Abstract
The massive amount of human genetic information already available has accelerated the identification of target genes, making gene and nucleic acid therapy the next generation of medicine. Nanoparticle (NP)-based anticancer gene therapy treatment has received significant interest in this evolving field. Recent advances in vector technology have improved gene transfection efficiencies of nonviral vectors to a level similar to viruses. This review serves as an introduction to surface modifications of NPs based on polymeric structural improvements and target moieties. A discussion regarding the future perspective of multifunctional NPs in cancer therapy is also included.
Keywords: gene delivery, cancer therapy, nanoparticle
1. Introduction
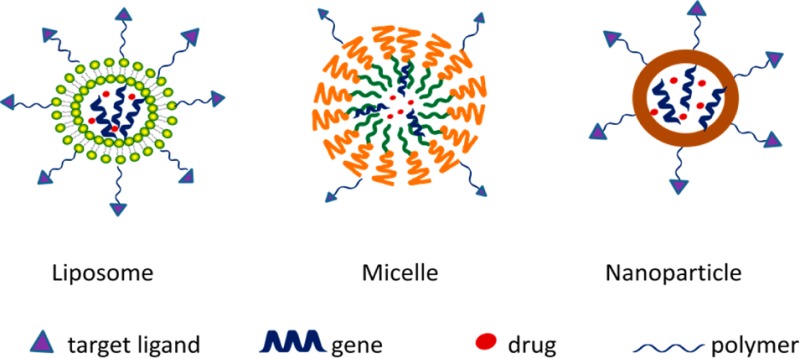
With the morbidity rate increasing over the past few decades, malignant tumors have become the biggest threat to human health. More than 200 different types of cancers have been discovered. More than 10 million cases were diagnosed, and over 7 million people died from cancer in 2008.1 Conventional cancer therapies, including chemotherapy and radiotherapy, are limited by their inability to distinguish malignant from noncancerous organs and tissues. The toxicity of chemotherapy often results in serious side effects accompanied by drug resistance. Modern therapy has been trending toward customized treatments that require high efficiency, specific targeted delivery, and minimal side effects for advanced cancer therapy. Various drug delivery systems, such as liposomes, micelles, vesicles, and nanoparticles, have been developed to improve the bioavailability of chemodrugs and to fulfill efficient drug delivery (Figure 1). The current desires in cancer therapy can be satisfied through the development of new drug delivery systems. With advances in molecular biology and biotechnology as well as the completion of the human genome project, it has been realized that most cancers are caused by genetic mutations, which has given rise to a growing list of genetic disease targets and gene therapy as a potential approach to cancer treatment.
Figure 1.
Various drug delivery systems for drug and gene delivery.
Gene therapy can be defined as the treatment of human disease using the transfer of genetic materials into specific cells of the patient.2 In cancer therapy, the delivered genes can be used in different approaches, such as mutation correction, enhancement of the immune response against tumor cells,3 RNA interference,4 antiangiogenesis, and the production of cytotoxic proteins or prodrug-activating enzymes. Prodrug-activating enzymes alter the expression of existing genes, facilitating a desired cellular or tissue response. Because gene therapy involves the intracellular transfer of nucleic acid drugs, most of them are vulnerable to nucleases, which makes traditional carriers incapable of achieving the expected biological effects. Numerous obstacles emerge during the transportation of the drugs from the site of administration to localization in the cell nucleus. These obstacles include the physical and chemical stability of DNA, the extracellular, cellular, and intracellular biological membranes, uptake by endocytosis, and the necessary escape from endosomal and nuclear localization. Generally, there are two different categories of gene transferring methods depending on the nature of the carrier: gene transfer mediated by viruses and nonviral gene delivery using artificial carriers. While viral vectors are able to mediate gene transfer with high efficiency and long-term gene expression, there are also several limitations. Viral vectors give rise to serious safety concerns, including limit in the size of carried gene, vector antigenicity, inflammation induced by the vector, and possible insertional mutagenesis. Viral vectors are also difficult to produce on a large scale. Nonviral vectors as alternative gene transfer vehicles also have benefits, including a lack of immunogenicity, low toxicity, and potential specific tissue targets.5 However, the gene transfer efficiency of the nonviral vectors has been far below that of the viral vectors.
Ideally, a gene delivery system should be stable, biocompatible, nontoxic, and capable of high transfection efficiency with specificity. Among the various nonviral gene carriers, nanoparticles (NPs) are an ideal platform that incorporates all desirable characteristics into a single gene delivery system. Their nanometer size allows for more efficient vector penetration into the target tissue. NPs also provide unlimited DNA packaging capacity, well-defined physicochemical properties, and a high degree of molecular diversity that allows extensive modifications to overcome extracellular and intracellular barriers.
In this paper, we focus on the factors for novel multifunctional NPs, specifically design, and illustrate their potential application for cancer therapy. The current limitations and toxicological risks are also discussed.
2. Definition of Multifunctional NPs for Gene Therapy
Nanotechnology refers to the creation and application of materials at the nanometer scale. Nanodevices have experienced significant development and improvement since the emergence of nanotechnology. They are being used in a wide array of fields ranging from electronics and communications to chemistry, energy, and biology. Nanomedicine combines nanotechnology with healthcare and has significant promise for medical treatments and therapies in certain areas, such as imaging, faster diagnosis, drug delivery, tissue regeneration, and the development of new medical products. Several NPs are already approved for clinical use, and numerous products are being evaluated in clinical trials.6
Multifunctional NPs combine different functionalities into a single stable construct, which can codeliver multiple components with high delivery efficiency and realize therapy and diagnosis simultaneously. For example, a core particle could be linked to a specific targeting function to recognize the unique surface signatures of their target cells or be modified with an imaging agent to monitor the drug transport process.
Ideally, the vectors appropriate for gene delivery should possess the following merits. First, the vectors have to compact genetic material into particles and protect them from degradation and undesired interactions with the biological environment. Second, the vectors have to be capable of overcoming the extracellular and intracellular barriers to transfer the molecules into the target cells, e.g., endosomal escape and localizing in the nucleus. Third, the vectors should have very little to no toxicity and avoid stimulating the immune system. Other than these properties, the vectors should also be biodegradable and able to induce sustained expression with high transfection efficiency over a defined period of time.
3. Design and Fabrication of Gene Loaded-Multifunctional NPs for Cancer Therapy
The design of a highly efficient multifunctional NP that meets all of the above requirements in a single carrier is an elaborate process that requires multiple steps. First, various functional units are synthesized. These functional units are then procedurally assembled using supramolecular assembly technologies into a nanosystem with a suitable size, controllable structure, and good biocompatibility. The supporting NP cores are mainly modified with biocompatible materials to stabilize the NP and different linkers to achieve target specific delivery.
NPs can be made from a variety of materials, such as compositing polymers, lipids, proteins, metals, or semiconductors with well-defined shapes. NPs can be designed and synthesized using top-down or bottom-up engineering techniques.7,8 The current nanocarrier platforms targeting tumors can be classified into three major categories: organic vectors (e.g., lipid-based NPs and polymer-based NPs), inorganic vectors (e.g., magnetic NPs, gold NPs, and quantum dots) and hybrid vectors (e.g., theranostic NPs that contain both organic and inorganic materials). We list some examples of currently available NP platforms for tumors in Table 1.
Table 1. Examples of NP Platforms for Tumor Gene Delivery.
| materials | type of NPs | main component | main applications | ref |
|---|---|---|---|---|
| organic | liposomes | DSPE,a protein | drug carrier | (9) |
| micelles | PEO-b-PCL | drug carrier | (10) | |
| polymer NPs | Pluronic F127/PEI | drug carrier | (11) | |
| dendrimers | CMCht/PAMAM | drug carrier | (12) | |
| inorganic | gold NPs | gold | drug carrier; photothermal therapy; imaging | (13) |
| silica NPs | polycation-modified mesoporous silica | drug carrier | (14) | |
| magnetic NPs | iron oxide | drug carrier; MRI | (15) | |
| quantum dots | ZnO; PDMAEMA-co-PMAA | drug carrier; real-time imaging | (16) | |
| hybrid | LCPsb | CaPc cores, lipid membranes | drug carrier | (17) |
DSPE: 1,2-dioctadecanoyl-sn-glycero-3-phosphoethanolamine.
LCPs: liposome calcium phosphate NPs.
CaP: calcium phosphate nanoprecipitate.
Although nonviral carriers have many advantages, they nonetheless possess some problems, such as low intracellular gene-transferring efficiencies and transient gene expression. Polymer-based gene delivery systems have attracted significant attention as a means of addressing specificity toward the target cells and the capacity of gene transduction. Among the various polymer materials, the most frequently used strategy is incorporating DNA into condensed particles based on cationic lipids or cationic polymers.18 Under certain conditions, cationic polymers containing several positively charged amine groups in their backbone will interact with negatively charged DNA, leading to the self-assembling and condensing of DNA or RNA into compact NPs. The compact structure of the charge-neutralized core provides excellent protection of the enclosed nucleic acid drugs from nucleases. Once the genes have been wrapped up by polycations, the cores should maintain their stability, allowing for facile cellular uptake and localization inside the cell. Thus, these vectors should inevitably surpass the numerous obstacles encountered from the site of administration to the final localization in the cell nucleus.19 This result means that the carriers should possess efficient gene delivery capacities.
3.1. The Design of Supporting Polymeric Nanocores
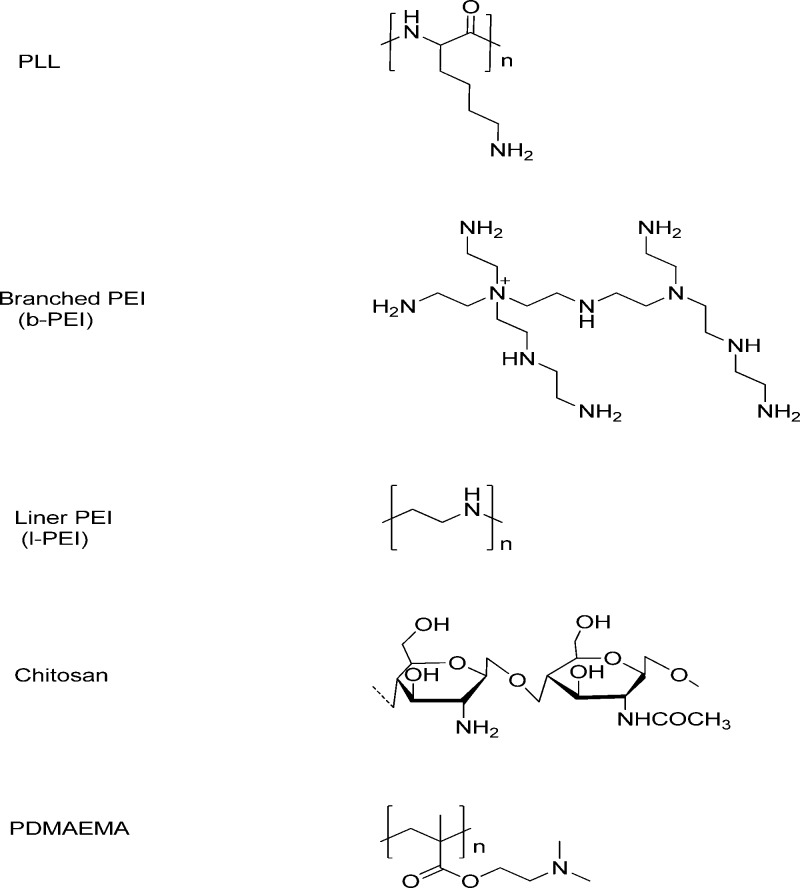
To date, polyethylenimine (PEI),20,21 poly l-lysine (PLL),22 chitosan,23,24 poly(2-N-(dimethylaminoethyl) methacrylate) (pDMAEMA),22,25 and poly(amidoamine) (PAMAM)26,27 are the most frequently used polycations (Figure 2). PLLs can interact with negatively charged DNA through electrostatic forces but with inefficient endosomal escape and transfection. PLL is relatively cytotoxic. Although they are not biodegradable, PEI and PAMAM can mediate endosomal disruption via the proton sponge effect.28 Chitosan is a linear biodegradable polycation, but its application is limited due to its limited transfection efficiency. Among these polymers, PEI is the most studied material due to its strong buffering range from 5.1 to 7.4 along with its high binding capability toward DNA and relatively high transfection efficiency. The primary, secondary, and tertiary amines in the structure of PEI may influence the DNA binding and toxicity. High molecular weight PEIs (branched, 25 kDa) are regarded as the gold standard of gene transfection but often have high toxicities. Low molecular weight (LMW) PEIs (MW < 2000) may be an alternative to reduce toxicity, but they are not satisfactory gene vectors due to their limited efficiency.
Figure 2.
Chemical structures of polycations.
Various modifications have been explored to improve gene delivery efficacy. Polymers are chosen and designed to address one of the perceived barriers during the gene delivery process for the purpose of effective transfection, reduced toxicity, and increased biocompatibility.29,30 Hydrophobic/hydrophilic modifications and the proton sponge effect are the basic strategies for achieving a balance between gene delivery efficacy and toxicity. These strategies help address the key factors involved in gene delivery, such as DNA compacting, cell surface binding and uptake, endosomal and lysosomal escape, localization in the nucleus, and vector unpacking. Therefore, modifications should affect most of the steps involved in the entire gene delivery process.
The modification of the periphery of PEI will enhance the stability of the polyplex. The introduction of hydroxyl groups can markedly improve the serum-tolerant capacity. PEGylation is the most utilized method and creates a hydrophilic exterior that reduces nonspecific interactions with serum components and clearance by phagocytic systems. This method is best known as the stealth effect. However, the “PEG dilemma”, the crucial issue caused by the use of PEG, also reduces gene expression by decreasing the surface charges of the copolymers, which results in disadvantages in terms of controlling intracellular trafficking of cellular uptake and endosomal escape.31,32 A series of PEI derivatives, obtained by treating PEI 25 kDa with tris(hydroxymethyl)acrylamidomethane (THA) via the Michael addition,33 are called PEI-g-THAn (PTns, where n represents the average THA units per PEI molecule). The PTns show lower cytotoxicity and better serum-resistant capacity than PEI25 kDa. Specifically, the transfection efficiency of PT26/DNA is 29-fold higher than that of PEI25 kDa in HeLa cells in serum-containing medium. In vitro flow cytometry analysis shows that the PTns can efficiently mediate the nucleic acids located in the cell. Xiao et al. reported a bioreducible PEI-based/p65 shRNA complex NP used for the treatment of breast cancer.34 In this system, Tween 85 improves the stability of complex NPs in the circulation system and increases cellular uptake by interacting with low-density lipoprotein receptors. The introduction of a disulfide bond guarantees the rapid release of shRNA due to the high concentration of glutathione in the intracellular tumor environment.
Hydrophobic segments that are conjugated to polycations may influence the steps in gene delivery by some of the following mechanisms: (i) increasing the physical encapsulation of genetic materials, (ii) promoting complex charge inversion, (iii) enhancing adsorption to the cell membrane, (iv) alleviating serum inhibition, (v) facilitating gene dissociation from polycation carriers, and (vi) reducing toxicity. Liu et al. reviewed several hydrophobic molecules for PEI modification in 2010,35 including linear alkyl chains of fatty acids (acetate, butanoate, hexanoate, butyric anhydrides, myristate, etc.), conjugates of Pluronic (PPO-PEO and Pluronic-123), and cyclic hydrophobic molecules (cholesteryl chloroformate, aldehyde PEG-cholesterol ether, and dexamethasone mesylate). These modifications are mainly based on the principles that acylation reduces the basicity and availability of free amine groups and the steric hindrance of PEI.36,37
An effective way to hydrolytically or reductively form degradable PEI polymers is through coupling of low molecular weight PEIs. Xun et al. modified PEI 600 with polyesters.38 Briefly, linear biodegradable polyesters with carbon–carbon double bonds were prepared and subsequently appended with PEI 600. Agarose gel retardation and fluorescence quenching assays showed that DNA was completely retarded by these materials at a weight ratio of 0.8. The resulting polyplex sizes were approximately 275 nm, and the zeta-potential values were about +20–35 mV. An MTT assay suggested that the cytotoxicity of these polymers was much lower than that of 25 kDa PEI. In vitro transfection toward 7402, HEK293, and U-2OS cells showed that these novel vectors exhibited much higher transfection efficiencies when compared to 25 kDa PEI, especially in U-2OS cells. The results suggest that biodegradable ester bonds ensure better biocompatibility and lower cytotoxicity. Several cationic polymers derived from PEI 600 linked with poly(amino alcohol esters) have also been introduced as promising nonviral biodegradable vectors.39 Three polymers were prepared by linking PEI 600 with diglycidyl adipate (DA-PEI), diglycidyl succinate (DS-PEI), and diglycidyl oxalate (DO-PEI), respectively. These polymers exhibited good DNA condensing ability with a size of 120–250 nm and zeta-potentials around +10–20 mV, while the weight ratio (polymer/DNA) was from 0.5 to 32. Agarose gel retardation showed that DNA could be released from the polyplexes after being preincubated for 30 h. In vitro experiments found that DS-PEI (weight ratio of 1) showed transfection efficiency approximately 5 times higher than the PEI in A549 cells. Meanwhile, the cytotoxicity of these three diglycidyl PEIs assayed using MTT is lower than that of 25 kDa PEI in HEK293 cells.
Adding low molecular weight PEIs onto biocompatible polymers can significantly improve the biocompatibility of PEIs. The most frequently used polymers include cyclodextrin,40−43 chistosan,44−46 polycarbonate,47 and dextrans.48,49 These materials are excellent candidates for PEI modification due to their biocompatibility, biodegradability, and low toxicity. The amphipathic PEIs also play an important role in gene transfection. Amphipathic deoxycholic acid (DA)-modified polyethylenimine (PEI 1.8) (DA-PEI 1.8) was found to have a high membrane permeability, enhancing both cellular internalization and target gene silencing.50 This PEI has been conjugated with hydrophobic polylactide (PLA) to form amphiphilic PEI for the construction of NPs.51 PEI-PCL (polycaprolactone) uses amphiphilic diblock copolymers, which assemble as biodegradable nanocarriers for codelivery of BCL-2 siRNA and doxorubicin (DOX) and modified with folic acid as the targeted unit.52 A series of experiments showed that the hierarchical nanoassembly platform was effective for siRNA and hydrophobic drug codelivery. Furthermore, in vivo transfection was observed using an in situ rat C6 glioma model, and the animal study showed that the folate-targeted PEI-PCL multifunctional nanoparticles could inhibit tumor growth and prolong the rat survival time.53
3.2. The Specific Design Strategies of Nanocarriers for Cancer Therapy
The complexity of tumorigenesis, the heterogeneity of cancer cells, and various physiological barriers have been the biggest obstacles in cancer therapy. Microenvironment changes around the tumor cells are complicated. Changes such as increased interstitial fluid pressure caused by leaky vasculature, increased acidity among tumors, poor lymphatic drainage, and a high density of stroma and cells significantly impede drug penetration and gradually induce drug resistance. Additionally, most cancers are caused by gene mutations or loss of function. Therefore, the combined delivery of drugs and genes has emerged as an exciting method for treating cancer. Combined delivery possesses a synergistic effect that can increase drug efficacy or enhance gene transfection efficiency. However, one of the most important challenges for highly efficient delivery is the specificity of the delivery systems. Thus, targeting moieties on the surface of a nanosystem have been developed to meet these limitations.
The mechanism for NP targeting can be either passive or active (Figure 3). Most NPs are expected to accumulate in tumors due to incomplete tumor vasculature. Tumors also tend to retain compounds, especially macromolecules, more than normal tissues. These pathophysiologic characteristics contribute to the enhanced permeability and retention effect54−56 and form the basis of passive targeting. However, passive targeting suffers from several limitations, e.g., the PEG dilemma. Attaching specific moieties on the NP surfaces can effectively improve the binding affinity toward target cells and help overcome these limitations. The targeting moieties can be classified as therapeutic agents, diagnostic agents, or barrier-avoiding agents according to their functions. These materials can also be categorized by their composition, i.e., proteins (mainly antibodies and their fragments), peptides, aptamers, small molecules, or others (vitamins or carbohydrates).
Figure 3.
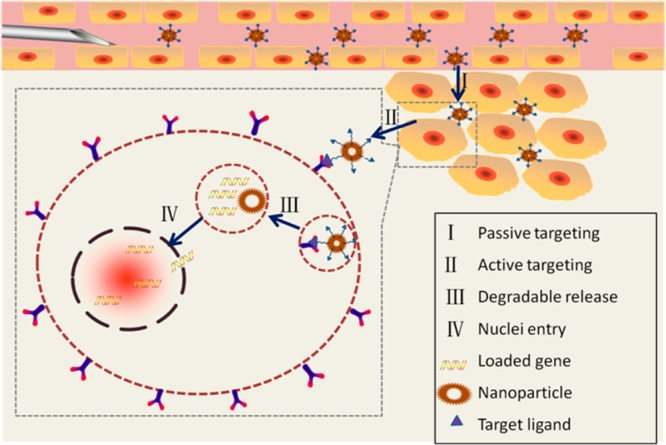
Targeting mechanism of gene delivery in nanoparticle systems.
Multiple gene mutations or abnormalities of gene expression can lead to the development of tumors. To effectively treat the broad spectrum of gene mutations and abnormalities, different gene therapy approaches are used. Some examples of these are mutation correction, immune response enhancement, RNA interference, targeted lysis of tumor cells using selective replicative viruses, antiangiogenesis, suicide gene transfer, and bone marrow protection using drug resistance genes.57,58 Each type of cancer has its own specific characteristics. For example, the brain is protected by the blood–brain barrier (BBB), which provides a barrier for brain tumors, referring to a heterogeneous group of primary and metastatic neoplasms in the central nervous system. The BBB is composed of tight junctions between endothelial cells, pericytes, abasement membrane, and the feet of astrocytes. Additionally, the ATP-binding cassette transporters, such as P-glycoprotein, are highly expressed in the brain. Additionally, the blood–cerebrospinal fluid barrier and the blood–tumor barrier act as a second and third barrier, respectively. Therefore, targeted design in gene delivery should take these factors into consideration.
Prostate stem cell antigen (PSCA) is a prostate-specific glycosyl phosphatidylinositol-anchored glycoprotein, which can be used for prostate cancer targeted imaging and therapy.59,60 In approximately 25% of non-small-cell lung cancer patients, c-Met, and the hepatocyte growth factor (HGF) receptor, were abundantly expressed. Wu et al. site-direct conjugated anti-c-Met antibody via cystine residues with liposomal and dual targeting properties. The inhibition of tumor growth and prevention of angiogenesis were observed for c-Met expression in angiogenic endothelium and tumor cells.61,62 Chlorotoxin (CLTx) is a 36 amino acid peptide, which can permeate intact BBB and has a strong affinity for tumors of neuroectodermal origin.63 Zhang et al. developed an in vivo brain tumor targeting magnetic/optical nanoprobe based on CLTx. In vivo MRI contrast enhancement and optical imaging were used for evaluation and found that this multifunctional platform may be further developed for brain tumor targeted therapeutic NP systems.64 Tumor-associated underglycosylated mucin-1 (uMUC-1) antigen is overexpressed in more than 90% of breast cancers. Medarova et al. have bound nanodrugs with uMUC-1 targeting EPPT synthetic peptides for selective breast tumor targeting.65,66 HER-2 is another overexpressed gene commonly found in some tumors, such as breast and ovarian. Herceptin (HER), a monoclonal antibody able to selectively recognize HER-2, was used by Mattu et al. as the targeting unit when modifying biodegradable NPs through hydrophilic/hydrophobic interactions for breast cancer therapy.67 Due to differing gene expression levels in various types of breast cancer cells, Chen et al. utilized HER in a different approach. They prepared PEO-b-PγMPS-coated magnetic iron oxide NPs (IONPs) before further conjugating HER or a single chain fragment (ScFv) of an antibody against epidermal growth factor receptor to PEO-b-PγMPS-coated IONPs. These two types of NPs could bind specifically to different types of breast cancer cells and enable active receptor-targeted imaging of xenografted breast tumors in nude mice using MRI.68
3.3. Other Factors That Influence the Therapeutic Effectiveness of Multifunctional NPs
Efforts have been made to develop less toxic and more biodegradable materials for vectors. Targeting moieties have been added to the surface of NPs for tumor cell recognition. Studies have revealed that some properties of NPs, such as the small size, large surface area, and geometry, could be partially responsible for their potential hazard to human health. Because of these factors, the size and concentration should be carefully controlled according to the NP platform. Several studies have reported that there is an inverse relationship between quantum dot size and concentration and the adverse effects of NPs: smaller sizes and higher concentrations are more cytotoxic.69,70 Geometry also influences the NP toxicity, especially in carbon nanomaterials, with single-walled nanotubes being the most toxic and nano-60 fullerenes the least toxic.71,72 Unfortunately, surface modification also seems to have a role in the cytotoxicity. Studies with quantum dots and gold NPs have indicated that toxicity varies depending on the nature of the surface coating applied to the NP.72−74
Not only do variations in NP characteristics induce toxicity but the density of non-biofouling moieties and targeting ligands on the surfaces of NPs can also influence their efficacy.56 Shielding materials, such as PEG and polysaccharide dextrans, provide a steric barrier that prevents nonspecific protein absorption and modifies the surface properties of the NPs to avoid recognition based on the RES.75 Few studies have reported that targeting ligands on the surface of NPs must be present at concentrations that exceed a minimum threshold for effective binding.76 In contrast, some studies insist that high ligand densities can promote nonspecific interactions with endothelial and other noncancerous cells, which increases immunogenicity, thereby causing opsonization-mediated clearance of the NPs.77 Therefore, the optimization of the density of the modification moieties plays a critical role in NP fabrication.
4. Conclusions and Future Perspectives
The advantages provided by NPs have led to an accelerated development of gene therapy for cancer in the past decades. The emergence of various biocompatible materials and the development of gene technology have deepened our understanding and enabled us to develop multifunctional NPs for tumor diagnosis and therapy. The ultimate goal of multifunctional NPs is to enhance patient survival and improve quality of life, especially for multidrug resistance patients. The materials chosen as vectors, preparation methods, and modification strategies should all be taken into consideration when attempting to achieve the maximization of the therapeutic efficacy in a NP system. Despite enormous efforts, multifunctional NPs have not yet met the standard requirements to achieve clinical significance.
Currently, the codelivery of genes and chemotherapeutics has been proposed as an exciting method for treating cancer. This method possesses a synergistic effect that can increase drug efficacy or enhance gene transfection, which in turn increases the efficiency of cancer treatment and prolongs the survival time of cancer patients. A similar concept, hybrid NPs, has been introduced and rapidly developed. Hybrid NPs combine different NP platforms into one system as a potential theranostic platform. This platform offers noninvasive visualization of drug distribution and accumulation at target sites, real-time monitoring of therapeutic responses, and individualized dosing regimens.78−80 Theranostic systems usually combine MRI and optical imaging with therapeutics. The high-resolution image can help accurately determine a clear tumor boundary by eye and recognize the pseudoprogression after radiotherapy and antiangiogenesis therapies.81,82 Theranostic NPs will open even more opportunities to create innovative NPs for tumor therapy.
Apoptosis, or programmed cell death, is widely associated with its important role in cancer therapy.83 Therefore, many novel approaches for successful treatment of cancers have been established through targeted pro-apoptotic therapeutic protocols and the development of apoptosis-inducing drugs that target the tumor without causing severe impairment to the normal tissue.84 The genes for clinical cancer therapy that target apoptotic machinery mainly include TNF-α, TRAIL, caspase-9, Bik, Bcl-2, and XIAP. The apoptotic machinery can be targeted via the introduction of a gene encoding an inducer, mediator, or executioner of apoptotic cell death or by inhibiting antiapoptotic gene expression. These methods have significant potential for efficient and specific gene delivery and administration systems.
The evaluation of risks associated with exposure to NPs is not well studied, and the present results are inconclusive. It has been suggested that NPs affect biological behaviors at the cellular, subcellular, protein, and gene levels, but other claims indicate that NPs are biologically inert materials and therefore are safe for in vivo application. Nanotoxicology has emerged to investigate the safety of nanotechnologies, but only limited statistics have been obtained so far. The regulatory issues surrounding NPs are often unclear and difficult to navigate, adding another hindrance to the field of nanotoxicology. The inherent complexity of NP systems establishes a need for specific regulations and official guidelines.71,85,86
The unique properties of multifunctional NPs allow for the selection of the best possible combination of factors for maximum effectiveness. There is still a long way to go before multifunctional NPs can be used in clinical applications for cancer therapy.
Acknowledgments
The Huang lab has been supported by NIH Grants CA149363, CA151652, DK100664, and CA149387. We thank Andrew Blair for editing the manuscript. This work was also supported by the National Natural Science Foundation of China (Grant No. 21203112) and the Natural Science Foundation of Shandong Province (Grant No. ZR2012BQ002).
Supporting Information Available
An abbreviation table of the materials. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Jemal A.; Center M. M.; DeSantis C.; Ward E. M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomarkers Prev. 2010, 1981893–1907. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science 1993, 260, 926–932. [DOI] [PubMed] [Google Scholar]
- U J.; Donnelly J. J.; Shiver J. W.; Liu M. A. DNA vaccines. Annu. Rev. Immunol. 1997, 15, 617–648. [DOI] [PubMed] [Google Scholar]
- Fire A.; Xu S. Q.; Montgomery M. K.; Kostas S. A.; Driver S. E.; Mello C. C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [DOI] [PubMed] [Google Scholar]
- Davis M. E. Non-viral gene delivery systems. Curr. Opin Biotechnol. 2002, 13, 128–131. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Gu F. X.; Chan J. M.; Wang A. Z.; Langer R. S.; Farokhzad O. C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [DOI] [PubMed] [Google Scholar]
- Rajasekhar A.; Gimi B.; Hu W. Applications of Semiconductor Fabrication Methods to Nanomedicine: A Review of Recent Inventions and Techniques. Recent Pat. Nanomed. 2013, 319–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesena R. N.; Tissera N.; Kannangara Y. Y.; Lin Y.; et al. A method for top down preparation of chitosan nanoparticles and nanofibers. Carbohydr. Polym. 2014, 10.1016/j.carbpol/2012/10.055. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Zhang L.; Guo S.; Hatefi A.; Huang L. Incorporation of histone derived recombinant protein for enhanced disassembly of core-membrane structured liposomal nanoparticles for efficient siRNA delivery. J. Controlled Release 2013, 1721179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X. B.; Lavasanifar A. Traceable Multifunctional Micellar Nanocarriers for Cancer-Targeted Co-delivery of MDR-1 siRNA and Doxorubicin. ACS Nano 2011, 5, 5202–5213. [DOI] [PubMed] [Google Scholar]
- Li N.; Yang X. G.; Zhai G. X.; Li L. B. Multifunctional pluronic/poly(ethylenimine) nanoparticles for anticancer drug. J. Colloid Interface Sci. 2010, 350, 117–125. [DOI] [PubMed] [Google Scholar]
- Cerqueira S. R.; Silva B. L.; Oliveira J. M.; Mano J. F.; Sousa N.; Salgado A. J.; Reis R. L. Multifunctionalized CMCht/PAMAM Dendrimer Nanoparticles Modulate the Cellular Uptake by Astrocytes and Oligodendrocytes in Primary Cultures of Glial Cells. Macromol. Biosci. 2012, 12, 591–597. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Meyers J. D.; Agnes R. S.; Doane T. L.; Kenney M. E.; Broome A. M.; Burda C.; Basilion J. P. Addressing Brain Tumors with Targeted Gold Nanoparticles: A New Gold Standard for Hydrophobic Drug Delivery?. Small 2011, 7, 2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai S. R.; Muthuswamy E.; Wani A.; Brichacek M.; Castaneda A. L.; Brock S. L.; Oupicky D. Enhanced Gene and siRNA Delivery by Polycation-Modified Mesoporous Silica Nanoparticles Loaded with Chloroquine. Pharm. Res. 2010, 27, 2556–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit F. M.; Wang F. Y.; Fang C.; Mok H.; Wang K.; Silber J. R.; Ellenbogen R. G.; Zhang M. Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J. Controlled Release 2011, 152176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Liu W. G. ZnO QD@PMAA-co-PDMAEMA nonviral vector for plasmid DNA delivery and bioimaging. Biomaterials 2010, 31, 3087–3094. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Haynes M. T.; Wang Y.; Liu F.; Huang L. A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano 2013, 765376–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.; Kim K. S.; Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007, 9, E92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethoff C. M.; Middaugh C. R. Barriers to nonviral gene delivery. J. Pharm. Sci. 2003, 92, 203–217. [DOI] [PubMed] [Google Scholar]
- Kircheis R.; Wightman L.; Wagner E. Design and gene delivery activity of modified polyethylenimines. Adv. Drug Delivery Rev. 2001, 53, 341–358. [DOI] [PubMed] [Google Scholar]
- Liu C. X.; Liu F. X.; Feng L. X.; Li M.; Zhang J.; Zhang N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials 2013, 34, 2547–2564. [DOI] [PubMed] [Google Scholar]
- Park T. G.; Jeong J. H.; Kim S. W. Current status of polymeric gene delivery systems. Adv. Drug Delivery Rev. 2006, 58, 467–486. [DOI] [PubMed] [Google Scholar]
- Liu Z. H.; Jiao Y. P.; Wang Y. F.; Zhou C. R.; Zhang Z. Y. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Delivery Rev. 2008, 60, 1650–1662. [DOI] [PubMed] [Google Scholar]
- Kim T. H.; Jiang H. L.; Jere D.; Park I. K.; Cho M. H.; Nah J. W.; Choi Y. J.; Akaike T.; Cho C. S. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog. Polym. Sci. 2007, 32, 726–753. [Google Scholar]
- van de Wetering P.; Cherng J. Y.; Talsma H.; Crommelin D. J. A.; Hennink W. E. 2-(dimethylamino)ethyl methacrylate based (co)polymers as gene transfer agents. J. Controlled Release 1998, 53, 145–153. [DOI] [PubMed] [Google Scholar]
- Choi J. S.; Nam K.; Park J.; Kim J. B.; Lee J. K.; Park J. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with L-arginine. J. Controlled Release 2004, 99, 445–456. [DOI] [PubMed] [Google Scholar]
- B H.; Tomalia D. A.; Dewald J. R.; Hall M.; Kallos G.; Martin S.; et al. A new class of polymers: starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar]
- Varkouhi A. K.; Scholte M.; Storm G.; Haisma H. J. Endosomal escape pathways for delivery of biologicals. J. Controlled Release 2011, 151, 220–228. [DOI] [PubMed] [Google Scholar]
- Hara Y.; Maeda S.; Hashimoto S.; Yoshida R. Molecular design and functional control of novel self-oscillating polymers. Int. J. Mol. Sci. 2010, 11, 704–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P. P.; Sharma V. Synthetic polymeric vectors in gene therapy. Curr. Opin. Solid State Mater. Sci. 2008, 12, 89–102. [Google Scholar]
- Hatakeyama H.; Akita H.; Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: A strategy for overcoming the PEG dilemma. Adv. Drug Delivery Rev. 2011, 63, 152–160. [DOI] [PubMed] [Google Scholar]
- Morille M.; Passirani C.; Vonarbourg A.; Clavreul A.; Benoit J. P. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 2008, 29, 3477–3496. [DOI] [PubMed] [Google Scholar]
- Dong X.; Lin L.; Chen J.; Guo Z. P.; Tian H. Y.; Li Y. C.; Wei Y.; Chen X. S. A Serum-Tolerant Hydroxyl-Modified Polyethylenimine as Versatile Carriers of pDNA/siRNA. Macromol. Biosci. 2013, 13, 512–522. [DOI] [PubMed] [Google Scholar]
- Xiao J. S.; Duan X. P.; Yin Q.; Miao Z. H.; Yu H. J.; Chen C. Y.; Zhang Z. W.; Wang J.; Li Y. P. The inhibition of metastasis and growth of breast cancer by blocking the NF-kappa B signaling pathway using bioreducible PEI-based/p65 shRNA complex nanoparticles. Biomaterials 2013, 34, 5381–5390. [DOI] [PubMed] [Google Scholar]
- Liu Z. H.; Zhang Z. Y.; Zhou C. R.; Jiao Y. P. Hydrophobic modifications of cationic polymers for gene delivery. Prog. Polym. Sci. 2010, 35, 1144–1162. [Google Scholar]
- Masotti A.; Moretti F.; Mancini F.; Russo G.; Di Lauro N.; Checchia P.; Marianecci C.; Carafa M.; Santucci E.; Ortaggi G. Physicochemical and biological study of selected hydrophobic polyethylenimine-based polycationic liposomes and their complexes with DNA. Bioorg. Med. Chem. 2007, 15, 1504–1515. [DOI] [PubMed] [Google Scholar]
- Neamnark A.; Suwantong O.; Bahadur K. C. R.; Hsu C. Y. M.; Supaphol P.; Uludag H. Aliphatic Lipid Substitution on 2 kDa Polyethylenimine Improves Plasmid Delivery and Transgene Expression. Mol. Pharmaceutics 2009, 6, 1798–1815. [DOI] [PubMed] [Google Scholar]
- Xun M. M.; Liu Y. H.; Guo Q.; Zhang J.; Zhang Q. F.; Wu W. X.; Yu X. Q. Low molecular weight PEI-appended polyesters as non-viral gene delivery vectors. Eur. J. Med. Chem. 2014, 78, 118–125. [DOI] [PubMed] [Google Scholar]
- Li S.; Wang Y.; Zhang J.; Yang W. H.; Dai Z. H.; Zhu W.; Yu X. Q. Biodegradable cross-linked poly(amino alcohol esters) based on LMW PEI for gene delivery. Mol. Biosyst. 2011, 7, 1254–1262. [DOI] [PubMed] [Google Scholar]
- Tang G. P.; Guo H. Y.; Alexis F.; Wang X.; Zeng S.; Lim T. M.; Ding J.; Yang Y. Y.; Wang S. Low molecular weight polyethylenimines linked by beta-cyclodextrin for gene transfer into the nervous system. J. Gene Med. 2006, 8, 736–744. [DOI] [PubMed] [Google Scholar]
- Lai W. F.; Tang G. P.; Wang X.; Li G.; Yao H.; Shen Z.; Lu G.; Poon W. S.; Kung H. F.; Lin M. C. Cyclodextrin-PEI-Tat Polymer as a Vector for Plasmid DNA Delivery to Placenta Mesenchymal Stem Cells. BioNanoScience 2011, 1, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L. H.; Wei W.; Qi X. T.; Shan Y. H.; Zhang F. J.; Chen X.; Zhu Q. Y.; Yu L.; Liang W. Q.; Gao J. Q. Epidermal stem cells manipulated by pDNA-VEGF165/CYD-PEI nanoparticles loaded gelatin/beta-TCP matrix as a therapeutic agent and gene delivery vehicle for wound healing. Mol. Pharmaceutics 2013, 10, 3090–3102. [DOI] [PubMed] [Google Scholar]
- Shen J.; Kim H. C.; Su H.; Wang F.; Wolfram J.; Kirui D.; Mai J.; Mu C.; Ji L. N.; Mao Z. W.; Shen H. Cyclodextrin and Polyethylenimine Functionalized Mesoporous Silica Nanoparticles for Delivery of siRNA Cancer Therapeutics. Theranostics 2014, 4, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.; Dai Y.; Lv L.; Zhao H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS One 2014, 9, e84703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. L.; Kwon J. T.; Kim Y. K.; Kim E. M.; Arote R.; Jeong H. J.; Nah J. W.; Choi Y. J.; Akaike T.; Cho M. H.; Cho C. S. Galactosylated chitosan-graft-polyethylenimine as a gene carrier for hepatocyte targeting. Gene Ther. 2007, 14, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Jiang H. L.; Kim Y. K.; Arote R.; Nah J. W.; Cho M. H.; Choi Y. J.; Akaike T.; Cho C. S. Chitosan-graft-polyethylenimine as a gene carrier. J. Controlled Release 2007, 117, 273–280. [DOI] [PubMed] [Google Scholar]
- Seow W. Y.; Yang Y. Y. Functional polycarbonates and their self-assemblies as promising non-viral vectors. J. Controlled Release 2009, 139, 40–47. [DOI] [PubMed] [Google Scholar]
- Tseng W. C.; Jong C. M. Improved stability of polycationic vector by dextran-grafted branched polyethylenimine. Biomacromolecules 2003, 4, 1277–1284. [DOI] [PubMed] [Google Scholar]
- Jiang D.; Salem A. K. Optimized dextran-polyethylenimine conjugates are efficient non-viral vectors with reduced cytotoxicity when used in serum containing environments. Int. J. Pharm. 2012, 427, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.; Lee D.; Jang Y. L.; Chae S. Y.; Choi D.; Jeong J. H.; Kim S. H. Facial amphipathic deoxycholic acid-modified polyethyleneimine for efficient MMP-2 siRNA delivery in vascular smooth muscle cells. Eur. J. Pharm. Biopharm. 2012, 81, 14–23. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Cao W.; Li S.; Jin S.; Hu K.; Hu L.; Huang Y.; Gao X.; Wu Y.; Liang X. J. Ultrabright and multicolorful fluorescence of amphiphilic polyethyleneimine polymer dots for efficiently combined imaging and therapy. Sci. Rep. 2013, 3, 3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao N.; Cheng D.; Zou S.; Ai H.; Gao J.; Shuai X. The synergistic effect of hierarchical assemblies of siRNA and chemotherapeutic drugs co-delivered into hepatic cancer cells. Biomaterials 2011, 32, 2222–2232. [DOI] [PubMed] [Google Scholar]
- Cheng D.; Cao N.; Chen J.; Yu X.; Shuai X. Multifunctionl nanocarrier mediated co-delivery of doxorubicin and siRNA for synergistic enhancement of glioma apoptosis in rat. Biomaterials 2012, 33, 1170–1179. [DOI] [PubMed] [Google Scholar]
- Hobbs S. K.; Monsky W. L.; Yuan F.; Roberts W. G.; Griffith L.; Torchilin V. P.; et al. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. U.S.A. 1998, 9584607–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. M.; Cullis P. R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 30356651818–1822. [DOI] [PubMed] [Google Scholar]
- Yu M. K.; Park J.; Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 213–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S.; Ito Y.; Hatake K.; Sugimoto Y. Gene therapy for breast cancer. --Review of clinical gene therapy trials for breast cancer and MDR1 gene therapy trial in Cancer Institute Hospital. Breast Cancer 2006, 13, 8–15. [DOI] [PubMed] [Google Scholar]
- Vassaux G.; Martin-Duque P. Use of suicide genes for cancer gene therapy: study of the different approaches. Expert Opin. Biol. Ther. 2004, 4, 519–530. [DOI] [PubMed] [Google Scholar]
- Wente M. N.; Jain A.; Kono E.; Berberat P. O.; Giese T.; Reber H. A.; Friess H.; Buchler M. W.; Reiter R. E.; Hines O. J. Prostate stem cell antigen is a putative target for immunotherapy in pancreatic cancer. Pancreas 2005, 31, 119–125. [DOI] [PubMed] [Google Scholar]
- Ling Y.; Wei K.; Luo Y.; Gao X.; Zhong S. Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles for both targeting magnetic resonance imaging and cancer therapy. Biomaterials 2011, 32, 7139–7150. [DOI] [PubMed] [Google Scholar]
- Lu R. M.; Chang Y. L.; Chen M. S.; Wu H. C. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials 2011, 32, 3265–3274. [DOI] [PubMed] [Google Scholar]
- Yu M. K.; Park J.; Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons S. A.; O’Neal J.; Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia 2002, 39, 162–173. [DOI] [PubMed] [Google Scholar]
- Veiseh O.; Sun C.; Fang C.; Bhattarai N.; Gunn J.; Kievit F.; Du K.; Pullar B.; Lee D.; Ellenbogen R. G.; Olson J.; Zhang M. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009, 69, 6200–6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.; Yigit M.; Dai G.; Moore A.; Medarova Z. Image-guided breast tumor therapy using a small interfering RNA nanodrug. Cancer Res. 2010, 70, 7553–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perey L.; Hayes D. F.; Maimonis P.; Abe M.; O’Hara C.; Kufe D. W. Tumor selective reactivity of a monoclonal antibody prepared against a recombinant peptide derived from the DF3 human breast carcinoma-associated antigen. Cancer Res. 1992, 52, 2563–2568. [PubMed] [Google Scholar]
- Mattu C.; Pabari R. M.; Boffito M.; Sartori S.; Ciardelli G.; Ramtoola Z. Comparative evaluation of novel biodegradable nanoparticles for the drug targeting to breast cancer cells. Eur. J. Pharm. Biopharm. 2013, 85, 463–472. [DOI] [PubMed] [Google Scholar]
- Chen H.; Wang L.; Yu Q.; Qian W.; Tiwari D.; Yi H.; Wang A. Y.; Huang J.; Yang L.; Mao H. Anti-HER2 antibody and ScFvEGFR-conjugated antifouling magnetic iron oxide nanoparticles for targeting and magnetic resonance imaging of breast cancer. Int. J. Nanomed. 2013, 8, 3781–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Chen W.; Zhang J.; Liu J.; Chen G.; Pope C. In vitro and in vivo toxicity of CdTe nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 497–503. [DOI] [PubMed] [Google Scholar]
- Kirchner C.; Liedl T.; Kudera S.; Pellegrino T.; Munoz Javier A.; Gaub H. E.; Stolzle S.; Fertig N.; Parak W. J. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005, 5, 331–338. [DOI] [PubMed] [Google Scholar]
- Sanvicens N.; Marco M. P. Multifunctional nanoparticles--properties and prospects for their use in human medicine. Trends Biotechnol. 2008, 26, 425–433. [DOI] [PubMed] [Google Scholar]
- Jia G.; Wang H.; Yan L.; Wang X.; Pei R.; Yan T.; Zhao Y.; Guo X. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [DOI] [PubMed] [Google Scholar]
- Hoshino A.; Manabe N.; Fujioka K.; Suzuki K.; Yasuhara M.; Yamamoto K. Use of fluorescent quantum dot bioconjugates for cellular imaging of immune cells, cell organelle labeling, and nanomedicine: surface modification regulates biological function, including cytotoxicity. J. Artif. Organs 2007, 10, 149–157. [DOI] [PubMed] [Google Scholar]
- Connor E. E.; Mwamuka J.; Gole A.; Murphy C. J.; Wyatt M. D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [DOI] [PubMed] [Google Scholar]
- Knop K.; Hoogenboom R.; Fischer D.; Schubert U. S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem., Int. Ed. 2010, 49, 6288–6308. [DOI] [PubMed] [Google Scholar]
- Olivier V.; Meisen I.; Meckelein B.; Hirst T. R.; Peter-Katalinic J.; Schmidt M. A.; Frey A. Influence of targeting ligand flexibility on receptor binding of particulate drug delivery systems. Bioconjugate Chem. 2003, 14, 1203–1208. [DOI] [PubMed] [Google Scholar]
- Ferrari M. Nanogeometry: beyond drug delivery. Nat. Nanotechnol. 2008, 3, 131–132. [DOI] [PubMed] [Google Scholar]
- Pene F.; Courtine E.; Cariou A.; Mira J. P. Toward theragnostics. Crit. Care Med. 2009, 37, S50–58. [DOI] [PubMed] [Google Scholar]
- Janib S. M.; Moses A. S.; MacKay J. A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Delivery Rev. 2010, 62, 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Feng L.; Liu T.; Zhang L.; Yao Y.; Yu D.; Wang L.; Zhang N. Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale 2014, 6, 3231–3242. [DOI] [PubMed] [Google Scholar]
- Brandsma D.; Stalpers L.; Taal W.; Sminia P.; van den Bent M. J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008, 9, 453–461. [DOI] [PubMed] [Google Scholar]
- van den Bent M. J.; Vogelbaum M. A.; Wen P. Y.; Macdonald D. R.; Chang S. M. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald’s Criteria. J. Clin. Oncol. 2009, 27, 2905–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portt L.; Norman G.; Clapp C.; Greenwood M.; Greenwood M. T. Anti-apoptosis and cell survival: a review. Biochim. Biophys. Acta 2011, 1813, 238–259. [DOI] [PubMed] [Google Scholar]
- Jia L. T.; Chen S. Y.; Yang A. G. Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat. Rev. 2012, 38, 868–876. [DOI] [PubMed] [Google Scholar]
- Oberdorster G.; Oberdorster E.; Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. C.; Chan W. C. W. Nanotoxicity: the growing need for in vivo study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.