman DE, Heller LJ. Cardiovascular Physiology. 6th ed. New York: McGraw Hill; 2006. [Google Scholar]
- 2.Sato T, Kawada T, Inagaki M, et al. New analytic framework for understanding sympathetic baroreflex control of arterial pressure. Am J Physiol. 1999;276:H2251–61. doi: 10.1152/ajpheart.1999.276.6.H2251. [DOI] [PubMed] [Google Scholar]
- 3.Shoukas AA, Callahan CA, Lash JM, Haase EB. New technique to completely isolate carotid sinus baroreceptor regions in rats. Am J Physiol. 1991;260:H300–3. doi: 10.1152/ajpheart.1991.260.1.H300. [DOI] [PubMed] [Google Scholar]
- 4.Sato T, Kawada T, Miyano H, et al. New simple methods for isolating baroreceptor regions of carotid sinus and aortic depressor nerves in rats. Am J Physiol. 1999;276:H326–32. doi: 10.1152/ajpheart.1999.276.1.H326. [DOI] [PubMed] [Google Scholar]
- 5.Kawada T, Kamiya A, Li M, et al. High levels of circulating angiotensin II shift the open-loop baroreflex control of splanchnic sympathetic nerve activity, heart rate and arterial pressure in anesthetized rats. J Physiol Sci. 2009;59:447–55. doi: 10.1007/s12576-009-0055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawada T, Li M, Kamiya A, et al. Open-loop dynamic and static characteristics of the carotid sinus baroreflex in rats with chronic heart failure after myocardial infarction. J Physiol Sci. 2010;60:283–98. doi: 10.1007/s12576-010-0096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto H, Kawada T, Shimizu S, Kamiya A, Miyazaki S, Sugimachi M. Effects of cilnidipine on sympathetic outflow and sympathetic arterial pressure and heart rate regulations in rats. Life Sci. 2013;92:1202–7. doi: 10.1016/j.lfs.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Kawada T, Shishido T, Inagaki M, et al. Estimation of baroreflex gain using a baroreflex equilibrium diagram. Jpn J Physiol. 2002;52:21–9. doi: 10.2170/jjphysiol.52.21. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto K, Kawada T, Kamiya A, Takaki H, Sugimachi M, Sunagawa K. Static interaction between muscle mechanoreflex and arterial baroreflex in determining efferent sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2005;289:H1604–9. doi: 10.1152/ajpheart.00053.2005. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya A, Kawada T, Yamamoto K, et al. Resetting of the arterial baroreflex increases orthostatic sympathetic activation and prevents postural hypotension in rabbits. J Physiol. 2005;566:237–46. doi: 10.1113/jphysiol.2005.086512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michikami D, Kamiya A, Kawada T, et al. Short-term electroacupuncture at Zusanli resets the arterial baroreflex neural arc toward lower sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2006;291:H318–26. doi: 10.1152/ajpheart.00975.2005. [DOI] [PubMed] [Google Scholar]
- 12.White CW. Abnormalities in baroreflex control of heart rate in canine heart failure. Am J Physiol. 1981;240:H793–9. doi: 10.1152/ajpheart.1981.240.5.H793. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Chen JS, Zucker IH. Carotid sinus baroreceptor sensitivity in experimental heart failure. Circulation. 1990;81:1959–66. doi: 10.1161/01.cir.81.6.1959. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Chen JS, Zucker IH. Carotid sinus baroreceptor reflex in dogs with experimental heart failure. Circ Res. 1991;68:1294–301. doi: 10.1161/01.res.68.5.1294. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Brändle M, Zucker IH. Influence of vagotomy on the baroreflex sensitivity in anesthetized dogs with experimental heart failure. Am J Physiol. 1993;265:H1310–7. doi: 10.1152/ajpheart.1993.265.4.H1310. [DOI] [PubMed] [Google Scholar]
- 16.Kawada T, Li M, Zheng C, et al. Chronic vagal nerve stimulation improves baroreflex neural arc function in heart failure rats. J Appl Physiol. 2014;116:1308–14. doi: 10.1152/japplphysiol.00140.2014. [DOI] [PubMed] [Google Scholar]
- 17.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation. 1988;77:721–30. doi: 10.1161/01.cir.77.4.721. [DOI] [PubMed] [Google Scholar]
- 18.Colucci WS, Sawyer DB, Singh K, Communal C. Adrenergic overload and apoptosis in heart failure: implications for therapy. J Card Fail. 2000;6(2 suppl 1):1–7. [PubMed] [Google Scholar]
- 19.Feng Q, Sun X, Lu X, Edvinsson L, Hedner T. Decreased responsiveness of vascular postjunctional α1-, α2-adrenoceptors and neuropeptide Y1 receptors in rats with heart failure. Acta Physiol Scand. 1999;166:285–91. doi: 10.1046/j.1365-201x.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–4. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zheng C, Kawada T, et al. Donepezil markedly improves long-term survival in rats with chronic heart failure after extensive myocardial infarction. Circ J. 2013;77:2519–25. doi: 10.1253/circj.cj-13-0476. [DOI] [PubMed] [Google Scholar]
- 22.Glantz S, Slinker B. Primer of Applied Regression and Analysis of Variance. 2nd ed. New York: McGraw-Hill; 2001. [Google Scholar]
- 23.Forster C, Carter SL, Armstrong PW. Vascular smooth muscle responsiveness to noradrenaline and phenylephrine following experimental heart failure in dogs. Cardiovasc Res. 1989;23:489–97. doi: 10.1093/cvr/23.6.489. [DOI] [PubMed] [Google Scholar]
- 24.Koida S, Ohyanagi M, Ueda A, Mori Y, Iwasaka T. Mechanism of increased alpha-adrenoceptor-mediated contraction in small resistance arteries of rats with heart failure. Clin Exp Pharmacol Physiol. 2006;33:47–52. doi: 10.1111/j.1440-1681.2006.04322.x. [DOI] [PubMed] [Google Scholar]
- 25.DiBona GF, Sawin LL. Frequency response of the renal vasculature in congestive heart failure. Circulation. 2003;107:2159–64. doi: 10.1161/01.CIR.0000062647.30366.98. [DOI] [PubMed] [Google Scholar]
- 26.Stassen FR, Willemsen MJ, Janssen GM, et al. Reduced responsiveness of rat mesenteric resistance artery smooth muscle to phenylephrine and calcium following myocardial infarction. Br J Pharmacol. 1997;120:1505–12. doi: 10.1038/sj.bjp.0701089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doyle MP, Walker BR. Attenuation of systemic vasoreactivity in chronically hypoxic rats. Am J Physiol. 1991;260:R1114–22. doi: 10.1152/ajpregu.1991.260.6.R1114. [DOI] [PubMed] [Google Scholar]
- 28.Grant TL, McGrath JC, O’Brien JW. The influence of blood gases on α1- and α2- adrenoceptors-mediated pressor responses in the pithed rat. Br J Pharmacol. 1985;86:69–77. doi: 10.1111/j.1476-5381.1985.tb09436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao M, Hagler HK, Muntz KH. Regulation of α1-, β1-, and β2-adrenergic receptors in rat heart by norepinephrine. Am J Physiol. 1996;271:H1762–8. doi: 10.1152/ajpheart.1996.271.5.H1762. [DOI] [PubMed] [Google Scholar]
- 30.Bristow MR, Ginsburg R, Umans V, et al. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 31.Huang BS, Yuan B, Leenen FH. Blockade of brain ‘ouabain’ prevents the impairment of baroreflexes in rats after myocardial infarction. Circulation. 1997;96:1654–9. doi: 10.1161/01.cir.96.5.1654. [DOI] [PubMed] [Google Scholar]
- 32.Kar S, Gao L, Belatti DA, Curry PL, Zucker IH. Central angiotensin 1–7 enhances baroreflex gain in conscious rabbits with heart failure. Hypertension. 2011;58:627–34. doi: 10.1161/HYPERTENSIONAHA.111.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abe C, Kawada T, Sugimachi M, Morita H. Interaction between vestibulo-cardiovascular reflex and arterial baroreflex during postural change in rats. J Appl Physiol. 2011;111:1614–21. doi: 10.1152/japplphysiol.00501.2011. [DOI] [PubMed] [Google Scholar]
- 34.Kawada T, Shimizu S, Li M, et al. Contrasting effects of moderate vagal stimulation on heart rate and carotid sinus baroreflex-mediated sympathetic arterial pressure regulation in rats. Life Sci. 2011;89:498–503. doi: 10.1016/j.lfs.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 35.Saku K, Kishi T, Sakamoto K, et al. Afferent vagal nerve stimulation resets baroreflex neural arc and inhibits sympathetic nerve activity. Physiol Rep. 2014;2(9):e12136. doi: 10.14814/phy2.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashihara K, Kawada T, Li M, Sugimachi M, Sunagawa K. Bezold-Jarisch reflex blunts arterial baroreflex via the shift of neural arc toward lower sympathetic nerve activity. Jpn J Physiol. 2004;54:395–404. doi: 10.2170/jjphysiol.54.395. [DOI] [PubMed] [Google Scholar]
- 37.Igawa A, Nozawa T, Fujii N, Kato B, Asanoi H, Inoue H. Long-term treatment with low-dose, but not high-dose, guanethidine improves ventricular function and survival of rats with heart failure after myocardial infarction. J Am Coll Cardiol. 2003;42:541–8. doi: 10.1016/s0735-1097(03)00650-8. [DOI] [PubMed] [Google Scholar]
- 38.Sia YT, Lapointe N, Parker TG, et al. Beneficial effects of long-term use of the antioxidant probucol in heart failure in the rat. Circulation. 2002;105:2549–55. doi: 10.1161/01.cir.0000016721.84535.00. [DOI] [PubMed] [Google Scholar]
- 39.Backs J, Haunstetter A, Gerber SH, et al. The neuronal norepinephrine transporter in experimental heart failure: evidence for a posttranscriptional down-regulation. J Mol Cell Cardiol. 2001;33:461–72. doi: 10.1006/jmcc.2000.1319. [DOI] [PubMed] [Google Scholar]
- 40.Eisenhofer G, Saigusa T, Esler MD, Cox HS, Angus JA, Dorward PK. Central sympathoinhibition and peripheral neuronal uptake blockade after desipramine in rabbits. Am J Physiol. 1991;260:R824–32. doi: 10.1152/ajpregu.1991.260.4.R824. [DOI] [PubMed] [Google Scholar]
- 41.Majewski H, Rand MJ, Tung LH. Activation of prejunctional β-adrenoceptors in rat atria by adrenaline applied exogenously or released as a co-transmitter. Br J Pharmacol. 1981;73:669–79. doi: 10.1111/j.1476-5381.1981.tb16802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floras JS. Epinephrine and the genesis of hypertension. Hypertension. 1992;19:1–18. doi: 10.1161/01.hyp.19.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal A, Esler MD, Socratous F, Kaye DM. Evidence for functional presynaptic alpha-2 adrenoceptors and their down-regulation in human heart failure. J Am Coll Cardiol. 2001;37:1246–51. doi: 10.1016/s0735-1097(01)01121-4. [DOI] [PubMed] [Google Scholar]
- 44.Viquerat CE, Daly P, Swedberg K, et al. Endogenous catecholamine levels in chronic heart failure. Relation to the severity of hemodynamic abnormalities. Am J Med. 1985;78:455–60. doi: 10.1016/0002-9343(85)90338-9. [DOI] [PubMed] [Google Scholar]
- 45.Roig Minguell E. Clinical use of markers of neurohormonal activation in heart failure. Rev Esp Cardiol. 2004;57:347–56. [PubMed] [Google Scholar]
- 46.Ninomiya I, Nisimaru N, Irisawa H. Sympathetic nerve activity to the spleen, kidney and heart in response to baroceptor input. Am J Physiol. 1971;221:1346–51. doi: 10.1152/ajplegacy.1971.221.5.1346. [DOI] [PubMed] [Google Scholar]