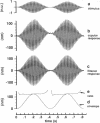
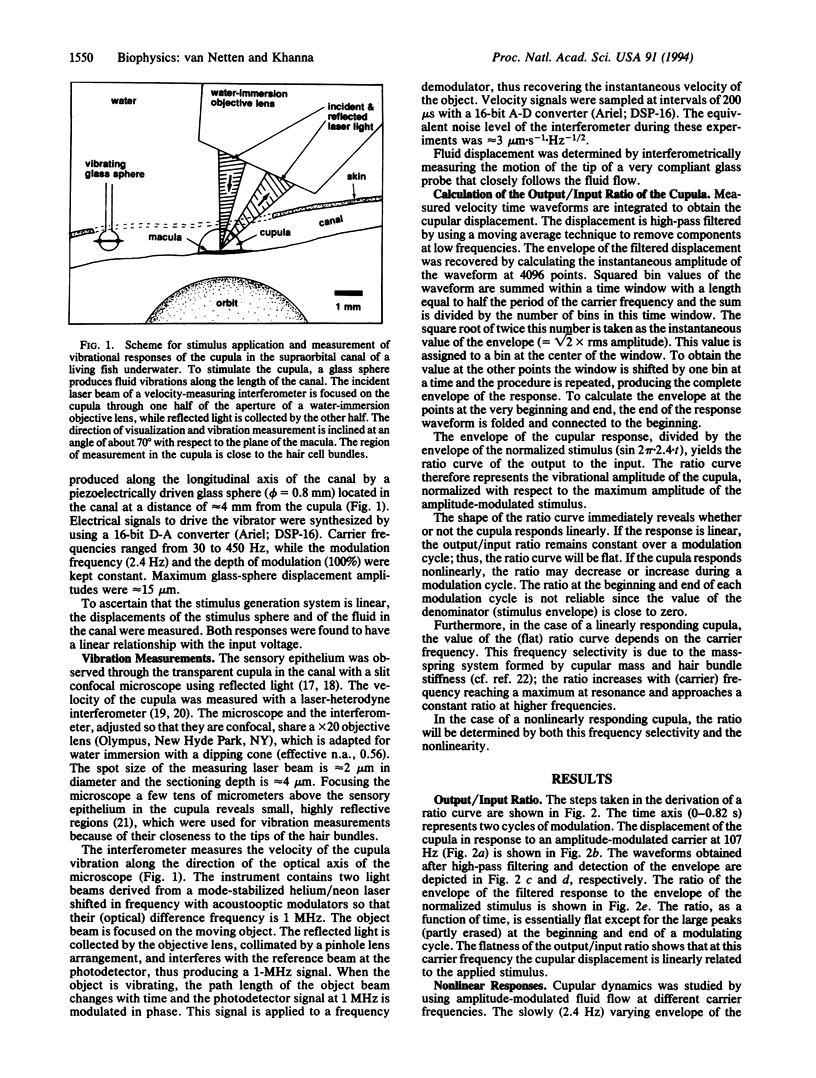
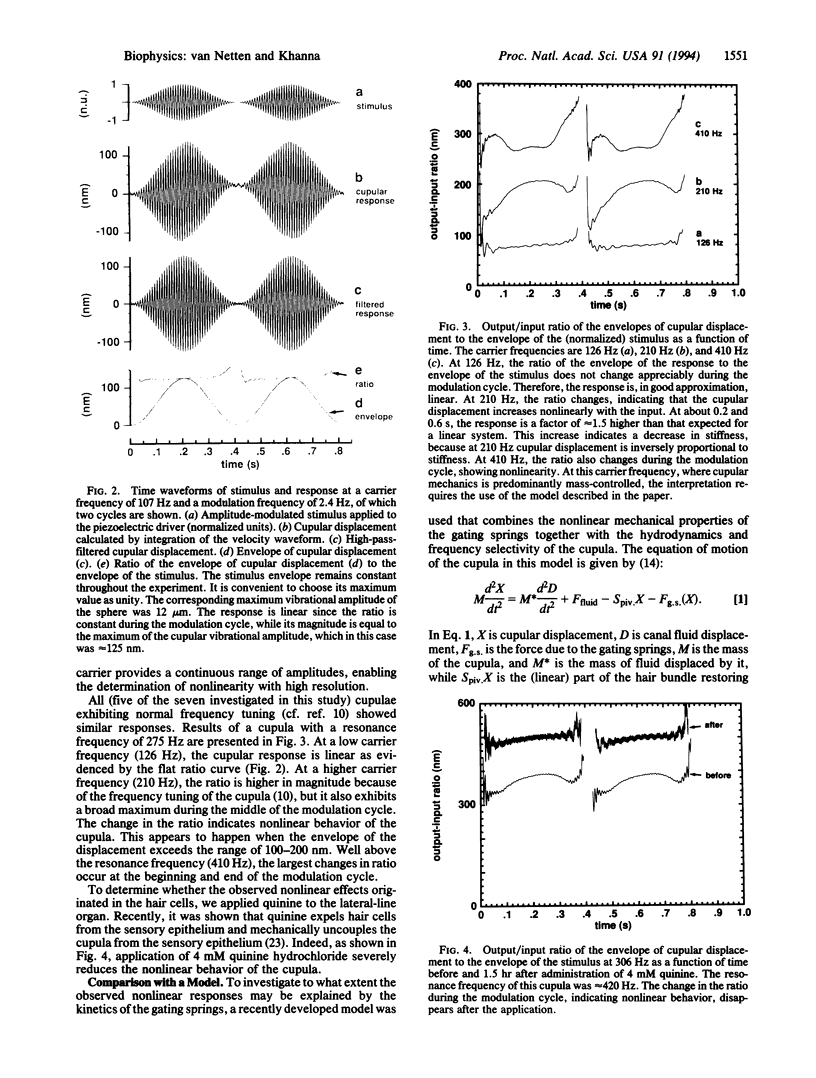
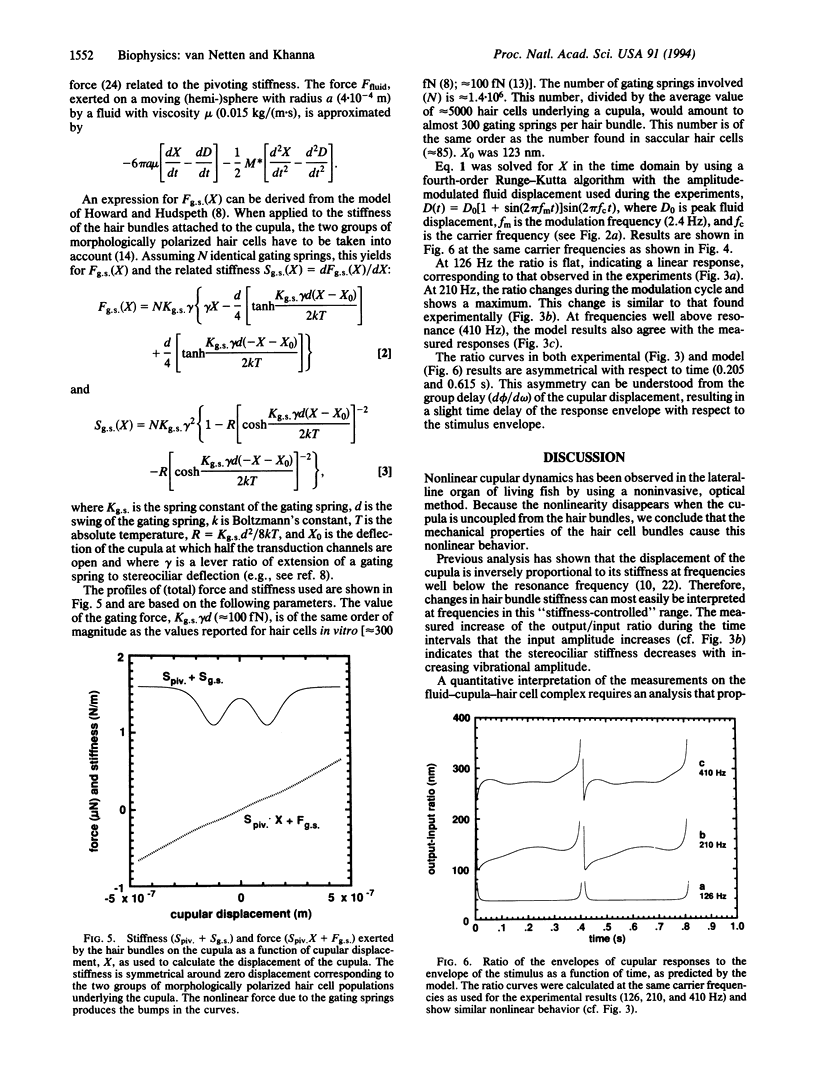
Abstract
Cupular vibration in the lateral-line canal of fish was measured in response to motion of the fluid in the canal by laser-heterodyne interferometry. The results show that the mechanical output/input ratio of the cupula depends on the stimulus amplitude; the cupula thus behaves nonlinearly. The nonlinearity is due to the hair bundles, since it disappears when the cupula is uncoupled from the underlying hair cells. A model of cupular dynamics in which the behavior of the gating springs of the hair cells is incorporated predicts nonlinear responses that are similar to the measurements, suggesting that the nonlinear behavior of the cupula may be attributed to the opening and closing of the transduction channels of the hair cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corey D. P., Hudspeth A. J. Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci. 1983 May;3(5):962–976. doi: 10.1523/JNEUROSCI.03-05-00962.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol. 1985 Jul;364:359–379. doi: 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J., Hudspeth A. J. Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog's saccular hair cell. Neuron. 1988 May;1(3):189–199. doi: 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Howard J., Roberts W. M., Hudspeth A. J. Mechanoelectrical transduction by hair cells. Annu Rev Biophys Biophys Chem. 1988;17:99–124. doi: 10.1146/annurev.bb.17.060188.000531. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. How the ear's works work. Nature. 1989 Oct 5;341(6241):397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Hudspeth A. J. Mechanoelectrical transduction by hair cells in the acousticolateralis sensory system. Annu Rev Neurosci. 1983;6:187–215. doi: 10.1146/annurev.ne.06.030183.001155. [DOI] [PubMed] [Google Scholar]
- Jaramillo F., Hudspeth A. J. Displacement-clamp measurement of the forces exerted by gating springs in the hair bundle. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1330–1334. doi: 10.1073/pnas.90.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo F., Hudspeth A. J. Localization of the hair cell's transduction channels at the hair bundle's top by iontophoretic application of a channel blocker. Neuron. 1991 Sep;7(3):409–420. doi: 10.1016/0896-6273(91)90293-9. [DOI] [PubMed] [Google Scholar]
- Kelly J. P., van Netten S. M. Topography and mechanics of the cupula in the fish lateral line. I. Variation of cupular structure and composition in three dimensions. J Morphol. 1991 Jan;207(1):23–36. doi: 10.1002/jmor.1052070105. [DOI] [PubMed] [Google Scholar]
- Khanna S. M., Rosskothen H., Koester C. J. Mechanical design of the measurement and micropositioning systems. Acta Otolaryngol Suppl. 1989;467:51–59. doi: 10.3109/00016488909138321. [DOI] [PubMed] [Google Scholar]
- Koester C. J., Khanna S. M., Rosskothen H., Tackaberry R. B. Incident light optical sectioning microscope for visualization of cellular structures in the inner ear. Acta Otolaryngol Suppl. 1989;467:27–33. doi: 10.3109/00016488909138318. [DOI] [PubMed] [Google Scholar]
- Oswald R. L. Injection anaesthesia for experimental studies in fish. Comp Biochem Physiol C. 1978;60(1):19–26. doi: 10.1016/0306-4492(78)90021-7. [DOI] [PubMed] [Google Scholar]
- Pickles J. O., Comis S. D., Osborne M. P. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984 Aug;15(2):103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Pickles J. O., Corey D. P. Mechanoelectrical transduction by hair cells. Trends Neurosci. 1992 Jul;15(7):254–259. doi: 10.1016/0166-2236(92)90066-h. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Kössl M., Richardson G. P. Nonlinear mechanical responses of mouse cochlear hair bundles. Proc Biol Sci. 1992 Dec 22;250(1329):217–227. doi: 10.1098/rspb.1992.0152. [DOI] [PubMed] [Google Scholar]
- Willemin J. F., Dändliker R., Khanna S. M. Heterodyne interferometer for submicroscopic vibration measurements in the inner ear. J Acoust Soc Am. 1988 Feb;83(2):787–795. doi: 10.1121/1.396122. [DOI] [PubMed] [Google Scholar]
- Willemin J. F., Khanna S. M., Dändliker R. Heterodyne interferometer for cellular vibration measurement. Acta Otolaryngol Suppl. 1989;467:35–42. doi: 10.3109/00016488909138319. [DOI] [PubMed] [Google Scholar]
- van Netten S. M., Kroese A. B. Laser interferometric measurements on the dynamic behaviour of the cupula in the fish lateral line. Hear Res. 1987;29(1):55–61. doi: 10.1016/0378-5955(87)90205-x. [DOI] [PubMed] [Google Scholar]