Abstract
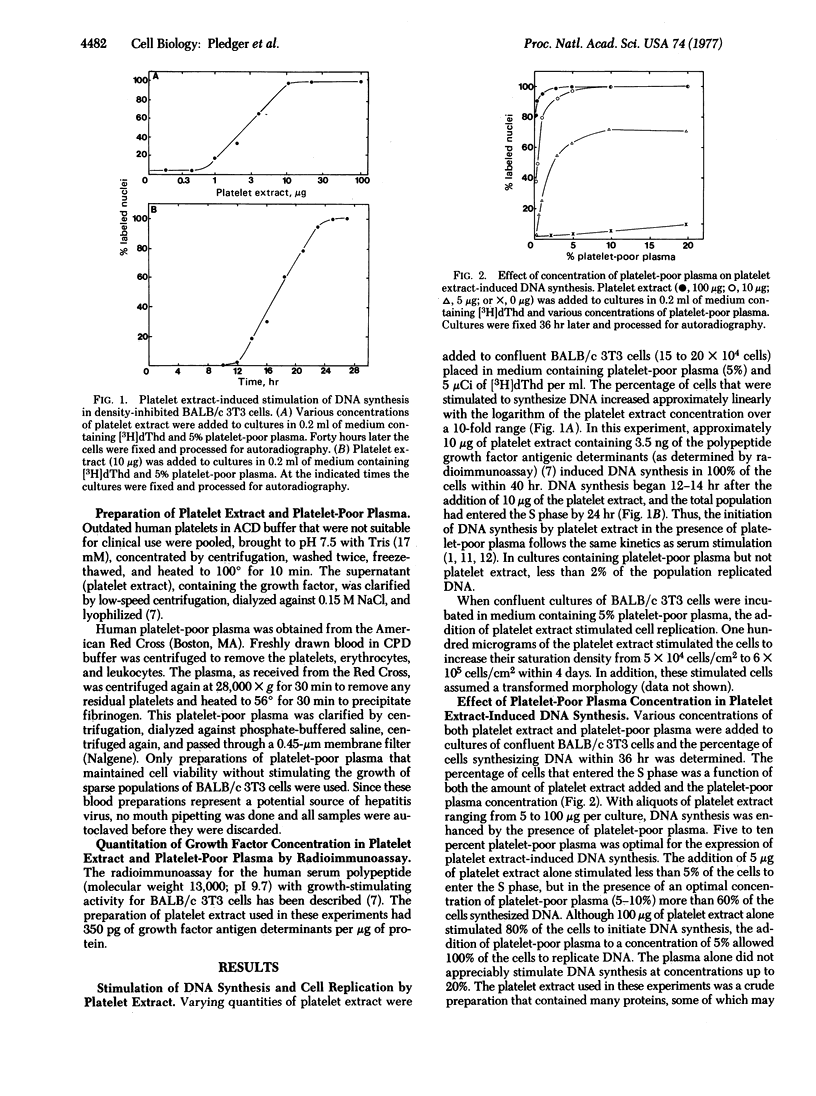
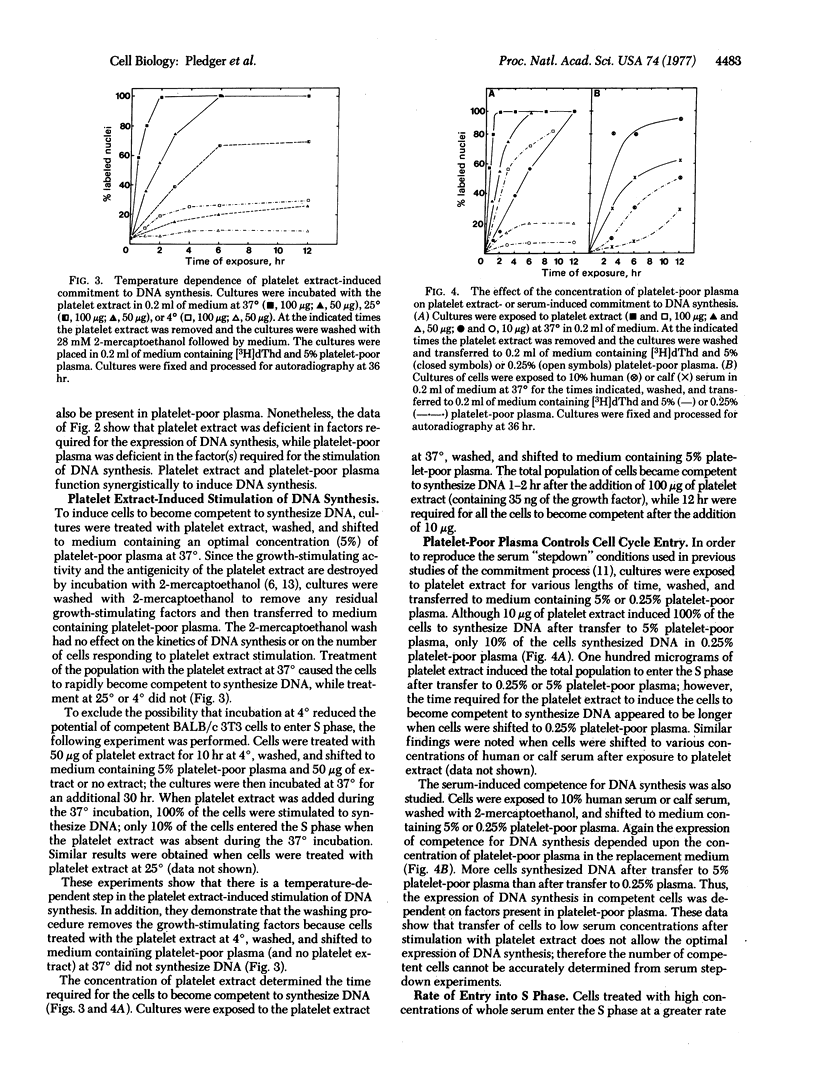
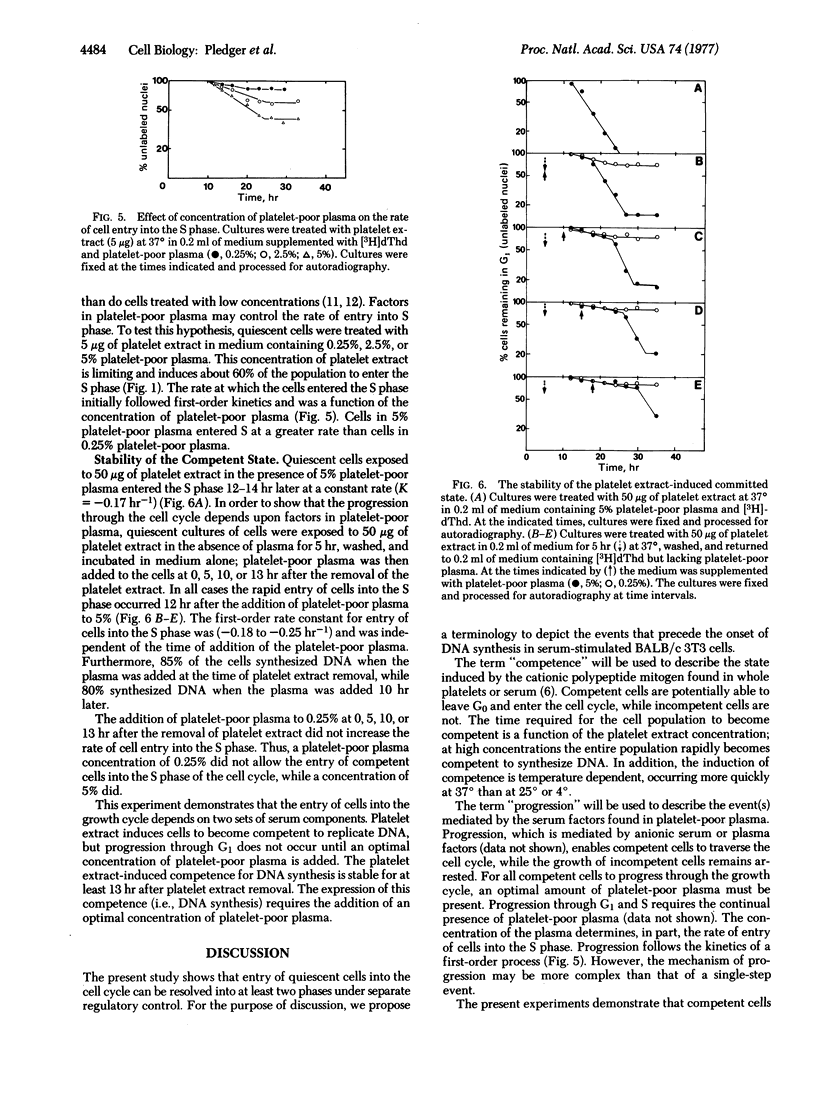
Serum contains a growth factor derived from platelets and also growth factors derived from platelet-poor plasma. Extracts of heated (100°) human platelets function synergistically with platelet-poor plasma to induce DNA synthesis in quiescent, density-inhibited BALB/c 3T3 cells. Platelet-poor plasma alone did not induce DNA synthesis. Cells exposed to platelet extracts became competent to enter the cell cycle, but the rate of entry into the S phase depended upon the concentration of platelet-poor plasma. The time required for the induction of this competent state was a function of the concentration of the platelet extract. A 2-hr exposure to 100 μg of the platelet extract at 37° caused the entire cell population to become competent to enter the S phase. At 4° or 25° the cells did not become competent to synthesize DNA. The platelet extract-induced competent state was stable for at least 13 hr after removal of the platelet extract; however, in the absence of platelet-poor plasma, these competent cells did not progress through the cell cycle. The addition of an optimal concentration of platelet-poor plasma (5%) to these competent cells initiated cell cycle traverse with a rapid, first-order entry of cells into the S phase beginning 12 hr after addition of the plasma. The addition of a suboptimal concentration of the plasma (0.25%) did not increase the rate of cell entry into the S phase. Thus, the induction of DNA synthesis in quiescent BALB/c 3T3 cells can be resolved into at least two phases, controlled by different serum components: (i) competence, induced by the platelet-derived growth factor; and (ii) progression of competent cells into the cell cycle, mediated by factors in platelet-poor plasma.
Keywords: platelets, plasma, cell cycle, transitional probability
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D. Radioimmunoassay of a human serum growth factor for Balb/c-3T3 cells: derivation from platelets. Proc Natl Acad Sci U S A. 1977 May;74(5):1973–1977. doi: 10.1073/pnas.74.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Stathakos D., Scher C. D. Isolation of a cationic polypeptide from human serum that stimulates proliferation of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2635–2639. doi: 10.1073/pnas.72.7.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. F. Regulation of fibroblast cell cycle by serum. Nature. 1976 Mar 18;260(5548):248–250. doi: 10.1038/260248a0. [DOI] [PubMed] [Google Scholar]
- Kohler N., Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974 Aug;87(2):297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- Pardee A. B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rierdan J., Brooks R. Verbal conditioning of middle and lower socioeconomic class schizophrenics. J Abnorm Psychol. 1977 Aug;86(4):369–378. doi: 10.1037//0021-843x.86.4.369. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Control by factors in serum of multiplication of uninfected cells and cells infected and converted by avian sarcoma viruses. Wistar Inst Symp Monogr. 1967;7:103–116. [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]


