Abstract
Exosomes released from different types of cells have been proposed to contribute to intercellular communication. We report that thymic exosome-like particles (ELPs) released from cells of the thymus can induce the development of Foxp3+ regulatory T (Treg) cells in the lung and liver. Thymic ELPs also induce the conversion of thymic CD4+CD25− T cells into Tregs. Tregs induced by thymic ELPs suppress the proliferation of CD4+CD25− T cells in vitro and in vivo. We further show that neutralization of TGF-β in ELPs partially reverses thymic ELP-mediated induction of CD4+Foxp3+ T cells in the lung and liver. This study demonstrates that thymic ELPs participate in the induction of Foxp3+ Tregs. Also, TGF-β of thymic ELPs might be required for the generation of Tregs in the peripheral tissues.
Exosomes are membrane vesicles that are released by cells upon fusion of multivesicular bodies (MVB)3 with the plasma membrane. In contrast to the fate of proteins trafficked for degradation to the lysosomal system, secreted exosomes are biologically active entities that are important for a variety of pathways, particularly of the immune system (1–4). Exosomes can be taken up by other cells and modulate the activity of recipient cells in vitro (5–7) and in vivo (3, 8–10). However, whether this establishes a novel mechanism of in vivo cell-cell communication is an intriguing but unanswered question.
Regulatory T (Treg) cells are known to play an important role in the control of host immune responses. The most frequently studied are CD4+CD25+Foxp3+ Treg cells that develop in the thymus. These cells actively suppress autoreactive T cells, thereby limiting aberrant immune responses and maintaining tolerance toward self tissues (11–13). Foxp3 has been characterized as the master regulator of Treg development and function and is used as a highly specific marker for Tregs (13); however, the origin of the signals necessary for Foxp3+ Treg development has not been determined under nonpathological (physiological) conditions.
TGF-β has been shown in in vitro assays to have a crucial role in immunological self-tolerance by induction of Foxp3 (14). Ablation of the TGF-β receptor type II gene in T cells produces catastrophic autoimmunity associated with decreased Foxp3+ Treg (15). Therefore, endogenous TGF-β appears to be required for Treg function; however, the source of TGF-β required for Treg maintenance is yet to be identified.
We have demonstrated that exosome-like particles (ELPs) released from thymic cells can promote the conversion of naive T cells into Foxp3+ Treg cells. This occurs without any further manipulation of the released ELPs. These results highlight one pathway regulated by thymic ELPs by which Treg cells may be generated in the thymus under nonpathological conditions.
Materials and Methods
Mice
Female BALB/c, DBA/2j, and C57BL/6 mice were purchased from The Jackson Laboratory and maintained under specific pathogen-free conditions at the University of Alabama at Birmingham. This study was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham, and animals were cared for in accordance to institutional and National Institutes of Health guidelines.
Isolation of CD4+ CD25− from the spleen
Freshly isolated spleens were placed in cold PBS and homogenized immediately. The cells were filtered through cotton gauze to remove tissue debris and RBCs were lysed using ACK solution. For splenocyte sorting, cells were stained with CD4-FITC and CD25-PE Abs. CD4+CD25− and CD4+CD25+ T cells were sorted using a FACSCalibur (BD Biosciences). Sorted cells were > 95% pure. Naive and expanded Treg cells were phenotyped using CD4-allophycocyanin, CD4-FITC, and CD25-PE. Fluorescently labeled isotype-matched Abs were used as controls. Intracellular Foxp3 staining was performed according to the manufacturer’s protocol (eBioscience).
Sorting of CD4+ CD25− from the thymus
Freshly isolated thymi were placed in cold PBS and homogenized immediately. A two-step procedure for the isolation of CD4+CD25− single positive T cells from the thymus was performed. To deplete the whole cell suspension of pooled thymic cells of CD4−CD8+ and CD4+CD8+ thymocytes, the thymocytes were suspended in an appropriate volume of sorting buffer and incubated with microbeads conjugated with monoclonal anti-mouse CD8 Abs. After incubation, the cells were passed through a MACS column. The procedure was conducted according to the manufacture’s directions (Miltenyi Biotec). Nonadherent thymocytes passing through the column (CD4+CD8− and CD4−CD8−) were stained with anti-CD4/PerCP and anti-CD25/PE (BD Pharmingen) mAbs and sorted using a FACSCalibur for isolation of CD4+CD25− and CD4+CD25+ T cells. The viability of the sorted cells was estimated using the trypan blue exclusion method. The purity of CD4+CD25+ and CD4+CD25− populations exceeded 98%.
Propagation of spleen accessory cells (ACs)
T cell-depleted spleen cells were prepared by first lysing the erythrocytes with ACK lysis buffer, followed by treatment with anti-CD3 Ab and rabbit complement for 45 min at 37°C. The cells were washed and used as ACs for induction of Treg cells in vitro.
Induction of Treg cells in vitro
CD4+CD25− cells were sorted from the thymus and spleen of naive BALB/c mice. They were cocultured with T cell-depleted irradiated (3000 rads) splenic cells used as ACs, plus 0.5 µg/ml anti-CD3, in the presence of varying concentrations of thymic ELPs. Controls consisted of splenic ELPs at the same concentrations as the thymic ELPs or nonbanded fraction 6 collected from the thymic ELP sucrose gradient (non-ELPF6) using a method as described previously (10). After 3 days in culture, the CD4+CD25+ T cells were sorted, phenotyped (CD4+CD25+Foxp3+), and assayed for function (suppression of CD4+CD25− T cell proliferation).
In vivo T cell expansion
Freshly isolated splenic CD4+CD25− T cells (1 × 107 cells/ml) were washed once in RPMI 1640 medium containing 10% FCS (v/v) and labeled with CFSE (1 µM; Molecular Probes) on ice for 5 min. Subsequently, cells were washed three times in RPMI 1640 medium/10% FCS and rested for 30 min at 37°C. The cells were washed again, and 5 × 106 cells injected i.v. per BALB/c mouse that had been treated with thymic or splenic ELPs (100 µg/mouse by i.v. injection) for 3 days. Proliferation of injected CFSE-labeled CD4+CD25− T cells was analyzed 3 days after the injection by flow cytometry. The data were expressed as percent of dividing CFSE-labeled CD4+CD25− T cells.
T cell suppression assays
For in vitro suppression of CD4+CD25−, a T cell proliferation assay was done. FACS-sorted CD4+CD25+ cells (5 × 104) were cultured in 96-well plates (0.2 ml) with irradiated ACs (5 × 104), 0.5 µg/ml anti-CD3, and the indicated numbers of CD4+CD25− cells for 72 h. Cultures were pulsed with 1 µCi [3H]thymidine (Amersham Biosciences) for the final 16 h of culture. Cells were collected using a Filtermate harvester (Packard-PerkinElmer), and radioactivity was measured using a TopCount liquid scintillation counter (Packard-PerkinElmer). Results were expressed as mean cpm of triplicate wells.
ELP preparation and electron microscopic examination
Upon sacrificing mice, the thymi were removed and weighed. Thymocyte and splenocyte suspensions from 2-mo-old female mice were prepared by crushing the thymus or spleen between two pieces of ground glass and resuspending the resulting cells in PBS. After centrifugation at 1,000×g for 5 min, the cell supernatants were collected and used for exosome purification by sucrose differential centrifugation using a previously described method (10). Protein content of purified ELPs was quantified using a MicroBCA Protein Assay Kit (Pierce). Morphological analysis was done by visualizing the ELPs using a Hitachi H7000 electron microscope (Electronic Instruments) as previously described (16). In addition, the non-banded fraction 6 collected from thymic ELP propagation (non-ELPF6) was collected using a method as described previously (10) and used as a control for in vivo Treg induction.
Antibodies
Rabbit polyclonal anti-CD81 and mouse mAbs anti-CD9, anti-CD63, and anti-TSG101 were obtained from Santa Cruz Biotechnology. The mouse mAb anti-TGF-β was purchased from R&D Systems and titrated to determine the optimal concentration of anti-TGF-β Ab that blocked thymic ELP-mediated induction of CD4+CD25+Foxp3+ T cells in an in vitro assay. Dilutions of anti-TGF-β Ab ranged from 0.1 to 2 µg/ml in the assay.
Western blot analysis of ELP proteins
ELP protein, ~50 µg/sample, was resolved by SDS-PAGE (12%) and subsequently electrotransferred onto a nitrocellulose membrane. After transfer, analysis of membranes was performed using an Odyssey infrared imaging system (LI-COR Biosciences) as described previously (17).
In vivo localization of thymic ELPs
Thymic ELPs were labeled using an IRDye 800CW labeling (NHS ester) kit according to the instructions provided by Odyssey. Unreacted dye was removed by dialysis. To localize ELPs injected into mice, the IRDye 800CW-labeled ELPs (100 µg) were injected into BALB/c mice via the tail vein and mice were imaged 3 h later using a prototype LI-COR Biosciences small animal imager. Five mice received PBS but no ELPs and served as controls. After 3 h, mice were sacrificed and each organ was imaged using a selection of excitation and detection optical fluorescent filters tuned specifically for IRDye 800CW. Images were acquired and analyzed with software specified by Odyssey. Signal-to-noise ratios were calculated as described below to determine the intensity of the signal for each organ analyzed.
Each organ was weighed, measured, and snap-frozen in OCT compound for cryosectioning. Sections (50-µm thickness) were scanned at 800-nm, for the IRDye 800CW fluorescence signal using the Odyssey laser-scanning imager. The area-weighted fluorescence signal in the 800-nm channel of sectioned tissues for control mice was compared with the ELP injected groups and the ELP:PBS ratio was calculated. This experiment was repeated once with five additional mice.
Coupling of ELPs to latex beads and immunofluorescence analysis
ELPs or non-ELPF6 (30 µg) in PBS were incubated with 4-µm diameter aldehyde/sulfate latex beads (Invitrogen) for 15 min at 20°C in a final volume of 100 µl. After an additional 2-h incubation period in PBS with gentle rotation, the reaction was stopped by the addition of 100 mmol/L glycine. ELP-coated beads were washed twice in PBS containing 10% FCS, stained with different Abs directed against CD9, MHCII, CD4, CD8, and CD80 (eBioscience), and analyzed using a BD Biosciences FACSCalibur and Cell Quest software. A “bead-only” control and an isotype-matched Ab control were prepared and fluorescence intensity was normalized for each Ab based on the controls.
Isolation of leukocytes from liver and lung, and FACS analysis of Tregs
Leukocytes from liver or lung were isolated using a Percoll gradient method described previously (10). The isolated leukocytes were washed with PBS and adjusted to 2 × 107/ml in staining buffer. Fifty µl of the cell suspension was used for staining with fluorescent Abs.
Induction of Tregs in liver and lung of mice injected i.v. with thymic ELPs, and the effects of ELP TGF-β on the induction of Tregs
To induce CD4+Foxp3+ T cells in liver and lung, ELPs (10 µg/mouse in PBS) were injected in the tail veins of 7-wk-old female BALB/c mice twice a week for 2 wk. Seven days after the final injection, mice were sacrificed and leukocytes in liver and lung were isolated and used in FACS analysis for CD4+Foxp3+ cells. Liver or lung CD4+CD25+ T cells were isolated via sorting by FACS and were used for an in vitro assay to determine the capacity of the cells to suppress the proliferation of spleen CD4+CD25− T cells. To determine the effect of ELP TGF-β on the induction of CD4+CD25+ T cells (Treg cells), thymic or splenic ELPs were incubated for 1 h at 22°C with mouse anti-TGF-β Ab (R&D System) or mouse IgG at 1 µg per ml final concentration. This concentration was chosen because higher concentrations of anti-TGF-β did not further reverse ELP-mediated induction of CD4+CD25+Foxp3+ T cells in an in vitro assay. The ELPs were then washed once at 100,000×g for 1 h, and resuspended in 200 µl of PBS. Ten female BALB/c mice per group were injected via the tail vein with thymic or splenic ELPs (100 µg) in PBS preincubated with anti-TGF-β Ab or mouse IgG. The injection volume was 100 µl. Five mice were sacrificed on day 3 for FACS analysis for the induction of CD4+CD25+Foxp3+ T cells in the liver or lung. The remaining mice were injected i.v. with 5 × 106 CFSE-labeled CD4+CD25− T cells and 3 days later sacrificed. The suppressive effect of Tregs (induced by the injected thymic ELPs) on the proliferation of CD4+CD25− T cells injected into mice was analyzed by FACS.
Statistics
Statistical differences between groups were determined by ANOVA with multiple comparisons. The Student’s t test was used for comparisons when only two parameters were evaluated. Proliferation/suppression assays were analyzed using the Mann-Whitney U test. p values < 0.05 were considered significant.
Results
Characterization of ELPs isolated from thymus
Exosomes play an important role in cell-cell communication, in particular the communication between immune cells (3, 9, 18, 19).
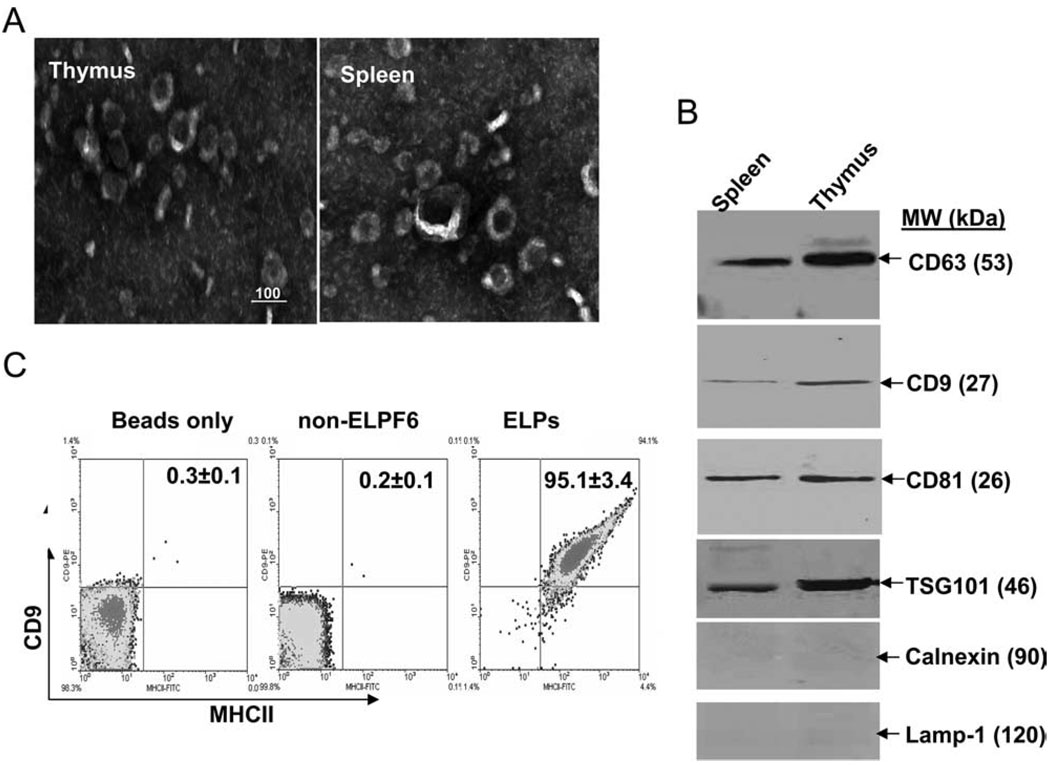
Exosomes are released from cells by fusion of MVBs with the plasma membrane and express specific marker proteins including CD63, CD9, CD81, and TSG101 (3, 18). To identify ELPs present in the thymus, the vesicles from the suspension of thymi was fractionated on sucrose density gradients. ELPs purified from thymocyte suspensions were analyzed for protein content, density, and morphology. Electron microscopy demonstrated that the vesicles present in our preparations were cup-shaped and measured ~60–100 nm in diameter (Fig. 1A) with a density of 1.06–1.11 g/ml, implying that ELPs can be released under physiological conditions in vivo. Immunoblot analysis revealed that TSG101 and CD63 were relatively abundant in ELPs (Fig. 1B) of thymic or splenic origin. CD81 and CD9 were detectable, but not abundant, in our ELP preparations. By contrast, calnexin and Lamp-1 were not detected in the purified ELP preparations (Fig. 1B), indicating that our ELP preparations were free of contamination with non-ELP membrane proteins. To further characterize the ELP surface markers, bead-based FACS analysis of a number of thymocyte cell surface markers was performed. The results revealed that the ELPs purified from the thymus are MHCII+CD9+ (Fig. 1C) but not positive for CD4 and CD8 (data not shown), suggesting that these ELPs may be released from CD4, CD8 double negative nonthymocytes. Staining is ELP specific, as no MHCII+CD9+ staining was detected on beads only or beads coated with nonbanded ELP fraction 6 (non-ELPF6) (Fig. 1C). CD9- and MHCII-positive ELPs with similar characteristics were also identified in the thymus of other strains of mice including C57BL/6j and DBA/2j (data not shown). Taken together, the results strongly support the idea that ELPs are present in the thymus and released from nonthymocyte cells.
Figure 1.
Characterization of thymic ELPs. A, Representative electron micrograph of sucrose gradient purified exosomes (magnification, × 80000; scale bar = 100 nm). Images were acquired with a Hitachi H7000 electron microscope. B, A total of 50 µg of ELPs isolated from the spleen or thymus were lysed and immunoblotted with Abs as indicated on Fig. 1B. Results are representative of two separate experiments. C, Surface phenotyping of ELPs by flow cytometry. Sulfate/aldehyde latex beads coated with thymic ELPs or non-ELPF6 preparations were probed for CD9 and MHCII. Data represent the means of three separate experiments (insets).
Thymic ELPs induced thymus Treg T cells
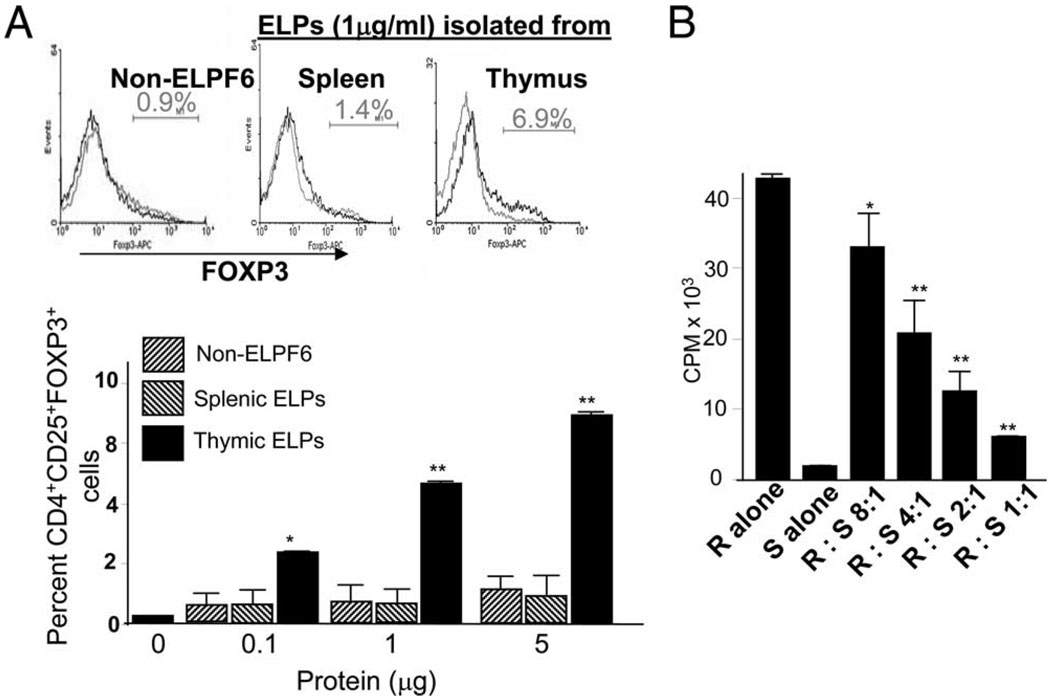
To test the function of thymic ELPs, CD4+CD25− T cells isolated from the thymus of female BALB/c mice were cultivated in the presence of varying concentrations of thymic ELPs or equimolar concentrations of splenic ELPs or non-ELPF6 (controls) for 3 days in the presence of T cell-depleted irradiated splenocytes (5 × 104) and anti-CD3 Ab (0.5 µg/ml). Foxp3 expression by thymus CD4+ T cells was determined by staining with Abs against CD4, CD25, and Foxp3. Results (Fig. 2A) showed that thymic ELPs induced CD4+ T cells to express Foxp3. Costaining with CD25 and Foxp3 indicated that Foxp3 was exclusively expressed in CD4+CD25+ T cell subsets. The CD4+CD25+Foxp3+ T cells were induced by thymic ELPs in a dose-dependent manner (Fig. 2A). In contrast, no induction of CD4+CD25+Foxp3+ T cells was observed when splenic ELPs or non-ELPF6 was added to the culture (Fig. 2A), suggesting that the induction of CD4+CD25+Foxp3+ T cells is specific to thymic ELP. To determine whether CD4+CD25+Foxp3+ T cells induced by ELPs can suppress the proliferation of CD4+CD25− T cells, thymic ELP-stimulated CD4+CD25+ suppressor T cells (S) were sorted and cocultured with splenic CD4+CD25− T cells (R, T effector cells (Teff)) at varying ratios, and [3H]thymidine incorporation was determined to evaluate the proliferation of Teff. Sorted CD4+CD25+ suppressor cells from thymic ELPs-stimulated thymus CD4 T cells markedly inhibited the proliferative response of CD4+CD25− Teff cells induced by CD3 stimulation (Fig. 2B). Inhibition of the proliferative response in CD4+CD25− Teff cells was dependent on the ratios of suppressor/response T cells added to the culture.
Figure 2.
Thymic ELPs induce Treg cells and suppress the proliferation of CD4+CD25− T cells. Thymic CD4+CD25− T cells were treated with thymic ELPs at different concentrations for 3 days, and the resulting CD4+CD25+Foxp3+ T cells were analyzed by FACS as described in the Materials and Methods. Splenic ELPs or non-ELPF6 served as controls. A representative FACS analysis is shown in A (top panel). Data represent three independent experiments performed in triplicate (**, p < 0.01) (A). B, After 3 days in culture in the presence of thymic ELPs (5 µg/ml), thymic CD4+CD25+ T cells were sorted and CD4+CD25+ (S) T cells (5 × 104 per well) were cocultured with varying numbers of CD4+CD25− (R) T cells for 3 days. Cultures were stimulated with anti-CD3 (0.5 µg/ml) in the presence of splenic ACs (5 × 104/well). Cell proliferation was measured by [3H]thymidine incorporation after 72 h in culture. Values are the average counts (±SD) of triplicate wells for three individual experiments (**, p < 0.01).
Thymic ELPs induce lung and liver Treg cells
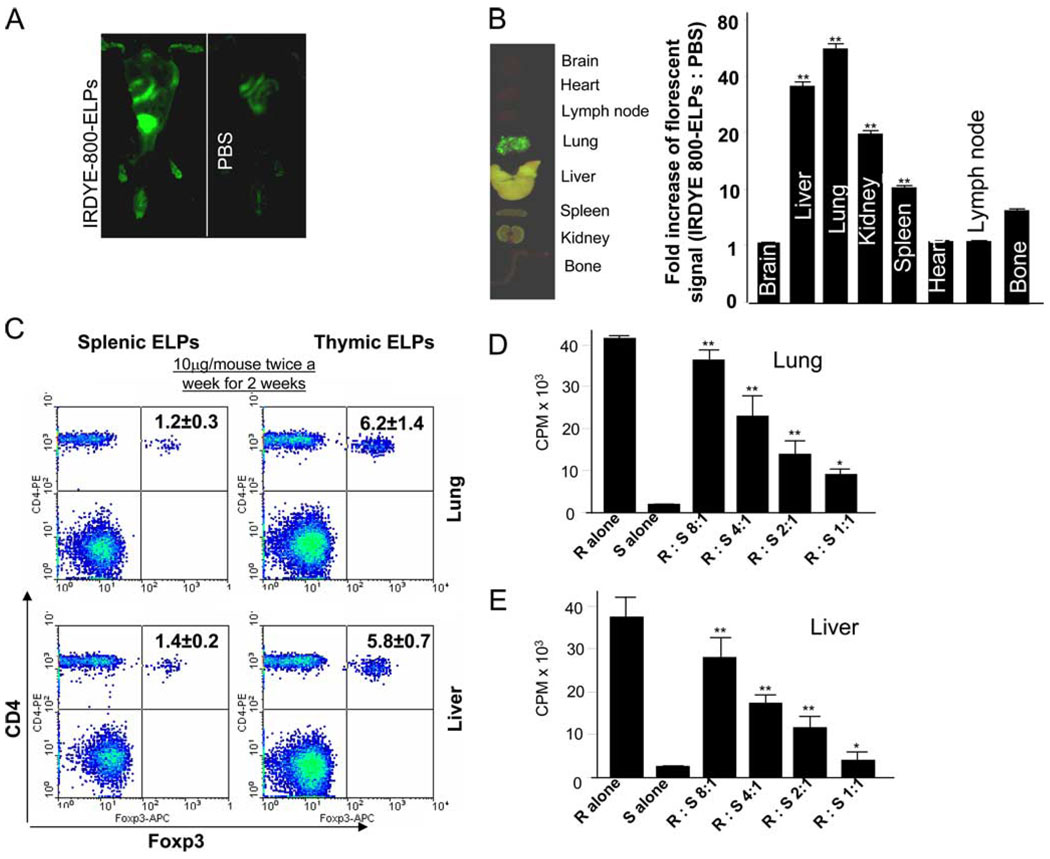
Many T cells develop their regulatory activity in the thymus, but there is also evidence for development of Foxp3+ Tregs from naive precursors in other organs. Thymic ELPs could migrate into peripheral organs and induce Treg cells. Analysis of in vivo imaging indicated that thymic ELPs were predominantly taken up in the liver and lung (Fig. 3, A and B). Based on this fact, we sought to determine whether Treg cells were induced in the liver and lung of BALB/c mice having been injected i.v. with thymic ELPs. To determine the concentration of ELPs that would be physiologically relevant for an in vivo stimulation of induction of CD4+CD25+Foxp3+ T cells, we first determined that the amounts of thymic ELPs that are released constantly over a given time. Analysis of the production of ELPs in the thymus of 7-wk-old BALB/c mice revealed that ~10.1 ± 0.2 µg of ELPs are released from a thymus (180 ± 1.5 mg of weight per thymus). Therefore, we assume that 10 µg of thymic ELPs are released to the peripheral tissues continuously. Based on these data, we observed that i.v. injection of thymic but not splenic ELPs (10 µg/mouse) induced expression of Foxp3 in CD4+CD25+ T cells (Figs. 3C). These CD4+CD25+ T cells inhibited CD4+CD25− proliferation (Fig. 3, D and E). Injecting mice with increasing concentrations (up to 100 µg/mouse) of thymic ELPs did not lead to an increased induction of CD4+CD25+Foxp3+ T cells in lung or liver tissue (data not shown).
Figure 3.
i.v. injection of thymic ELPs causes the induction of Tregs. A total of 100 µg of IRDye 800-labeled thymic ELPs (green) were injected into BALB/c mice via the tail vein. Whole body imaging was done 3 h post injection (A). Upon completion of the in vivo imaging studies, mice were sacrificed and each tissue was imaged (B, left panel) and then frozen for sectioning. Sections (50-µm thickness) were scanned using the Odyssey laser-scanning imager. The area-weighted fluorescence signal in the 800-nm channel of sectioned tissues for PBS- vs thymic ELP-injected groups was compared. Data represent 4 independent experiments with 10 slides for each treatment and the means were calculated. The fluorescence signal ratios of ELPs:PBS was calculated (**, p < 0.01) (B, right panel). Mice were injected i.v. with thymic or splenic ELPs (10 µg/mouse in 200 µl of PBS) twice a week for 2 wk, and, 7 days after the last injection, the mice were sacrificed and the percentages of CD4+Foxp3+ T cells in lung and liver tissues were determined by FACS analysis. A representative figure from one of five mice in each group is shown (C). Results were obtained from two independent experiments with five mice in each experiment pooled and are presented as the mean ± SEM (C, insets). CD4+CD25+ cells (5 × 104) were isolated from the lung (D) and liver (E) by MACS as described in the Materials and Methods and the isolated cells cocultured with splenic CD4+CD25− Teff cells at the indicated ratios and assayed as described in Fig. 2B. Data represent mean ± SEM of triplicate wells; **, p < 0.01.
Thymic ELPs TGF-β plays a role in induction of Treg cells
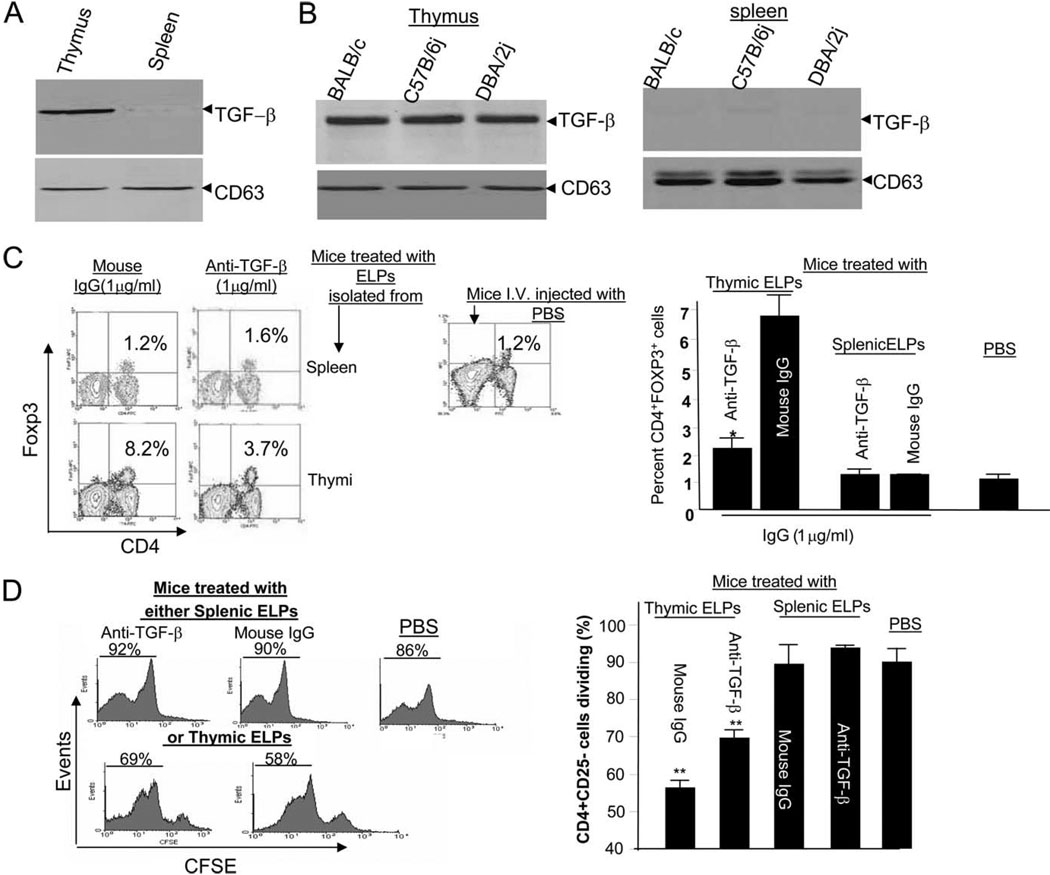
Recent studies have shown that TGF-β can promote Treg cell development. Western blotting of the ELPs released from thymic and splenic preparations indicated that TGF-β is associated with the ELPs isolated from the thymus but not the spleen (Fig. 4A). The presence of TGF-β on ELPs is also evident in thymic ELPs purified from other strains of mice (Fig. 4B). The CD63 protein is present in exosomes produced by many different types of cells (20–28) including thymus and spleen, and similar amounts of CD63 protein were detected in thymic and splenic ELPs (bottom panel, Fig. 4, A and B). This finding suggests that the absence of detectable membrane TGF-β on splenic ELPs is not due to loading lesser amounts of splenic ELP protein on electrophoretic gels.
Figure 4.
Thymic ELP TGF-β plays a role in induction of Treg cells. A total of 50 µg total proteins from thymic and splenic ELPs of BALB/c mice (A) or other strains of mice, as indicated in B, were lysed and each lysate was resolved by PAGE on a 10% SDS gel. The proteins were transferred to a nitrocellulose membrane and the membranes probed with the Abs as indicated. The data represent three independent experiments. CD63 served as an internal control to confirm equivalent protein loading. Thymic or splenic ELPs (100 µg) were incubated with anti-TGF-β Ab or a normal IgG at the final concentration of 1 µg/ml for 1 h at 20°C. ELPs were then washed by ultracentrifugation and resuspened in 100 µl of PBS. Mice (n = 10) received via i.v. route 100 µg of washed ELPs or PBS as a control. Five mice were sacrificed on day 3 post injection. The percentages of CD4+Foxp3+ T cells in the lung were determined. A representative figure from one of five mice in each group is shown (C, left panel). Data represent three independent experiments and are displayed as means ± SEM (C, right panel); **, p < 0.01. The remaining mice were injected i.v. with 5 × 106 CFSE-labeled CD4+CD25− T cells. The mice were sacrificed on day 3 post injection with CFSE-labeled T cells and the proliferating CFSE-labeled CD4+CD25− T cells in the lung tissue were determined by FACS analysis. A representative figure from one of five mice in each group is shown (D, left panel). The percent of proliferation of injected CFSE-labeled CD4+CD25− T cells was calculated as described in the Materials and Methods. Data represent mean ± SEM of five replicas (D); **, p < 0.01.
To determine whether the effect of the thymic ELPs on induction of Treg cells was dependent on thymic ELP TGF-β, thymic ELPs were preincubated with anti-TGF-β (1 µg/ml) for 1 h at 20°C before being injected i.v. into BALB/c mice (n = 10 mice). Three days after the injection, five mice were sacrificed for FACS analysis of Tregs in the lung, and the remaining mice were injected i.v. with CFSE-labeled CD4+CD25−T cells for determining the proliferation of injected T cells at day 3 post injection. The results of FACS analysis of CD4+CD25+Foxp3+ T cells in the lung indicated that the addition of anti-TGF-β Ab, but not a normal mouse IgG, blocked the thymic ELP-mediated induction of CD4+Foxp3+ cells (Fig. 4C). The induction of CD4+Foxp3+ cells in normal mice injected with IgG was 1.2 compared with 8.2% for mice injected with thymic ELPs alone (Fig. 4C, left panel). Furthermore, FACS analysis of CFSE-labeled CD4+ CD25− T cells injected into mice indicated a significant reduction in proliferation of CFSE-labeled CD4+CD25− T cells (p < 00.1) when mice were injected with thymic ELPs, but not splenic ELPs. Preneutralization of thymic ELPs with anti-TGF-β Ab led to a partial rescuing of proliferation of injected CFSE-labeled CD4+CD25− T cells (Fig. 4D). However, it was noticed that the thymic ELP-mediated induction of CD4+CD25+Foxp3+ T cells in the lung was not fully blocked by preincubation of these cells with optimal amounts of anti-TGF-β Ab (Fig. 4, C and D). Similar results were obtained when CD4+CD25+Foxp3+ T cells in the liver were analyzed (data not shown). The inability to fully block the induction of Treg cells in response to anti-TGF-β treatment suggests that additional exosomal molecules may participate in thymic ELP-mediated induction of Treg cells.
Discussion
In this study, we show that thymic ELPs have a size distribution similar to that seen for exosomes from other sources, which is consistent with the size criterion for exosomes proposed by Thery et al. (18). Western blot and FACS analyses of thymic ELPs demonstrated a number of associated proteins consistent with those identified in exosomes from other cellular types (10, 29–35) and indicates that the thymic ELPs are derived from MVBs. Notably, however, the thymic ELP proteins include TGF-β, which is absent from splenic ELPs. The presence of TGF-β was of particular interest because TGF-β is well known to play a role in the induction of Treg cells in peripheral lymphoid organs. The current investigation has shown that thymic ELPs have a role in the induction of both thymic and peripheral Treg cells. In agreement with other investigators’ data (14), our data show that induction of peripheral Treg cells is TGF-β dependent. Taken together, these results suggest that thymic ELPs are one of the endogenous drivers for inducing Treg cells under physiological conditions, an observation that has not been demonstrated previously.
There is little information available regarding which endogenous factors induce Treg cells in the thymus. Although it is clear that TGF-β can promote Treg cell development in culture, little is known about the cellular and molecular mechanisms that mediate this pathway under physiological or in vivo conditions. Our data suggest that thymic, but not splenic, ELPs can stimulate CD4 T cells to differentiate into CD4+CD25+Foxp3+ Treg cells. We found that TGF-β from thymic ELPs participate in the induction of Treg cells in organs such as the lung and liver, which is in agreement with previous studies (14, 36–41). Interestingly, blocking thymic ELP-associated TGF-β led to a partial reduction in the induction of Treg cells, suggesting that other molecules in thymic ELPs may play a role in the induction of Treg cells. Identification of additional molecules from thymic ELPs that contribute to the induction of Treg cells in the thymus, liver, and lung is needed to provide a comprehensive understanding of the role of thymic ELPs in immunological regulation. Additional experiments are needed to identify the thymic ELP molecule(s) that regulate induction of Foxp3. There are a number of proteins and/or pathways that have been identified for their role in the induction of Foxp3 (42, 43). Our proteomic profiles of thymic ELPs (G. Wang, Y. Liu, A. Qin, S. Shah, Z. Deng, X. Xiang, Z. Cheng, C. Liu, J. Wang, L. Zhang, W. Grizzle, and H. G. Zhang, manuscript in preparation) suggests that a group of molecules including TGF-β may be required for induction of Treg cells in the thymus.
In this study, although we did not address the role of MHCII presence on thymic ELPs, the data generated from MHCII knockout mice indicated that MHCII is essential for production of naturally occurring Treg cells in the thymus but not in the peripheral lymphoid organs (44, 45). It is possible that thymic ELPs enriched with the MHCII molecule might have an increased efficiency for inducing CD4+CD25+Foxp3+ T cells development/generation similar to the mechanism reported by other groups (44). In addition, MHCII present on thymic ELPs might also provide Ag-specific induction of CD4+CD25+Foxp3+ T cells. Mice with a deficiency of TGF-β have a defect(s) in the induction of Treg cells in the peripheral organs but not in the thymus (38). Our findings indicate that both MHCII and TGF-β are detected on the thymic ELPs. Additional experiments on the role of ELP MHCII in terms of Ag-specific induction of Treg cells in concert with ELP TGF-β could provide the basis for a new avenue for the suppression of unwanted immune responses in autoimmunity, allergy, and transplantation or depletion of tumor Ag-specific Treg cells in cancer.
Because the regulation of induction of Foxp3+ T cells is critical for maintaining immune tolerance, it is conceivable that molecules or pathways other than those involving ELP MHCII and TGF-β are required to keep the induction of Foxp3+ T cells balanced. Unlike a single molecule such as TGF-β, exosomes packed with 300–500 proteins plus additional mRNAs and microRNAs (46) would qualify for composing such a comprehensive network. The difference in terms of induction of Foxp3+ T cells between thymic and splenic ELPs also makes the identification of such molecules become possible by comparison of proteomic profiles of thymic and splenic ELPs.
Finally, caution should be exercised so as not to interpret our results too broadly in regard to the natural or “normal” route of thymic ELP circulation; however, our data indicate that liver and lung tissues retain a majority of thymic ELPs injected i.v. How thymic ELPs exit the thymus and what types of cells take up thymic ELPs under physiological conditions requires additional study. Future experiments of this nature will be fundamental to understanding how the thymus communicates with peripheral organs, including lymphoid and nonlymphoid organs, via thymic ELPs to regulate immune tolerance in a host.
Acknowledgments
We thank Dr. Jerald Ainsworth for editorial assistance.
Footnotes
This work was supported by National Institutes of Health Grants R01CA116092, R01CA107181, and R01AT004294; Birmingham Veterans Administration Medical Center Merit Review Grants (H.-G.Z.); and grants from Susan G. Komen Breast Cancer Foundation.
Abbreviations used in this paper: MVB, multivesicular body; ELP, thymes exo-some-like particle; Treg, regulatory T cell; AC, accessory cell; Teff, T effector cell.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Fader CM, Colombo MI. Multivesicular bodies and autophagy in erythrocyte maturation. Autophagy. 2006;2:122–125. doi: 10.4161/auto.2.2.2350. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RM. Revisiting the road to the discovery of exosomes. Blood Cells Mol Dis. 2005;34:214–219. doi: 10.1016/j.bcmd.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RM. Exosomes biological significance: a concise review. Blood Cells Mol. Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol. Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanc L, Barres C, Bette-Bobillo P, Vidal M. Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2. Blood. 2007;110:3407–3416. doi: 10.1182/blood-2007-04-085845. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths RE, Heesom KJ, Anstee DJ. Normal prion protein trafficking in cultured human erythroblasts. Blood. 2007;110:4518–4525. doi: 10.1182/blood-2007-04-085183. [DOI] [PubMed] [Google Scholar]
- 8.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 9.Li XB, Zhang ZR, Schluesener HJ, Xu SQ. Role of exosomes in immune regulation. J. Cell. Mol. Med. 2006;10:364–375. doi: 10.1111/j.1582-4934.2006.tb00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, Kappes JC, Barnes S, Kimberly RP, Grizzle WE, Zhang HG. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J. Immunol. 2006;176:1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- 11.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol. Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Sakaguchi S. Foxp3: a critical regulator of the development and function of regulatory T cells. Microbes Infect. 2004;6:745–751. doi: 10.1016/j.micinf.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 14.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Zhang HG, Liu C, Su K, Yu S, Zhang L, Zhang S, Wang J, Cao X, Grizzle W, Kimberly RP. A membrane form of TNF-α presented by exosomes delays T cell activation-induced cell death. J. Immunol. 2006;176:7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J. Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 18.Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Chapter 3: Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 19.Ohno H. Overview: membrane traffic in multicellular systems: more than just a housekeeper. J. Biochem. 2006;139:941–942. doi: 10.1093/jb/mvj119. [DOI] [PubMed] [Google Scholar]
- 20.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/ζ complex. J. Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 22.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 23.Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods. 2001;247:163–174. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- 24.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 25.Johansson SM, Admyre C, Scheynius A, Gabrielsson S. Different types of in vitro generated human monocyte-derived dendritic cells release exosomes with distinct phenotypes. Immunology. 2008;123:491–499. doi: 10.1111/j.1365-2567.2007.02714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 27.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 28.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am. J. Respir. Cell Mol. Biol. 2004;31:114–121. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- 30.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes: potential implications for their function and multivesicular body formation. J. Biol. Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 31.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis, and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 32.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 33.Skokos D, Le Panse S, Villa I, Rousselle JC, Peronet R, David B, Namane A, Mecheri S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 34.Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4β1. Eur. J. Biochem. 2000;267:583–590. doi: 10.1046/j.1432-1327.2000.01036.x. [DOI] [PubMed] [Google Scholar]
- 35.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahic M, Yaqub S, Bryn T, Henjum K, Eide DM, Torgersen KM, Aandahl EM, Tasken K. Differentiation of naive CD4+ T cells into CD4+CD25+FOXP3+ regulatory T cells by continuous antigen stimulation. J. Leukocyte Biol. 2008;83:1111–1117. doi: 10.1189/jlb.0507329. [DOI] [PubMed] [Google Scholar]
- 37.Huber S, Schramm C. TGF-β and CD4+CD25+ regulatory T cells. Front. Biosci. 2006;11:1014–1023. doi: 10.2741/1859. [DOI] [PubMed] [Google Scholar]
- 38.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-β induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J. Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 40.Horwitz DA, Zheng SG, Gray JD, Wang JH, Ohtsuka K, Yamagiwa S. Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease. Semin. Immunol. 2004;16:135–143. doi: 10.1016/j.smim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Chrobak P. Control of T cell responses, tolerance and autoimmunity by regulatory T cells: current concepts. Acta Medica. 2003;46:131–137. [PubMed] [Google Scholar]
- 42.Venuprasad K, Huang H, Harada Y, Elly C, Subramaniam M, Spelsberg T, Su J, Liu YC. The E3 ubiquitin ligase Itch regulates expression of transcription factor Foxp3 and airway inflammation by enhancing the function of transcription factor TIEG1. Nat. Immunol. 2008;9:245–253. doi: 10.1038/niXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, Roncarolo MG, Bacchetta R. STAT5-signaling cytokines regulate the expression of FOXP3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int. Immunol. 2008;20:421–431. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 44.Stephens GL, Andersson J, Shevach EM. Distinct subsets of FoxP3+ regulatory T cells participate in the control of immune responses. J. Immunol. 2007;178:6901–6911. doi: 10.4049/jimmunol.178.11.6901. [DOI] [PubMed] [Google Scholar]
- 45.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4+25+ immunoregulatory T cells. J. Exp. Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]