Abstract
Mice deficient in the angiotensin II type 1a (AT1a) receptor demonstrate a vasopressin-resistant nephrogenic diabetes insipidus. These knockout mice exhibit a threefold increase in 24-h urine excretion. Neither 2 weeks of exogenous vasopressin nor 5 days of fluid restriction reversed this polyuric state. This nephrogenic diabetes insipidus was associated with reductions in adenylyl cyclase protein and in the phosphorylated mitogen-activated protein kinase extracellular signal–regulated kinase 1/2. The results support an important interaction between vasopressin and angiotensin II in maximal urinary concentration.
There is substantial evidence that arterial underfilling, due to either decreased cardiac output, e.g., heart failure, or systemic arterial vasodilation, e.g., cirrhosis, is associated with activation of the neurohumoral axis, including the sympathetic nervous system, the renin–angiotensin system, and arginine vasopressin (AVP). This neurohumoral response involves compensatory systemic vasoconstriction and renal sodium and water restriction in order to attenuate the arterial underfilling, that is, the so-called decreased effective arterial blood volume. What is not known is whether there is an interaction at the cellular and molecular levels between these responses to arterial underfilling.
Li et al.1 (this issue) provide experimental evidence indicating an importance of angiotensin II in normal AVP-mediated urinary concentration. There are several potential mechanisms whereby angiotensin II could modulate water homeostasis, and thus angiotensin II receptor knockout (KO) mice would be expected to have impaired urine-concentrating capacity. There is substantial evidence that angiotensin II stimulates the thirst center in the brain, thereby leading to increased thirst and water intake.2 Thus, the angiotensin II type 1a (AT1a) receptor KO mice might be expected to decrease their water intake unless other intervening effects occur. In this regard, Li et al.1 demonstrated that these KO mice actually have an increase, not a decrease, in water intake. Although somewhat controversial, there is evidence that angiotensin II may stimulate AVP release. 3,4 Li et al.1 did demonstrate a decrease in basal plasma AVP concentrations in AT1a receptor KO mice, as compared with wild-type mice. This, however, has not been a consistent finding in these AT1a receptor KO mice.5 In order to demonstrate partial central diabetes insipidus in the AT1a KO mice, for which Li et al.1 propose a role, it would be necessary to perform fluid restriction—which maximally concentrates urine in wild-type mice—and then administer exogenous AVP Exogenous AVP would not increase urinary osmolality further after such fluid restriction in wild-type mice; however, a further increment in urinary osmolality after fluid restriction with exogenous AVP would be expected in mice with partial central diabetes insipidus. As these studies were not performed, evidence for a role of AVP-responsive partial central diabetes insipidus cannot be established in these AT1a KO mice.
A hemodynamic role of angiotensin II was apparent in these KO mice because their arterial blood pressures were significantly decreased compared with those of wild-type mice. Although renal hemodynamics were not reported in these KO mice, the observed decrease in renal arterial pressure was a likely factor in the observed diminished solute diuresis in response to sucrose administration, as compared with that of wild-type mice. In any case, increased solute excretion was excluded as a factor in the urine-concentrating defect in these AT1a KO mice.
What was very clear in the Li et al.1 study was the presence of an AVP-resistant nephrogenic diabetes insipidus in these AT1a KO mice. The lower blood pressure in these KO mice could stimulate thirst, independent of angiotensin II, and increased water ingestion. Increased water intake has been shown to cause a urine-concentrating defect.6 Decreasing the water intake in the AT1a KO mice to the level ingested by wild-type mice for 24 h or 5 days did not, however, correct the urine-concentrating defect. Although these results do not support a polydipsia-related urine-concentrating defect, perhaps a longer period of normalizing fluid intake to the level ingested by wild-type mice may be necessary to improve the urine-concentrating defect in AT1a KO mice, which have had lifelong polydipsia.
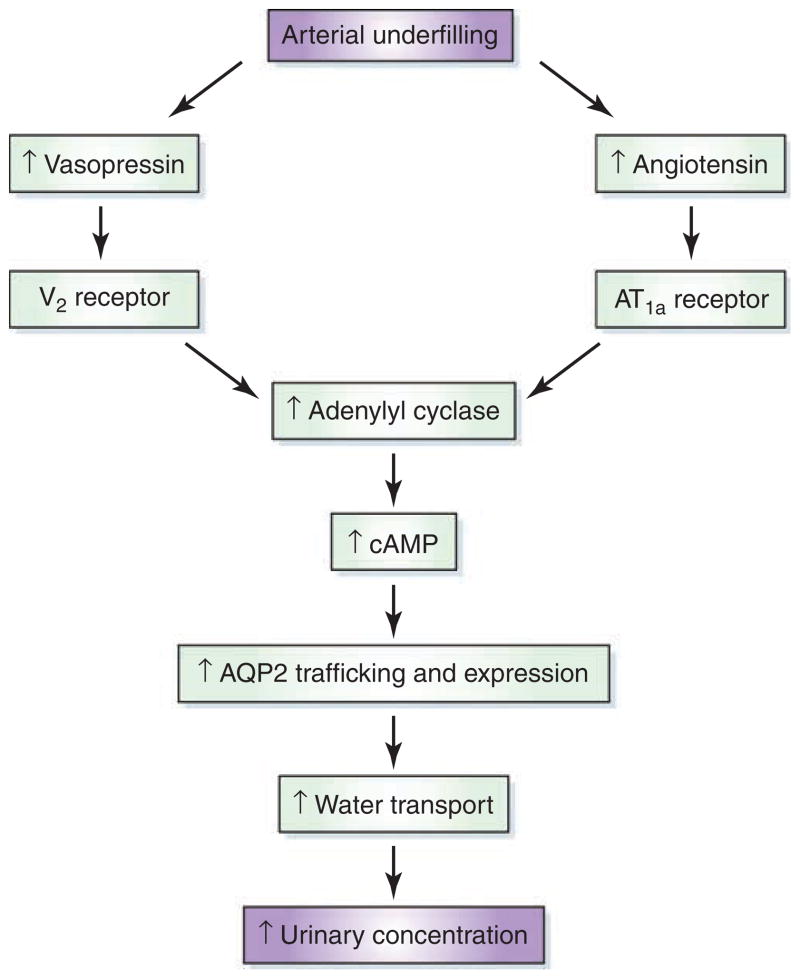
Of considerable interest was the observed defect in AVP signaling and aquaporin 2 (AQP2) trafficking to the apical membrane in the inner medulla of the AT1a KO mice. Although there was no evidence of decreased V2 vasopressin receptor binding in the inner medulla, there was a decline in adenylyl cyclase III and V/VI protein and in the phosphorylated mitogen-activated protein kinase extracellular signal–regulated kinase 1/2. In contrast to the defect in the AVP-mediated AQP2 trafficking, the AVP stimulation of AQP2 protein expression was less disparate as compared with that in the wild-type mice. This result suggests that angiotensin II may exert a more important interaction with AVP in AQP2 trafficking to the apical membrane of the principal cells of the medullary collecting duct as compared with the AVP-mediated upregulation of AQP2 expression (Figure 1).
Figure 1. Potential pathway for interaction between angiotensin II and arginine vasopressin in the modulation of urinary concentration.
AQP2, aquaporin 2, cAMP, cyclic adenosine monophosphate.
There are previously published in vitro and in vivo results that suggest an interaction between AVP and angiotensin II. Angiotensin has been shown to enhance the in vitro AVP-dependent cAMP (cyclic adenosine monophosphate) accumulation in Chinese hamster ovary cells transfected with AT1a and V2 receptors.7 In primary cell culture of inner medullary collecting duct cells, angiotensin II and Desmopressin acetate both increased phosphorylation of AQP2 protein and its trafficking to the apical membrane.8 Moreover, angiotensin receptor blockade in vitro has been shown to decrease urinary concentration and AQP2.9 Because there are many clinical disorders of water homeostasis in which arterial underfilling is present and associated with both increased AVP and angiotensin II, the potential interaction of these hormones at the molecular and cellular level has important implications.10
Acknowledgments
This work was supported by a grant from the US National Institutes of Health (PO1 DK19928). The author would like to acknowledge Jan Darling for her assistance.
Footnotes
DISCLOSURE
The author makes the following disclosures: educational grant from Astellas, review of applications with Amgen, and consulting agreement with Otsuka.
References
- 1.Li XC, Shao Y, Zhuo JL. AT1a receptor knockout in mice impairs urine concentration by reducing basal vasopressin levels and its receptor signaling proteins in the inner medulla. Kidney Int. 2009;76:169–177. doi: 10.1038/ki.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 3.Henrich WL, Walker BR, Handelman WA, et al. Effects of angiotensin II on plasma antidiuretic hormone and renal water excretion. Kidney Int. 1986;30:503–508. doi: 10.1038/ki.1986.214. [DOI] [PubMed] [Google Scholar]
- 4.Bonjour JP, Malvin RL. Stimulation of ADH release by the renin-angiotensin system. Am J Physiol. 1970;218:1555–1559. doi: 10.1152/ajplegacy.1970.218.6.1555. [DOI] [PubMed] [Google Scholar]
- 5.Oliverio MI, Delnomdedieu M, Best CF, et al. Abnormal water metabolism in mice lacking the type 1A receptor for ANG II. Am J Physiol Renal Physiol. 2000;278:F75–F82. doi: 10.1152/ajprenal.2000.278.1.F75. [DOI] [PubMed] [Google Scholar]
- 6.Cadnapaphornchai MA, Summer SN, Falk S, et al. Effect of primary polydipsia on aquaporin and sodium transporter abundance. Am J Physiol Renal Physiol. 2003;285:F965–F971. doi: 10.1152/ajprenal.00085.2003. [DOI] [PubMed] [Google Scholar]
- 7.Klingler C, Ancellin N, Barrault MB, et al. Angiotensin II potentiates vasopressin-dependent cAMP accumulation in CHO transfected cells. Mechanisms of cross-talk between AT1a and V2 receptors. Cell Signal. 1998;10:650–674. doi: 10.1016/s0898-6568(97)00077-6. [DOI] [PubMed] [Google Scholar]
- 8.Le YJ, Song IK, Jang KJ, et al. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol. 2007;292:F340–F350. doi: 10.1152/ajprenal.00090.2006. [DOI] [PubMed] [Google Scholar]
- 9.Kwon TH, Nielsen J, Knepper MA, et al. Angiotensin II AT1 receptor blockade decreases vasopressin-induced water reabsorption and AQP2 levels in NaCl-restricted rats. Am J Physiol Renal Physiol. 2005;288:F673–F684. doi: 10.1152/ajprenal.00304.2004. [DOI] [PubMed] [Google Scholar]
- 10.Schrier RW. Body water homeostasis: clinical disorders of urinary dilution and concentration. J Am Soc Nephrol. 2006;17:1820–1832. doi: 10.1681/ASN.2006030240. [DOI] [PubMed] [Google Scholar]