Abstract
Impaired urinary dilution leading to water retention and hyponatremia may occur in patients with cardiac failure, cirrhosis, pregnancy, hypothyroidism, glucocorticoid and mineralocorticoid deficiency. The mechanisms for these defects predominantly involve the non-osmotic stimulation of arginine vasopressin release with upregulation of aquaporin 2 water channel expression and trafficking to the apical membrane of the principal cells of the collecting duct. These perturbations are reversed by V2 vasopressin receptor antagonists. In contrast, urinary concentration defects leading to polyuria are vasopressin-resistant. They may involve several factors, such as impaired counter-current concentration secondary to downregulation of Na-K-2Cl co-transporter. Vasopressin-resistant downregulation of aquaporin 2 expression has also been described as a factor in impaired urinary concentration.
Keywords: vasopressin, water channels, hyponatremia, V2 receptor antagonists, aquaretics
Introduction
Professor Edward B. Verney from Cambridge University performed seminal experiments which demonstrated the presence of hypothalamic osmoreceptors regulating antidiuretic hormone release from the posterior pituitary (Fig. 1; Verney, 1947). An increase in central osmolality during hypertonic injections into the internal carotid artery of a water-diuresing dog, Nicky, caused an antidiuresis earlier than injection of the same solutions into an ankle vein. Hypertonic sodium chloride, sucrose and mannitol caused comparable antidiuresis while hypertonic urea, which readily passes across cell membranes, did not alter the diuresis. Du Vigneaud synthesized arginine vasopressin (AVP) which had the biological properties of the anti-diuretic hormone and he was awarded the Nobel Prize in Chemistry for this discovery (Fig. 2; Du Vigneaud et al., 1954). Our laboratory then performed a series of experiments which documented the non-osmotic, baroreceptor-mediated pathway for AVP release, which occurred independent of the osmoregulation of this antidiuretic hormone (Schrier and Berl, 1972, 1974; Schrier et al., 1972; Berl et al., 1974a, b). With the development of a radioimmunoassay, the sensitivity of the osmotic and non-osmotic pathways for AVP was demonstrated. The osmotic regulation of AVP was sensitive for 1–2% changes in plasma osmolality, while an 8–10% decrease in blood pressure was necessary to activate the non-osmotic baroreceptor pathway. Once the non-osmotic pathway is activated, however, the resultant plasma AVP levels were much higher than with osmotic stimulation (Fig. 3; Dunn et al., 1973). Neurophysiological studies have demonstrated that the same supraoptic neuron can be activated by either osmotic or non-osmotic baroreceptor stimulation (Fig. 4; Schrier et al., 1979). When opposing stimuli occur, such as a decrease in plasma osmolality which suppresses AVP and baroreceptor activation which stimulates AVP, a lower steady-state plasma osmolality may occur. With modest hyponatremia, these competing stimuli for AVP release may account for what has been termed a “reset osmostat”, since an acute water load (20 ml/kg) may further lower plasma osmolality, override the baroreceptor stimulus and lead to urinary dilution.
Fig. 1.

Edward B. Verney, FRS, Professor of Pharmacology, Cambridge, England. Adapted with permission from Verney, 1947.
Fig. 2.

Vicent du Vigneaud, Nobel Prize in Chemistry, 1955. Adapted with permission from Du Vigneaud et al., 1954.
Fig. 3.
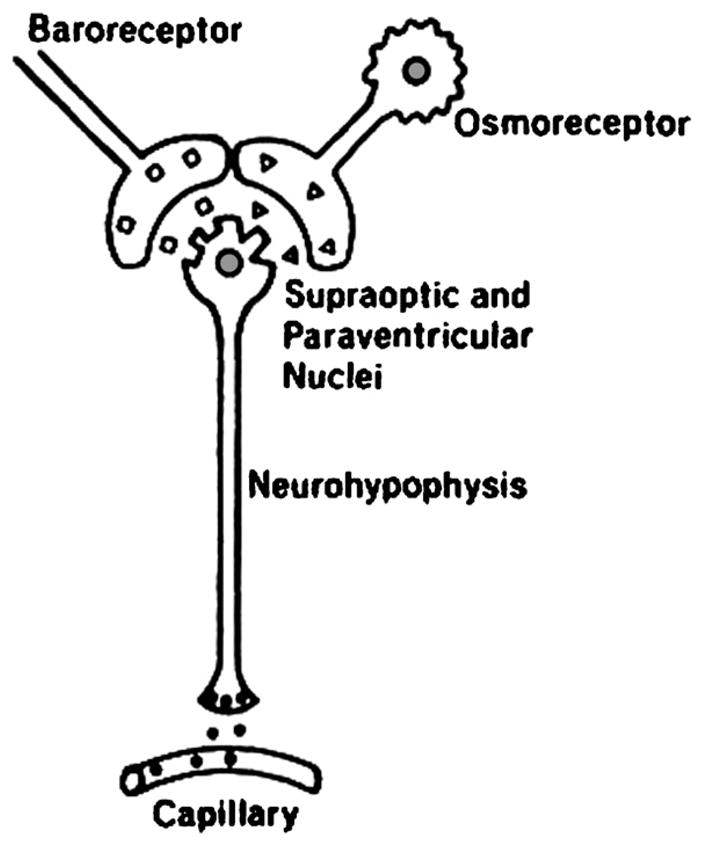
Interrelationship between non-osmotic (baroreceptor) and osmotic (osmoreceptor) pathways for AVP release. Adapted with permission from Dunn et al., 1973.
Fig. 4.
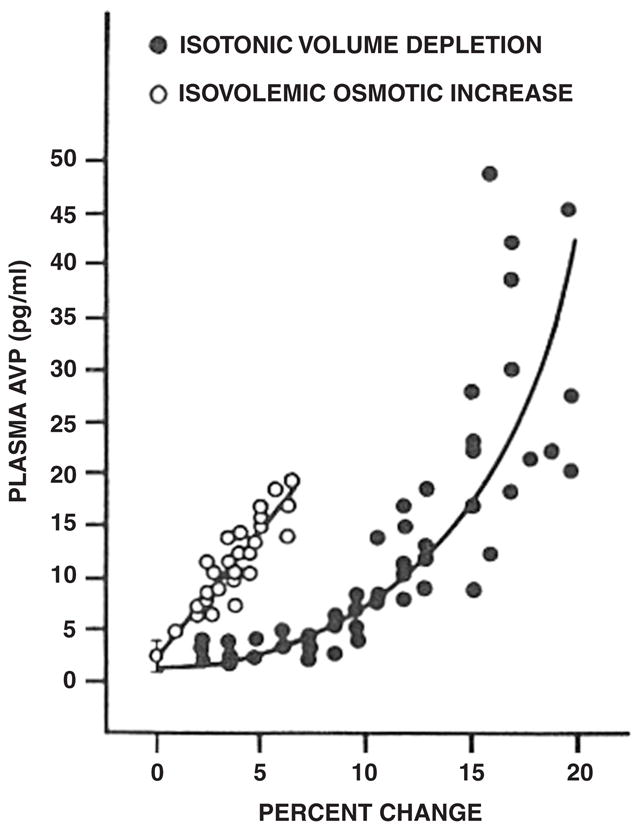
Relationship of plasma AVP to percentage of change in plasma osmolality (open circles) and volume depletion (solid circles). Adapted with permission from Schrier et al., 1979.
Water transport across bilipid plasma membranes was shown to be too rapid for diffusion alone; therefore, tissue water channels were hypothesized. A search for water channels in various tissues was unsuccessful until a haematologist, Peter C. Agre, found an abundant protein in red blood cells (Fig. 5; Agre et al., 1993). The protein was transfected into frog eggs (Xenopus laevis oocytes). After 3 min in a hypotonic solution, a marked expansion occurred in the transfected frog eggs but not in normal frog eggs (Fig. 6; Preston et al., 1992). The structure of this water channel, initially termed Chip 28 and then aquaporin 1, is shown in Fig. 7 (Nielsen, 2002) and was found to be present in the proximal tubule, descending limb of Henle’s loop and descending vasa rectae of the kidney. Subsequent studies by Sasaki and colleagues identified aquaporin 2 (AQP2) in the principal cells of the collecting duct (Fushimi et al., 1993). AQP2 was found to be regulated both within the short term and long term by AVP. Short-term regulation involves trafficking of AQP2 from cytoplasmic vesicles to the apical membrane of collecting duct principal cells. This involves activation of the V2 vasopressin receptor, adenylyl cyclase, cyclic AMP and protein kinase A pathway with phosphorylation of AQP2 (Fig. 8; Bichet, 2006). In the presence of the osmotic driving force initiated by the Na-K-2Cl co-transporter in the water-impermeable thick ascending limb and generated by the counter-current multiplier system, water transport enters principal cells via apical AQP2 water channels and exits via AQP3 and AQP4 water channels on the basolateral surface of these collecting duct cells. The long-term regulation of AQP2 by AVP involves a cyclic AMP response element in the AQP2 promoter with resultant increase in AQP2 protein expression. The V2 vasopressin receptor has been cloned, and recently several non-peptide V2 receptor antagonists have been developed (Schrier, 2007). On the background of these advances, the molecular mechanisms involved in a variety of clinical disorders of water homeostasis have been defined.
Fig. 5.

Peter C. Agre, MD, Professor of Medicine, Duke University. Nobel Prize in Chemistry, 2003 for the discovery of water channels.
Fig. 6.
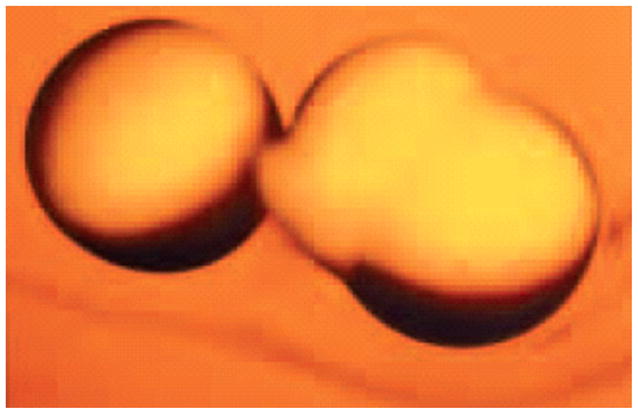
Functional expression of AQP1 water channels on Xenopus laaevis oocytes after 3 min in hypotonic solution. Adapted with permission from Preston et al., 1992.
Fig. 7.
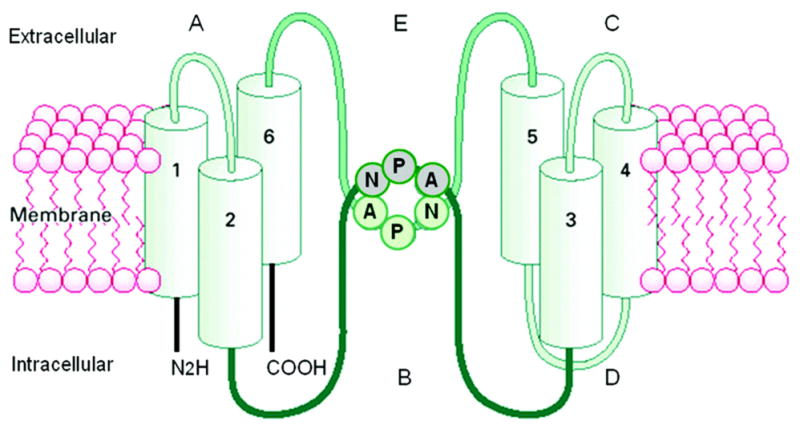
Schematic representation of the antidiuretic hormone, arginine-vasopressin. Adapted with permission from Bichet, 2006. (See Color Plate 41.7 in color plate section.)
Fig. 8.
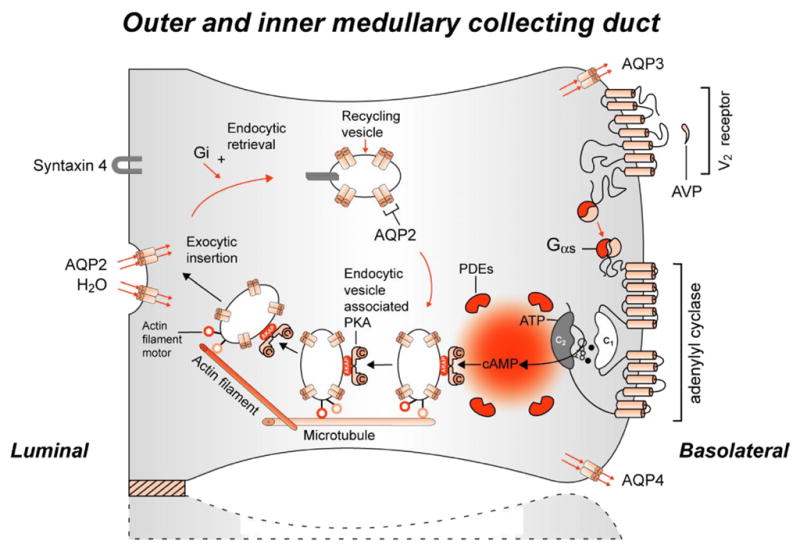
Intracellular action of the antidiuretic hormone, arginine-vasopressin. Adapted with permission from Sands and Bichet, 2006. (See Color Plate 41.8 in color plate section.)
Congenital nephrogenic diabetes insipidus
Mutations in theV2 vasopressin receptor have been found to account for 85–90% of congenital nephrogenic diabetes insipidus (Fig. 9; Sands and Bichet, 2006). The remaining 10–15% of congenital nephrogenic diabetes insipidus is associated with mutations in the AQP2 water channel gene (Fig. 10; Sands and Bichet, 2006). Acquired nephrogenic diabetes insipidus has been shown to be associated with a downregulation of AQP2 in a series of experimental studies from Aarhus, Denmark, including lithium toxicity, hypokalemia, hypercalcemia, ureteral obstruction, ischaemic kidney injury and chronic renal failure (Schrier, 2006).
Fig. 9.
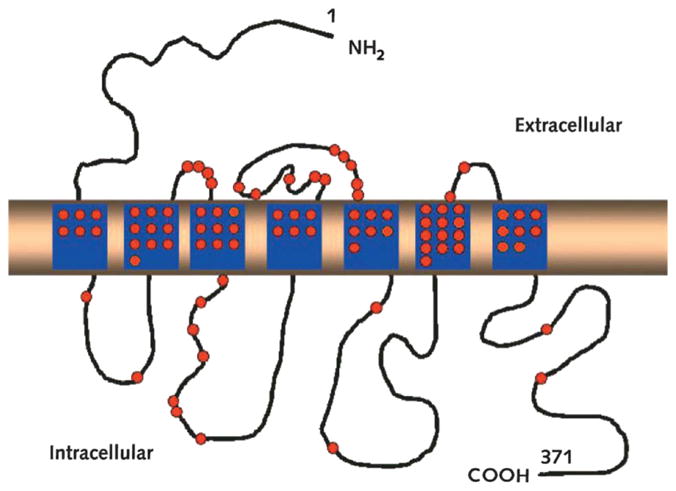
Mutations in V2 receptor causing congenital nephrogenic diabetes insipidus. Adapted with permission from Sands and Bichet, 2006. (See Color Plate 41.9 in color plate section.)
Fig. 10.
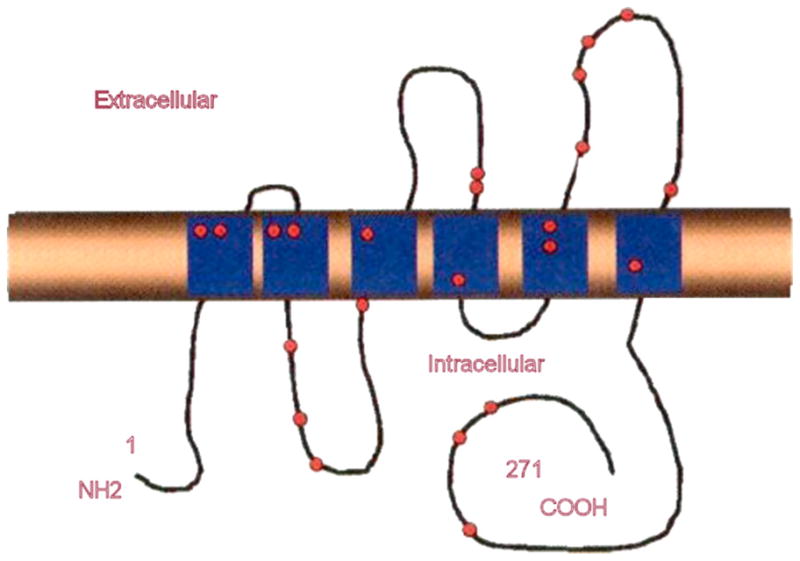
Mutations in AQP2 water channel causing congenital nephrogenic diabetes insipidus. Adapted with permission from Sands and Bichet, 2006. (See Color Plate 41.10 in color plate section).
Primary polydipsia
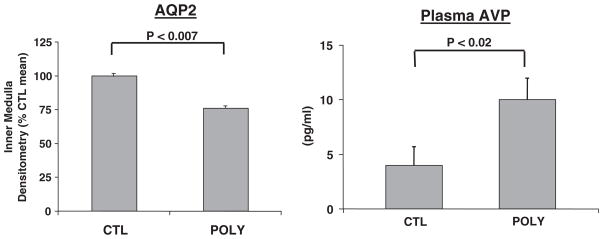
Excessive water drinking in humans has been found to be associated with a diminution in maximal urinary concentration (deWardener and Herxheimer, 1957). The urinary concentrating defect secondary to polydipsia has been reproduced in the rat, thus allowing a molecular analysis of the mechanisms (Cadnapaphornchai et al., 2003b). With fluid restriction in these polyuric rats, maximal urinary osmolality was significantly diminished in spite of no change in the Na-K-2Cl transporter, the initiator of the counter-current concentrating mechanism, or medullary osmolality as compared to control rats’ drinking ad libitum. During fluid deprivation, this concentrating defect was associated with elevated plasma AVP concentrations, and yet diminished AQP2 expression was observed in the polyuric rats as compared to control animals (Fig. 11; Cadnapaphornchai et al., 2003b). Thus, vasopressin-resistant downregulation of AQP2, rather than medullary “washout”, explained the concentrating defect of primary polydipsia.
Fig. 11.
Decreased AQP2 water channel expression in polydipsic rats during fluid restriction. Adapted with permission from Cadnapaphornchai et al., 2003b.
Hypothyroidism
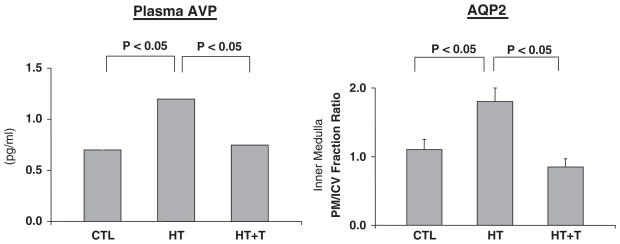
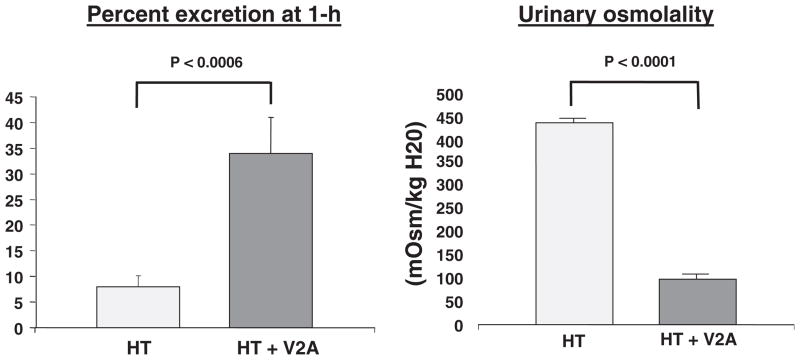
Advanced hypothyroidism, as occurs with myxo-edema, is associated with defects in both urinary concentration and dilution. Moreover, hypothyroid patients have been shown to not suppress plasma vasopressin during an acute water load (Skowsky and Kikuchi, 1978). An experimental hypothyroid rat has been developed which demonstrates a decrease in cardiac output and slower pulse rate compared to euthyroid animals (Chen et al., 2005b). The diminished maximal urinary osmolality during fluid deprivation has been shown to exhibit a decreased expression of the Na-K-2Cl transporter and diminished medullary osmolality as well as downregulation of AQP2 and 3. This occurred in the absence of decreased plasma AVP concentration (Cadnapaphornchai et al., 2003a). The urinary dilution defect during an acute water load was profoundly diminished in the hypothyroid as compared to euthyroid animals. Moreover, the plasma vasopressin concentration and AQP2 expression were significantly increased in the hypothyroid animals (Fig. 12; Chen et al., 2005b). The impaired response to an acute water load in hypothyroid rats was totally reversed including suppression of plasma vasopressin and AQP2 with thyroxin replacement (Fig. 12). The percentage of acute water load excreted and urinary osmolality were normalized with a V2 receptor antagonist (Fig. 13; Chen et al., 2005b).
Fig. 12.
Impaired water excretion in hypothyroidism: role of vasopressin-mediated AQP2. Adapted with permission from Chen et al., 2005b.
Fig. 13.
Reversal of impaired water excretion during hypothyroidism (HT) with V2 receptor antagonist (V2A). Adapted with permission from Chen et al., 2005b.
Glucocorticoid deficiency
Glucocorticoid deficiency occurs with hypopituitarism and may be associated with hyponatremia. Physiological doses of hydrocortisone have been shown to correct the hyponatremia associated with hypopituitarism (Agus and Goldberg, 1971). The hyponatremia has been shown to be mediated by the non-osmotic stimulation of vasopressin (Chen et al., 2005a). Studies have been undertaken in adrenalectomized rats replaced only with mineralocorticoid hormone, as compared to control adrenalectomized rats replaced with both glucocorticoid and miner-alocorticoid hormones (Chen et al., 2005a; Wang et al., 2006). Maximal urinary concentration was diminished in the glucocorticoid deficient rat in association with a significant decrease in Na-K-2Cl co-transporter, medullary osmolality, AQP1 and AQP2 and urea transporter 1 (UTA1) (Chen et al., 2005a). The response to an acute water load was profoundly impaired in association with an increase in plasma vasopressin and AQP2 expression. The administration of a V2 receptor antagonist normalized the defect in urinary dilution and the elevated AQP2 in glucocorticoid-deficient animals (Fig. 14; Wang et al., 2006).
Fig. 14.
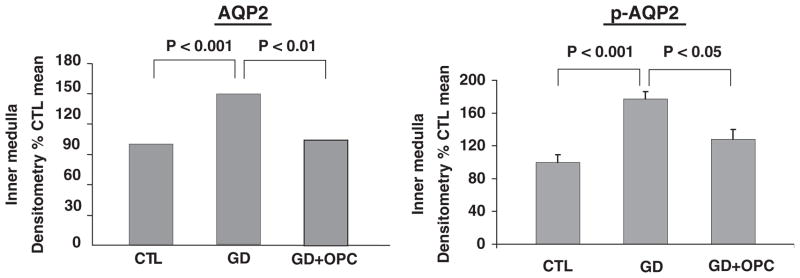
Reversal of impaired water excretion in glucocorticoid deficiency with V2 receptor antagonist (OPC). Adapted with permission from Chen et al., 2005a.
Mineralocorticoid deficiency
In contrast to glucocorticoid deficiency, miner-alocorticoid deficiency is a renal sodium wasting state, which may be associated with renal hyperkalemia and non-anion gap metabolic acidosis. In experimental mineralocorticoid deficiency, the impaired urinary concentrating defect was associated with a diminution in Na-K-2Cl co-transporter and an increase in AQP2 and AQP3 (Ohara et al., 2002). These perturbations were totally reversed by avoiding the negative sodium balance in mineralocorticoid-deficient animals with saline drinking water. The role of non-osmotic vasopressin in the hyponatremia of mineralocorticoid deficiency was supported by the improved urinary dilution with a V2 vasopressin antagonist (Ishikawa and Schrier, 1982).
Cardiac failure
Hyponatremia, independent of treatment, occurs in advanced cardiac failure and is a poor prognostic factor (Lee and Packer, 1986). Plasma vasopressin concentrations have been shown to be increased in hyponatremic heart failure patients (Szatalowicz et al., 1981). In experimental heart failure secondary to coronary ligation rats, an increase in AQP2 expression and membrane trafficking have been demonstrated (Nielsen et al., 1997; Xu et al., 1997). Administration of V2 vasopressin antagonist was shown to increase solute-free water excretion and correct hyponatremia in association with normalizing AQP2 expression and membrane trafficking (Xu et al., 1997). On this background, several V2 vasopressin antagonists have been shown to increase plasma sodium concentration in hyponatremic cardiac failure patients in association with an increase in solute-free water excretion (Gheorghiade et al., 2004) and a decrease in urinary AQP2 excretion (Martin et al., 1999).
Cirrhosis
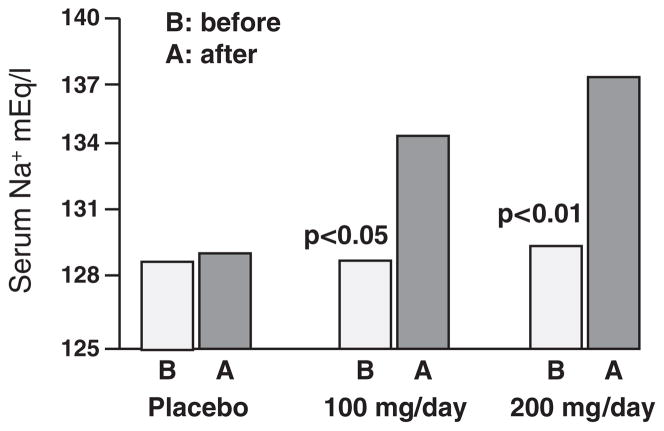
Pretreatment hyponatremia occurs in advanced cirrhosis and is a risk factor for progression to hepatorenal syndrome and mortality (Gines et al., 1993). Plasma vasopressin concentrations are not suppressed in these patients and the response to an acute water load is impaired (Bichet et al., 1982). This finding is compatible with non-osmotic baroreceptor-mediated vasopressin release, secondary to splanchnic arterial vasodilation and thus relative arterial underfilling. In experimental cirrhosis with intraperitoneal CC14, the impaired response to an acute water load is associated with failure to suppress AQP2 message (Asahina et al., 1995). On this background, studies in hyponatremic cirrhotic patients using different V2 vasopressin receptor antagonists have been undertaken (Gerbes et al., 2003; Schrier et al., 2006). In these studies, an increase in electrolyte-free water excretion and an increase in plasma sodium concentration have been observed (Fig. 15; Gerbes et al., 2003).
Fig. 15.
Lixivaptan in hyponatremic cirrhotic patients. Adapted with permission from Gerbes et al., 2003.
Pregnancy
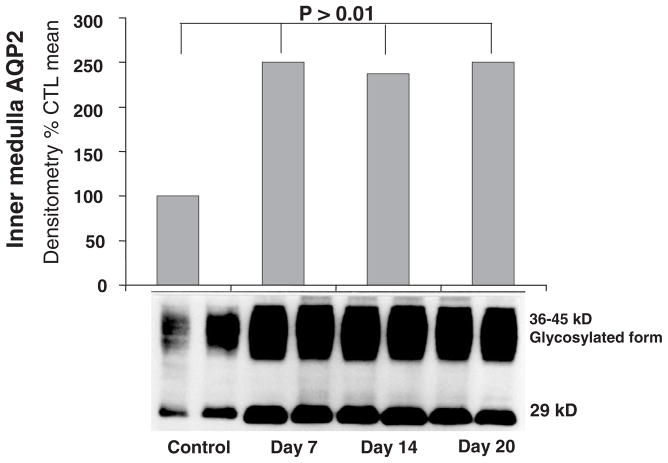
Human pregnancy is associated in the first trimester with arterial vasodilation, and this arterial underfilling is associated with a decrease in plasma osmolality, failure to suppress plasma vasopressin, activation of the renin–angiotensin–aldosterone system and a secondary rise in cardiac output (Chapman et al., 1998). Pregnancy in rats mimics these hormonal and haemodynamics of human pregnancy. In rat pregnancy, a profound increase in renal medullary AQP2 occurs (Fig. 16; Ohara et al., 1998). With a V2 vasopressin receptor antagonist, a reversal of the water retention and increase in electrolyte-free water excretion. In human pregnancy, an increase in urinary AQP2 has been observed (Buemi et al., 2001).
Fig. 16.
Protein expression of inner medullary AQP2 in pregnancy. Adapted with permission from Ohara et al., 1998.
Thus, in several hyponatremic states in humans and experimental animals has been shown to be associated with baroreceptor-mediated non-osmotic release of vasopressin in association with increased renal AQP2 expression and trafficking. These perturbations have been shown to be reversed with V2 vasopressin receptor antagonist.
Acknowledgments
This work has been supported by the NIH Vasopressin Grant at the University of Colorado. The author would like to thank Jan Darling for her editorial support.
References
- Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;265:F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Agus Z, Goldberg M. Role of antidiuretic hormone in the abnormal water diuresis of anterior hypopituitarism in man. J Clin Invest. 1971;50(7):1478–1489. doi: 10.1172/JCI106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina Y, Izumi N, Enomoto N, Sasaki S, Fushimi K, Marumo F, Sato C. Increased gene expression of water channel in cirrhotic rat kidneys. Hepatology. 1995;21:169–173. [PubMed] [Google Scholar]
- Berl T, Cadnapaphornchai P, Harbottle JA, Schrier RW. Mechanism of suppression of vasopressin during alpha-adrenergic stimulation with norepinephrine. J Clin Invest. 1974a;53:219–227. doi: 10.1172/JCI107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl T, Cadnapaphornchai P, Harbottle JA, Schrier RW. Mechanism of stimulation of vasopressin release during beta adrenergic stimulation with isoproterenol. J Clin Invest. 1974b;53:857–867. doi: 10.1172/JCI107626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet DG. Lithium, cyclic AMP signaling, A-kinase anchoring proteins, and aquaporin-2. J Am Soc Nephrol. 2006;17(4):920–922. doi: 10.1681/ASN.2006020135. [DOI] [PubMed] [Google Scholar]
- Bichet D, Szatalowicz V, Chaimovitz C, Schrier RW. Role of vasopressin in abnormal water excretion in cirrhotic patients. Ann Intern Med. 1982;96:413–417. doi: 10.7326/0003-4819-96-4-413. [DOI] [PubMed] [Google Scholar]
- Buemi M, D’Anna R, DiPasquale G, Di Pasquali G, Flocceri F, Ruello A, Aloisi C, Leonardi I, Frisina N, Corica F. Urinary excretion of aquaporin-2 water channel during pregnancy. Cell Physiol Biochem. 2001;11:203–208. doi: 10.1159/000047807. [DOI] [PubMed] [Google Scholar]
- Cadnapaphornchai M, Kim YW, Gurevich A, Summer S, Falk S, Schrier RW. Urinary concentrating defect in hypothyroid rats: role of sodium, potassium, 2-chloride cotransporter and aquaporins. J Am Soc Nephrol. 2003a;14(3):566–574. doi: 10.1097/01.asn.0000053417.33945.63. [DOI] [PubMed] [Google Scholar]
- Cadnapaphornchai MA, Summer S, Falk S, Thurman J, Knepper M, Schrier RW. Effect of primary polydipsia on aquaporin and sodium transporter abundance. Am J Physiol Renal Physiol. 2003b;285:F965–F97l. doi: 10.1152/ajprenal.00085.2003. [DOI] [PubMed] [Google Scholar]
- Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- Chen YC, Cadnapaphomchai MA, Summer S, Falk S, Li C, Wang W, Schrier RW. Molecular mechanisms of impaired urinary concentrating ability in glucocorticoid-deficient rats. J Am Soc Nephrol. 2005a;16:2864–2871. doi: 10.1681/ASN.2004110944. [DOI] [PubMed] [Google Scholar]
- Chen YC, Cadnapaphornchai MA, Yang J, Summer S, Falk S, Li C, Wang W, Schrier RW. Nonosmotic release of vasopressin and renal aquaporins in impaired urinary dilution in hypothyroidism. Am J Physiol Renal Physiol. 2005b;289:F672–F678. doi: 10.1152/ajprenal.00384.2004. [DOI] [PubMed] [Google Scholar]
- deWardener H, Herxheimer A. The effect of a high water intake on the kidney’s ability to concentrate urine in man. J Physiol. 1957;139:42–52. doi: 10.1113/jphysiol.1957.sp005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FL, Brennan TJ, Nelson AE. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973;52:3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Vigneaud V, Gish DT, Katsoyannis PG. A synthetic preparation possessing biological properties associated with arginine vasopressin. J Am Chem Soc. 1954;76:4751–4752. [Google Scholar]
- Fushimi K, Uchida S, Hara K, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature. 1993;361:549–552. doi: 10.1038/361549a0. [DOI] [PubMed] [Google Scholar]
- Gerbes AL, Gulerg V, Gines P, Decauz G, Gross P, Gandjini H, Dijan J VPA Study Group. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology. 2003;124:933–939. doi: 10.1053/gast.2003.50143. [DOI] [PubMed] [Google Scholar]
- Gheorghiade M, Gattis WA, O’Connor CM, Adams KF, Jr, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew FA, Klapholz M, Ouyang J, Orlandi C Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) Investigators. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291:1963–1971. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- Gines A, Escorsell A, Gines P, Salo J, Jimenez W, Inglada L, Navasa M, Claria J, Rimola A, Arroyo V, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Schrier RW. Effect of arginine vasopressin antagonist on renal water excretion in glucocorticoid and mineralocorticoid deficient rats. Kidney Int. 1982;22:587–593. doi: 10.1038/ki.1982.216. [DOI] [PubMed] [Google Scholar]
- Lee W, Packer M. Prognostic importance of serum concentration and its modification by converting-enzyme inhibition in patients with severe chronic heart failure. Circulation. 1986;73:257–267. doi: 10.1161/01.cir.73.2.257. [DOI] [PubMed] [Google Scholar]
- Martin PY, Abraham WT, Xu L, Olson BR, Oren RM, Ohara M, Schrier RW. Selective V2 receptor vasopressin antagonism decreases urinary aquaporin-2 excretion in patients with chronic heart failure. J Am Soc Nephrol. 1999;10:2165–2170. doi: 10.1681/ASN.V10102165. [DOI] [PubMed] [Google Scholar]
- Nielsen S. Renal aquaporins: an overview. BJU Int. 2002;90(Suppl 3):1–6. doi: 10.1046/j.1464-410x.90.s3.1.x. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Terris J, Andersen D, Ecelbarger C, Frokiaer J, Jonassen T, Marples D, Knepper MA, Petersen JS. Congestive heart failure in rats is associated with increased expression and targeting of aquaporin-2 water channel in collecting duct. Proc Natl Acad Sci USA. 1997;94:5450–5455. doi: 10.1073/pnas.94.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara M, Cadnapaphornchai MA, Summer S, Falk S, Yang J, Togawa T, Schrier RW. Effect of mineralocorticoid deficiency on ion and urea transporters and aquaporin water channels in the rat. Biochem Biophys Res Commun. 2002;299:285–290. doi: 10.1016/s0006-291x(02)02634-7. [DOI] [PubMed] [Google Scholar]
- Ohara M, Martin PY, Xu DL, St John J, Pattison T, Kim J, Schrier RW. Upregulation of aquaporin 2 water channel expression in pregnant rats. J Clin Invest. 1998;101:1076–1083. doi: 10.1172/JCI649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston GM, Carooll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;17:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Sands J, Bichet D. Nephrogenic diabetes insipidus. Ann Intern Med. 2006;144:186–194. doi: 10.7326/0003-4819-144-3-200602070-00007. [DOI] [PubMed] [Google Scholar]
- Schrier RW. Body water homeostasis: clinical disorders of urinary dilution and concentration. J Am Soc Nephrol. 2006;17(7):1820–1832. doi: 10.1681/ASN.2006030240. [DOI] [PubMed] [Google Scholar]
- Schrier RW. The sea within us: disorders of body water homeostasis. Curr Opin Invest Drugs. 2007;8(4):304–311. [PubMed] [Google Scholar]
- Schrier RW, Berl T. Mechanism of the antidiuretic effect associated with interruption of parasympathetic pathways. J Clin Invest. 1972;51:2613–2620. doi: 10.1172/JCI107079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RW, Berl T. Mechanism of effect of alpha adrenergic stimulation with norepinephrine on renal water excretion. J Clin Invest. 1974;52:502–511. doi: 10.1172/JCI107207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RW, Berl T, Anderson RJ. Osmotic and non-osmotic control of vasopressin release. Am J Physiol. 1979;236:F321–F322. doi: 10.1152/ajprenal.1979.236.4.F321. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C for the SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Lieberman RA, Ufferman RC. Mechanism of antidiuretic effect of beta adrenergic stimulation. J Clin Invest. 1972;51:97–111. doi: 10.1172/JCI106803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowsky WR, Kikuchi TA. The role of vasopressin in the impaired water excretion of myxedema. Am J Med. 1978;64:613–621. doi: 10.1016/0002-9343(78)90581-8. [DOI] [PubMed] [Google Scholar]
- Szatalowicz V, Arnold P, Chaimovitz C, Bichet D, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305:263–266. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]
- Verney EB. The antidiuretic hormone and the factors which determine its release. Proc R Soc Lond B Biol Sci. 1947;135:27–106. [PubMed] [Google Scholar]
- Wang W, Li C, Summer SN, Falk S, Cadnapaphornchai MA, Chen YC, Schrier RW. Molecular analysis of impaired urinary diluting capacity in glucocorticoid deficiency. Am J Physiol Renal Physiol. 2006;290:F1135–F1142. doi: 10.1152/ajprenal.00356.2005. [DOI] [PubMed] [Google Scholar]
- Xu D, Martin PY, Ohara M, St John J, Pattison T, Meng X, Morris K, Kim JK, Schrier RW. Upregulation of aquaporin-2 water channel expression in chronic heart failure. J Clin Invest. 1997;99:1500–1505. doi: 10.1172/JCI119312. [DOI] [PMC free article] [PubMed] [Google Scholar]