Abstract
Lung cancer is a deadly disease that is difficult to diagnose and even more difficult to treat effectively. Many pathways are known to affect tumor growth, and targeting these pathways provides the cornerstone by which cancer is treated. Somatostatin receptors (SSTR) are a family of G protein coupled receptors that signal to alter hormonal secretion, increase apoptosis, and decrease cellular proliferation. These receptors are expressed in many normal and malignant cells, including both small cell and non-small cell lung cancer. Synthetic analogs of SSTRs are commercially available, but their effects in lung cancer are still largely uncertain. Signaling pathway studies have shown that SSTRs signal through phosphotyrosine phosphatases to induce apoptosis as well as to decrease cell proliferation. Radiolabeled SSTR2 analogs are utilized for radiographic imaging of tumors, which, when combined with positron emission tomography-computed tomography (PET-CT) may improve detection of lung cancer. These radiolabeled SSTR2 analogs also hold promise for targeted chemotherapy as well as radiotherapy. In this review, we summarize what is known about SSTRs and focus our discussion on the knowledge as it relates to lung cancer biology, as well as discuss current and future uses of these receptors for imaging and therapy of lung cancer.
Keywords: Somatostatin receptors, Lung neoplasms, Neuroendocrine tumors, Molecular imaging
INTRODUCTION
Lung cancer is the leading cause of cancer-related death in the United States (1). It remains extremely challenging to diagnose in its early stages, and treatment is similarly difficult. Recent work has shown that new small molecular inhibitors targeted at specific mutated receptors, such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), improves outcomes for those selected patient populations (2). In this age of emphasis on genotyping tumors for personalized, more targeted therapies, evaluating the role of previously known receptors and proteins could assist in improving treatment and predicting outcomes. In this review, we will describe the different somatostatin receptor subtypes and the role they play in lung cancer biology, as well as discuss current and future utilization of these receptors for imaging and therapy in lung cancer.
SOMATOSTATIN RECEPTORS AND THEIR LIGANDS
Somatostatin receptors (SSTR) are members of the family of G protein coupled receptors (GPCRs) and are widely expressed in tissue throughout the body, including many tumors (3). There are five known subtypes of SSTRs (SSTR1-SSTR5) (Table 1). They bind the hormone somatostatin, of which there are two biologically active forms, SST-14 and SST-28. Somatostatin binds to all of the SSTRs without preference, but the result of somatostatin binding appears to be receptor specific as well as organ dependent, though there is some overlap between the different receptor subtypes. Somatostatin has several downstream effects, including influencing cellular secretion, motility, and even proliferation. It is a major endocrine “off switch” including control of hormone secretion and with significant antiproliverative properties (4-6). It has both autocrine and paracrine activity. SSTRs modulate several key enzymes involved in the cell cycle, including inhibition of adenylate cyclase, impairment of calcium influx, increasing p53 expression and Bax to initiate apoptosis, and also affecting ERK1/2 and AKT to decrease cell proliferation (5,7). For these reasons, not only do SSTRs play a major role in normal human biology, but also they are key players in cancer biology. Somatostatin and its receptors are found throughout the body with resulting physiologic activity, but for this review, we will try to limit our focus to their role in lung cancer.
Table 1.
Somatostatin Receptor Properties
| Receptor subtype | |||||
|---|---|---|---|---|---|
| Gene | SSTR1 | SSTR2 | SSTR3 | SSTR4 | SSTR5 |
| Chromosome | 14q13 | 17q24 | 22q13.1 | 20p11.2 | 16p13.3 |
| Molecular weight (kDa) | 42.7 | 41.3 | 45.9 | 41.9 | 39.2 |
| Glycosylation sites | 3 | 4 | 2 | 1 | 3 |
| Proliferation | ↓ | ↑ ↓ | ↓ | ↑ ↓ | ↓ |
| Apoptosis | ↑ | ↑ | |||
| Hormonal secretion | ↓ | ↓ | |||
This table is adapted from Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov 2003;2:999-1017. Reproduced with the permission of Nature.
The first synthetic analog for a SSTR was created against SSTR2 in the late 1970s (8), and now SSTR2 analogs such as octreotide and lanreotide are used clinically in several types of endocrine and neuroendocrine cancers. The half-life of the endogenously produced hormone is less than 3 minutes (9), so the creation of these synthetic peptides with longer half-lives has allowed for advances in our understanding of SSTRs. The inhibitory effects of somatostatin analogs such as octreotide are used in various tumors (e.g. neuroendocrine tumors, acromegaly, pancreatic tumors) to inhibit tumor hormone hyper-secretion, to decrease gastrointestinal bleeding, and to decrease symptoms of the dumping syndrome (10-12). Because these analogs are already used clinically, are readily available, and have shown to have a good safety profile, researchers are exploring other clinical applications of these compounds. Although all of the SSTRs have biologic importance and are expressed in lung cancers, SSTR2 is the most studied (partly because of the early creation of synthetic SSTR2 analogs), and therefore much of our discussion with regards to lung cancer will focus on it.
SOMATOSTATIN SIGNALING SWITCHES OFF PROLIFERATION
With respect to cancer biology, somatostatin receptors are involved in apoptosis and in cell cycle signaling. The signaling pathway is complex and not entirely understood, as receptor makeup and signaling differences appear to exist between various tissues. This signaling works via G protein coupled receptor activation of phosphotyrosine phosphatases, including SHP-1 and SHP-2, which then signals through ERK1/2 to induce the cyclin-dependent kinase inhibitor p27Kip1 to halt cell growth and induce arrest (Fig. 1) (5,13). Besides growth arrest, SSTRs are involved in apoptosis. SSTR3 induces apoptosis via SHP-1 activation of p53 (14,15), and SSTR2 induces apoptosis via an undetermined p53-independent pathway (16). Once activated, somatostatin receptors are internalized and recycled to the cell surface, with some trafficked for lysosomal degradation (17). Although much is known, further work in evaluating different mechanisms of SSTR-dependent cell cycle control is needed, paying close attention to tissue specificity.
Fig. 1.
Somatostatin receptor signaling cascade. SSTR activation decreases hormonal secretion in most cells by decreasing cyclic AMP and by altering voltage gated potassium and calcium channels. In a minority of cells (pancreatic B-cells), hormonal secretion is increased. SSTR signals through PTPases to alter ERK1/2 phosphorylation to impair p27kip1 degradation which leads to cell growth arrest. SSTR also signals through PTPases to induce apoptosis. Rarely SSTR2 induces proliferation. ERK: extracellular signal-regulated kinase, Gα, Gβ, Gγ: G-protein subunit, PLC: phospholi-apse C, cAMP: cyclic adenosine monophosphate, IP3: inositol tri-phosphate, PTPase: phosphotyro-sine phosphatase, SRIF: somatostatin, SHP: Src homology phosphatase, PTP-η: phosphotyrosine phosphatase η.
TARGETING SSTR2 IN HUMAN STUDIES
SSTR2 is widely expressed in pulmonary neuroendocrine tumors, including typical carcinoids, atypical carcinoids, large cell neuroendocrine, and small cell lung cancer (18-20). Altogether, these tumor types make up approximately 25% of all lung cancers (21). There are mixed reports of SSTR2 expression in non-neuroendocrine lung cancers, including adenocarcinoma and squamous cell carcinoma (18,22), with inconsistent and sometimes even lack of correlation between immunohistochemistry, PCR, and radiolabeled somatostatin analog uptake.
Early work using SSTR2 analogs evaluated their therapeutic potential to alter cell growth in SCLCs with promising results in cell culture and in nude mice (23); however, in subsequent therapeutic trials in human SCLC, the SSTR2 analogs did not improve patient survival (4,24). Although these results were discouraging, previous human studies have typically included small, heterogeneous patient cohorts of only 18 and 20 patients with varying stages of SCLC and with unknown degrees of SSTR2 expression; accordingly, these results are best considered as inconclusive (24,25). Future investigations in more tightly controlled patient trials are needed to determine if there is a roll for the use of SSTR analogs in the treatment of lung cancer, alone and in combination with other treatments and not limited to SSTR2 analogs. Several groups have recently evaluated whether targeting multiple SSTRs at the same time by using SSTR agonists with multiple SSTR binding affinity has any added benefit for inhibiting tumor cell growth. For example, Ono, et al. found that in a bronchial carcinoid cell line (NCI-H727), octreotide, having affinity primarily for SSTRs 2 and 5, did not alter cell proliferation, but pasireotide (SOM230), which binds strongly to SSTRs 1, 2, 3 and 5, did significantly reduce cell growth in vitro (26).
Accordingly, targeting multiple SSTRs may be necessary to produce clinical benefit. The rationale for improved tumor response to multiple somatostatin receptor subtype targeting is not entirely clear, but G protein receptors are known to cross-talk and undergo both homo- and hetero- dimerization. For example, it may be that SSTR2 may induce a certain response when the receptor is not dimerized, but the response may change entirely or in potency when the SSTR2 receptor dimerizes with another SSTR receptor or with an EGFR or dopamine receptor. Further work to understand the biology of SSTR dimerization is necessary to determine the potential of targeting various somatostatin receptors alone or in various combinations as therapeutic targets in lung cancer.
Exactly how somatostatin receptors influence tumor growth in vivo is still not entirely understood, and whether SSTR2 expression on tumor cells benefits or impairs cancer growth remains unknown. Oddstig et al. investigated SSTR2 mRNA expression of cells after the cells were irradiated and observed that SSTR2 expression was upregulated, suggesting an additional possible mechanism by which radiation therapy induces SCLC death, thereby implying a possible future therapeutic role of SSTR2 analogs coupled with already existing therapies (27). Several studies of neuroendocrine lung cancers have shown an inverse correlation between the intensity of SSTR2 expression in immunohistochemistry and the grade of the pulmonary neuroendocrine tumor, such that the more aggressive the tumor (higher grade), the lower the SSTR2 expression (28,29). A recent study by Muscarella et al. (30) evaluated the expression of various SSTRs by using real time quantitative PCR in the peripheral blood of patients with neuroendocrine lung tumors, including several patients with SCLC. They found that there were increased levels of SSTR2 and SSTR5 in the peripheral blood of these patients compared with controls, presenting an exciting new method of assessing a patient's SSTR expression status. Whether knowing a patient's SSTR expression status will have bearing on their clinical treatment or outcome will need further study.
SOMATOSTATIN RECEPTOR-RELATED IMAGING
IHC staining for specific somatostatin receptor subtypes has shown that SSTR2 is expressed by both the tumor cells and by the tumor vasculature, particularly in the endothelium of the venules (31). Accordingly, it is unknown whether the imaging modality is detecting SSTR2 expression in tumor cells and/or in the draining peri-tumoral veins. In the past two decades, significant advances in medical imaging, including development of PET-based somatostatin receptor imaging, have occurred. The use of PET and PET/CT imaging technology improves spatial and contrast resolution significantly over older planar and SPECT imaging, and also allows semi-quantitation with standardized uptake values (SUV).
Blum et al. published a study of 30 patients with solitary pulmonary nodules, finding that somatostatin receptor scintigraphy coupled with CT scan correctly differentiated benign from malignant lung nodules in 27 of 30 (90%) of patients (32). This study, reported in 1999, used 99mTc-based planar and SPECT imaging in patients with the broad-spectrum somatostatin analog (SSTR 2, 3 and 5) depreotide who were high-risk for lung cancer and in a region (Arizona, USA) with high endemic granulomatous nodules and with a concordant CT revealing an indeterminate lung nodule (33). True positive scans occurred in 12 patients (10 with adenocarcinoma, 1 with squamous cell carcinoma and 1 with malignant carcinoid). Two false positive patients had necrotizing granulomata due to Mycobacterium avium. Of the 16 patients with negative scans, biopsy showed necrotizing granulomatous infection in 13 (8 with coccidioidomycosis), one with a hamartoma, and one that was not biopsied but stable on 24 months follow-up. One false negative (no uptake) occurred with a nodule due to squamous cell carcinoma.
Another multicenter, phase III trial of somatostatin receptor imaging published in 2000, using 99mTc-depreotide for diagnosis of solitary lung nodules or masses, reported sensitivity (96.6%) and specificity (73.1%) with SPECT-imaging that were very similar to the results of 18F-FDG PET imaging reports contemporaneous to this investigation, prior to PET/CT (34). This trial evaluated 114 patients with indeterminate lung nodules or masses ranging in size from 8 mm to 6 cm. Histologically proven malignancy was present in 88 of 144 (61%) of patients, with 85 of these 88 cancers (96%) correctly identified as “probably malignant” by 99mTc-depreotide SPECT imaging. There were three false negative imaging results, all from adenocarcinoma (two from lung primary tumors and one from metastatic colon cancer). Another 31 adenocarcinomas were correctly diagnosed as “probably malignant” on the basis of the 99mTc-depreotide imaging study. False positive results included 7 granulomas and one hamartoma, though three other hamartomas were correctly classified as “probably benign.” “Probably benign” was correctly diagnosed in 19 of 26 (73%) of patients. The overall accuracy of differentiating benign from malignant etiology was 91% for these lesions that were indeterminate by other imaging criteria. These results are very impressive considering the technical limitations of the day (SPECT-only imaging instead of a modern PET/CT based scanner with easily performed SUV measurements). Despite these limitations, the lower resolution SPECT-based imaging was still able to identify as “probably malignant” a wide range of tumors, including NSCLC (adenocarcinoma, squamous cell carcinoma and large cell lung cancer), carcinoid tumors, small cell lung cancer and Hodgkin lymphoma.
Mena and colleagues demonstrated that 99mTc-depreotide scanning can successfully image both primary lung cancers and bony metastases (35). In 20 patients with newly diagnosed lung cancer undergoing both whole-body 99mTc-depreotide and conventional imaging (including bone scans and radiographs of suspected bone lesions), somatostatin-based imaging demonstrated uptake in 19 of 20 primary lung tumors with 55 bone lesions seen with conventional imaging, with 31 of these bone lesions determined to be malignant. Uptake of the somatostatin analog in the malignant bone lesions was seen in 28 of these 31 malignant bone lesions (90%). Importantly, none of the benign bone lesions showed somatostatin-analog uptake. In addition, 99mTc-depreotide imaging found six unsuspected extraosseous sites of metastases.
A seminal paper from Kahn and coworkers compared the use of somatostatin receptor imaging in a Veteran population of 166 patients imaged separately with 99mTc-depreotide SPECT and 18F-FDG PET (36). Nine patients were excluded, leaving 157 patients included in the analysis. Pathologic proof of diagnosis was obtained in 146 patients, with a benign diagnosis verified in the remainder by follow-up. The sensitivities for the two imaging methods were statistically equivalent for detection of malignancy (96% for 18F-FDG PET and 94% for 99mTcdepreotide SPECT) despite the typical superior spatial and contrast resolution of 18F-FDG PET imaging, though the specificity of 18F-FDG PET was superior to 99mTc-depreotide SPECT-based imaging for evaluation of the primary tumor (71% and 51%, respectively). FN results occurred with each imaging agent over a wide range of sizes (0.5~3 cm for 18F-FDG PET and 0.5~4 cm for 99mTc-depreotide SPECT). Of six patients with relatively low grade lung cancers, 3 of these missed by 18F-FDG PET and two were missed by 99mTc depreotide SPECT. Granulomatous disease occurred in 13 patients, 5 false positive (FP) by 18F-FDG PET, with 4 of these 5 FP by 99mTc-depreotide SPECT. Importantly, when the results from the two imaging modalities were combined in a multivariate model, only two patients were false negative (FN) for cancer, each with a sub-cm lesion below the reliable resolution limits of the then current clinical 18F-FDG PET or 99mTc-depreotide SPECT scanning equipment.
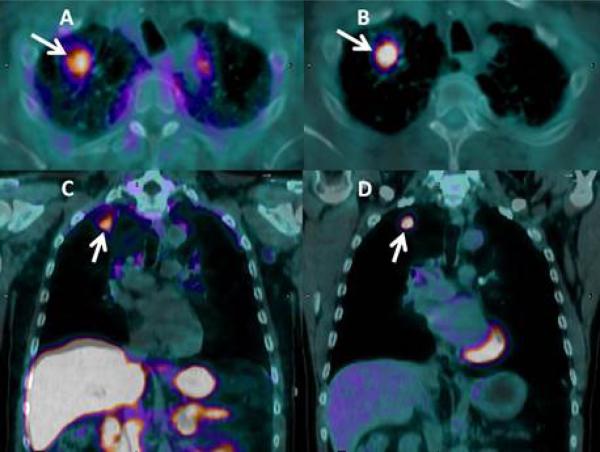
The expression of SSTR receptors in 9 patients with NSCLC (6 with squamous carcinoma and 3 with adenocarcinoma) has also been assessed using 68Ga-DOTATOC PET imaging, which binds to SSTR2 and 5 receptors, labeled with the PET-emitter gallium-68. Seven patients had received prior treatment with seven courses of chemotherapy with platinum-based regimens, and two patients were newly diagnosed and untreated. This study found moderate expression of SSTR2 in 7 of 9 primary NSCLC (squamous and adenocarcinoma) (37). An example of molecular imaging of a lung nodule using both modalities, 18F-FDG PET and 68Ga-DOTATATE PET imaging at our institution is provided in Fig. 2.
Fig. 2.
Use of a new synthetic somatostatin analog in lung cancer: Axial (A, B) and coronal (C, D) fused PET/CT images in a patient with newly diagnosed, untreated adenocarcinoma of the right upper lobe (arrows). The tumor is easily seen with both the somatostatin analog (68Ga-DOTATATE; A, C) and with conventional PET imaging (18F-FDG: B, D) with comparable uptake (each had an SUV value of 2.7). The combination of both imaging agents may help reduce false positive exams with 18F-FDG PET/CT imaging alone, decreasing unnecessary biopsies or thoracotomies. Images by the authors.
Ambrosini and colleagues reported on the sensitivity of 68Ga-labeled somatostatin receptor PET/CT imaging with the analog DOTA-NOC in 13 neuroendocrine tumor patients, including 2 lung tumors (one well-differentiated and one poorly differentiated lung carcinoid tumor) (38). The 68Ga-DOTA-NOC imaging was positive in all 13 patients, including in the two lung primary tumors.
Few studies correlating the results of SSTR2 nuclear imaging with in vivo SSTR2 expression as determined by immunohistochemistry or other laboratory assays is lacking at this time, but as the use of this new imaging technique becomes more available and increasingly studied, this will be determined. A recent study from 2009 correlated the in vivo expression of SSTR2 by immunohistochemistry and SUV intensity using 68Ga-DOTATOC PET imaging in 18 patients with neuroendocrine tumors, finding found strong correlation, including one lung tumor (typical carcinoid) (39).
PEPTIDE-RECEPTOR BASED RADIATION THERAPY
Researchers are beginning to exploit radiolabeled somatostatin analogs for therapy, called peptide-receptor radiation therapy (PRRT) (40,41). Imaging with PET-based somatostatin analogs prior to PRRT allows verification of both the presence of somatostatin receptors in sufficient quantity to allow effective uptake of the therapeutic agent and calculation of specific dosimetry in order to determine how much therapeutic agent to give.
Pless et al. (42) reported a pilot trial using the beta-emitter form yttrium (90Y) labeled to the somatostatin analog DOTATOC for PRRT treatment of small cell lung cancer. A total of six patients were treated. While there was no demonstrable improvement in survival, the number of patients was small, all patients had extensive disease that was progressing on standard treatment, and not all areas of extrathoracic disease demonstrated uptake on 111In-octreotide scanning which was done to determine suitability for enrollment. In another study, Virgolini et al. used 90Y-DOTA-lanreotide to target SSTR-expressing tumors with radiation therapy, with uptake in all 8 patients with small cell neuroendocrine lung cancer, all 17 with NSCLC and all 8 patients with SCLC (43). Suitability of uptake was verified with 111In-octreotide imaging prior to enrollment. All patients entered the trial with progressive disease. Of the 33 patients with lung cancer in this trial, 10 received a measurable response to the PRRT treatment. Other similar studies been performed, some with measurable success, though few have looked specifically at lung cancer. SSTR2 imaging is likely to see further study in lung cancer, both in neuroendocrine tumors as well as other NSCLCs. Hopefully this will aid in the diagnostic management of pulmonary nodules, especially if CT screening becomes standard in high risk individuals, and in targeted treatment of SSTR2-expressing tumors (44).
The detection of indeterminate pulmonary nodules (5~15 mm in diameter) is an increasingly frequent event given the increased use of chest CT imaging (45). Due to the recent encouraging results of the National Lung Screening Trial (NLST) showing a 20% reduction in lung cancer mortality in favor of low dose chest CT screening in high risk individuals (44). CT screening is likely to become standard clinical practice and further increase the detection rate of small, indeterminate lung nodules. These screening CTs in patients at high risk for lung cancer are reported to identify lung nodules worrisome enough to deserve a follow up in up to 27% of patients screened. Yet, only a small fraction of these nodules represent lung cancer (46). Given the costs and complications associated with diagnostic workup of pulmonary nodules, there is a critical need for effective tools to distinguish benign from malignant nodules non-invasively. PET-based somatostatin imaging, combined in a multivariate model with conventional imaging and 18F-FDG PET/CT, may help improve the discrimination between benign and malignant lung nodules.
CONCLUSION
Since their discovery in the 1970s, somatostatin and its receptors have received extensive study. Although our understanding has increased exponentially, much further research remains necessary to understand fully how SSTR signaling affects cell proliferation, particularly in cancerous tissue where mutated cells may respond differently to somatostatin receptor signaling than normal cells. The advances in medical imaging as well as therapeutics with somatostatin receptor-coupled ligands are particularly exciting. In the era of personalized medicine, future success in diagnosing and treating some lung cancers may incorporate the exploitation of somatostatin receptors and signaling pathways.
Acknowledgments
This study was supported by VA Merit Review I01BX007080 to RCW and CA 102353 to PPM.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reubi JC, Schaer JC, Markwalder R, Waser B, Horisberger U, Laissue J. Distribution of somatostatin receptors in normal and neoplastic human tissues: recent advances and potential relevance. Yale J Biol Med. 1997;70:471–479. [PMC free article] [PubMed] [Google Scholar]
- 4.Hejna M, Schmidinger M, Raderer M. The clinical role of somatostatin analogues as antineoplastic agents: much ado about nothing? Ann Oncol. 2002;13:653–668. doi: 10.1093/annonc/mdf142. [DOI] [PubMed] [Google Scholar]
- 5.Lahlou H, Saint-Laurent N, Estève JP, et al. sst2 Somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J Biol Chem. 2003;278:39356–39371. doi: 10.1074/jbc.M304524200. [DOI] [PubMed] [Google Scholar]
- 6.O'Byrne KJ, Schally AV, Thomas A, Carney DN, Steward WP. Somatostatin, its receptors and analogs, in lung cancer. Chemotherapy. 2001;47(Suppl 2):78–108. doi: 10.1159/000049163. [DOI] [PubMed] [Google Scholar]
- 7.Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2:999–1017. doi: 10.1038/nrd1255. [DOI] [PubMed] [Google Scholar]
- 8.Vale W, Rivier J, Ling N, Brown M. Biologic and immunologic activities and applications of somatostatin analogs. Metabolism. 1978;27(9 Suppl 1):1391–1401. doi: 10.1016/0026-0495(78)90081-1. [DOI] [PubMed] [Google Scholar]
- 9.Patel YC, Wheatley T. In vivo and in vitro plasma disappearance and metabolism of somatostatin-28 and somatostatin-14 in the rat. Endocrinology. 1983;112:220–225. doi: 10.1210/endo-112-1-220. [DOI] [PubMed] [Google Scholar]
- 10.Didden P, Penning C, Masclee AA. Octreotide therapy in dumping syndrome: Analysis of long-term results. Aliment Pharmacol Ther. 2006;24:1367–1375. doi: 10.1111/j.1365-2036.2006.03124.x. [DOI] [PubMed] [Google Scholar]
- 11.Thabut D, Bernard-Chabert B. Management of acute bleeding from portal hypertension. Best Pract Res Clin Gastroenterol. 2007;21:19–29. doi: 10.1016/j.bpg.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Chan JA, Kulke MH. New treatment options for patients with advanced neuroendocrine tumors. Curr Treat Options Oncol. 2011;12:136–148. doi: 10.1007/s11864-011-0148-2. [DOI] [PubMed] [Google Scholar]
- 13.Florio T. Somatostatin/somatostatin receptor signalling: phosphotyrosine phosphatases. Mol Cell Endocrinol. 2008;286:40–48. doi: 10.1016/j.mce.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Srikant CB. Cell cycle dependent induction of apoptosis by somatostatin analog SMS 201-995 in AtT-20 mouse pituitary cells. Biochem Biophys Res Commun. 1995;209:400–406. doi: 10.1006/bbrc.1995.1517. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Martino G, Thangaraju M, et al. Caspase-8-mediated intracellular acidification precedes mitochondrial dysfunction in somatostatin-induced apoptosis. J Biol Chem. 2000;275:9244–9250. doi: 10.1074/jbc.275.13.9244. [DOI] [PubMed] [Google Scholar]
- 16.Teijeiro R, Rios R, Costoya JA, et al. Activation of human somatostatin receptor 2 promotes apoptosis through a mechanism that is independent from induction of p53. Cell Physiol Biochem. 2002;12:31–38. doi: 10.1159/000047824. [DOI] [PubMed] [Google Scholar]
- 17.Reubi JC, Waser B, Cescato R, Gloor B, Stettler C, Christ E. Internalized somatostatin receptor subtype 2 in neuroendocrine tumors of octreotide-treated patients. J Clin Endocrinol Metab. 2010;95:2343–2350. doi: 10.1210/jc.2009-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papotti M, Croce S, Bellò M, et al. Expression of somatostatin receptor types 2, 3 and 5 in biopsies and surgical specimens of human lung tumours. Correlation with preoperative octreo-tide scintigraphy. Virchows Arch. 2001;439:787–797. doi: 10.1007/s004280100494. [DOI] [PubMed] [Google Scholar]
- 19.O'Byrne KJ, Halmos G, Pinski J, et al. Somatostatin receptor expression in lung cancer. Eur J Cancer. 1994;30A:1682–1687. doi: 10.1016/0959-8049(94)00351-5. [DOI] [PubMed] [Google Scholar]
- 20.Taylor JE, Theveniau MA, Bashirzadeh R, Reisine T, Eden PA. Detection of somatostatin receptor subtype 2 (SSTR2) in established tumors and tumor cell lines: evidence for SSTR2 heterogeneity. Peptides. 1994;15:1229–1236. doi: 10.1016/0196-9781(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 21.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628–1638. doi: 10.5858/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 22.Herlin G, Kölbeck KG, Menzel PL, et al. Quantitative assessment of 99mTc-depreotide uptake in patients with non-small-cell lung cancer: immunohistochemical correlations. Acta Radiol. 2009;50:902–908. doi: 10.1080/02841850903127477. [DOI] [PubMed] [Google Scholar]
- 23.Taylor JE, Bogden AE, Moreau JP, Coy DH. In vitro and in vivo inhibition of human small cell lung carcinoma (NCI-H69) growth by a somatostatin analogue. Biochem Biophys Res Commun. 1988;153:81–86. doi: 10.1016/s0006-291x(88)81192-6. [DOI] [PubMed] [Google Scholar]
- 24.Macaulay VM, Smith IE, Everard MJ, Teale JD, Reubi JC, Millar JL. Experimental and clinical studies with somatostatin analogue octreotide in small cell lung cancer. Br J Cancer. 1991;64:451–456. doi: 10.1038/bjc.1991.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotto C, Quoix E, Thomas F, Henane S, Trillet-Lenoir V. Phase I study of the somatostatin analogue somatuline in refractory small-cell lung carcinoma. Ann Oncol. 1994;5:290–291. doi: 10.1093/oxfordjournals.annonc.a058812. [DOI] [PubMed] [Google Scholar]
- 26.Ono K, Suzuki T, Miki Y, et al. Somatostatin receptor subtypes in human non-functioning neuroendocrine tumors and effects of somatostatin analogue SOM230 on cell proliferation in cell line NCI-H727. Anticancer Res. 2007;27:2231–2239. [PubMed] [Google Scholar]
- 27.Oddstig J, Bernhardt P, Nilsson O, Ahlman H, Forssell-Aronsson E. Radiation-induced up-regulation of somatostatin receptor expression in small cell lung cancer in vitro. Nucl Med Biol. 2006;33:841–846. doi: 10.1016/j.nucmedbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Papotti M, Croce S, Macrì L, et al. Correlative immunohisto-chemical and reverse transcriptase polymerase chain reaction analysis of somatostatin receptor type 2 in neuroendocrine tumors of the lung. Diagn Mol Pathol. 2000;9:47–57. doi: 10.1097/00019606-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Righi L, Volante M, Tavaglione V, et al. Somatostatin receptor tissue distribution in lung neuroendocrine tumours: a clinic-opathologic and immunohistochemical study of 218 ‘clinically aggressive' cases. Ann Oncol. 2010;21:548–555. doi: 10.1093/annonc/mdp334. [DOI] [PubMed] [Google Scholar]
- 30.Muscarella LA, D'Alessandro V, la Torre A, et al. Gene expression of somatostatin receptor subtypes SSTR2a, SSTR3 and SSTR5 in peripheral blood of neuroendocrine lung cancer affected patients. Cell Oncol (Dordr) 2011;34:435–441. doi: 10.1007/s13402-011-0025-9. [DOI] [PubMed] [Google Scholar]
- 31.Reubi JC, Schaer JC, Laissue JA, Waser B. Somatostatin receptors and their subtypes in human tumors and in peritumo ral vessels. Metabolism. 1996;45(8 Suppl 1):39–41. doi: 10.1016/s0026-0495(96)90077-3. [DOI] [PubMed] [Google Scholar]
- 32.Blum JE, Handmaker H, Rinne NA. The utility of a somatostatin-type receptor binding peptide radiopharmaceutical (P829) in the evaluation of solitary pulmonary nodules. Chest. 1999;115:224–232. doi: 10.1378/chest.115.1.224. [DOI] [PubMed] [Google Scholar]
- 33.Menda Y, Kahn D. Somatostatin receptor imaging of non-small cell lung cancer with 99mTc depreotide. Semin Nucl Med. 2002;32:92–96. doi: 10.1053/snuc.2002.31564. [DOI] [PubMed] [Google Scholar]
- 34.Blum J, Handmaker H, Lister-James J, Rinne N. A multicenter trial with a somatostatin analog (99m)Tc depreotide in the evaluation of solitary pulmonary nodules. Chest. 2000;117:1232–1238. doi: 10.1378/chest.117.5.1232. [DOI] [PubMed] [Google Scholar]
- 35.Mena E, Camacho V, Estorch M, Fuertes J, Flotats A, Carrió I. 99mTc-depreotide scintigraphy of bone lesions in patients with lung cancer. Eur J Nucl Med Mol Imaging. 2004;31:1399–1404. doi: 10.1007/s00259-004-1594-x. [DOI] [PubMed] [Google Scholar]
- 36.Kahn D, Menda Y, Kernstine K, et al. The utility of 99mTc depreotide compared with F-18 fluorodeoxyglucose positron emission tomography and surgical staging in patients with suspected non-small cell lung cancer. Chest. 2004;125:494–501. doi: 10.1378/chest.125.2.494. [DOI] [PubMed] [Google Scholar]
- 37.Dimitrakopoulou-Strauss A, Georgoulias V, Eisenhut M, et al. Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using (68)Ga-DOTATOC PET and comparison with (18)F-FDG PET. Eur J Nucl Med Mol Imaging. 2006;33:823–830. doi: 10.1007/s00259-005-0063-5. [DOI] [PubMed] [Google Scholar]
- 38.Ambrosini V, Tomassetti P, Castellucci P, et al. Comparison between 68Ga-DOTA-NOC and 18F-DOPA PET for the detection of gastro-entero-pancreatic and lung neuro-endocrine tumours. Eur J Nucl Med Mol Imaging. 2008;35:1431–1438. doi: 10.1007/s00259-008-0769-2. [DOI] [PubMed] [Google Scholar]
- 39.Miederer M, Seidl S, Buck A, et al. Correlation of immuno histopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:48–52. doi: 10.1007/s00259-008-0944-5. [DOI] [PubMed] [Google Scholar]
- 40.van Essen M, Krenning EP, Kooij PP, et al. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med. 2006;47:1599–1606. [PubMed] [Google Scholar]
- 41.van Essen M, Krenning EP, Kam BL, de Jong M, Valkema R, Kwekkeboom DJ. Peptide-receptor radionuclide therapy for endocrine tumors. Nat Rev Endocrinol. 2009;5:382–393. doi: 10.1038/nrendo.2009.105. [DOI] [PubMed] [Google Scholar]
- 42.Pless M, Waldherr C, Maecke H, Buitrago C, Herrmann R, Mueller-Brand J. Targeted radiotherapy for small cell lung cancer using 90Yttrium-DOTATOC, an Yttrium-labelled somatostatin analogue: a pilot trial. Lung Cancer. 2004;45:365–371. doi: 10.1016/j.lungcan.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 43.Virgolini I, Britton K, Buscombe J, Moncayo R, Paganelli G, Riva P. In- and Y-DOTA-lanreotide: results and implications of the MAURITIUS trial. Semin Nucl Med. 2002;32:148–155. doi: 10.1053/snuc.2002.31565. [DOI] [PubMed] [Google Scholar]
- 44.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McWilliams A, Mayo J. Computed tomography-detected noncalcified pulmonary nodules: a review of evidence for significance and management. Proc Am Thorac Soc. 2008;5:900–904. doi: 10.1513/pats.200809-111QC. [DOI] [PubMed] [Google Scholar]
- 46.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–2229. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]