Abstract
We hypothesized that tongue-palate pressure generation might directly facilitate hyoid movement in swallowing through the anatomical connections of the extrinsic tongue muscles. If true, noninvasive measures of tongue-palate pressure timing might serve as a proxy measure of hyoid excursion. We explored the timing relationships between events in the tongue-palate pressure and hyoid movement profiles during water and nectar-thick liquid swallowing in healthy adults. Concurrent intra-oral manometry and submental B-mode midsagittal ultrasound were recorded. We determined that there is no obligate sequence in the onsets, or offsets, of tongue-palate pressures and hyoid excursion. Timing lags (either of hyoid movement lagging tongue-palate pressures, or vice versa) fell within ½ second, on average. We conclude that tongue-palate pressure generation and hyoid movement are separate phenomena in the swallowing sequence and that non-invasive measures of tongue-pressure timing cannot be used reliably as proxy measures of hyoid movement timing.
Swallowing is a complex neurophysiological process, involving a sequence of muscle contractions in the upper aerodigestive tract. This sequence unfolds in a cascade-like chain reaction, beginning with contraction of the mylohyoid and geniohyoid muscles, and proceeding to the muscle of the posterior tongue, the pharynx, larynx and then the cervical esophagus (Jean, 2001). Contraction of the mylohyoid and geniohyoid muscles, which leads this sequence, serves to lift the hyolaryngeal complex (Pearson, Langmore and Zumwalt, 2010), and position the entrance to the airway out of the path of the bolus. This reconfiguration of the pharynx from a respiratory to a swallowing pathway is crucial for swallowing safety. The delivery and propulsion of the bolus into and thought the pharynx is thought to arise primarily from driving forces generated by the tongue, which rises towards the palate, and generates pressures that squeeze the liquid bolus backwards through the faucial isthmus into the pharynx (Chi-Fishman, Stone and McCall, 1998; Hiiemae and Palmer, 2003; Ono, Hori and Nokubi, 2004; Yeates, Steele and Pelletier, 2010; Youmans and Stierwalt, 2006).
The anatomy of the extrinsic tongue muscles includes several direct and first-order connections to the hyoid. The genioglossus muscle runs between the underside of the tongue and the floor of mouth (genioglossus), which also houses the mylohyoid and genioghoid muscles. The hyoglossus muscle runs directly from the hyoid to the tongue. Both the hyoid and the tongue have extrinsic muscles that insert into the styloid process, namely the stylohyoid and styloglossus (Sawczuk and Mosier, 2001). Based on these anatomical connections, it is logical to expect that movements of the tongue may involve or induce concurrent displacement of the hyoid (Hiiemae and Palmer, 2003; Hiiemae, Palmer, Medicis, Hegener, Jackson and Lieberman, 2002). Further, it is logical to think that positioning of the tongue against the palate serves not only to generate a pressure gradient that leads to liquid bolus squeeze-back, but also to provide mechanical stabilization that facilitates contraction of the submental musculature for hyoid elevation. The purpose of this study was to explore the timing relationships that exist between the generation of tongue pressures and hyoid movements in swallowing, in order to elucidate the possible facilitatory role that tongue-palate pressure generation might have in hyolaryngeal excursion. The demonstration of such a relationship would provide evidence in support of using tongue-palate pressure exercise as an intervention for patients who display reduced hyolaryngeal excursion in swallowing. Furthermore, if a consistent timing relationship can be demonstrated between tongue-pressure and hyoid events, then tongue-pressure measures may hold utility as proxy measures of hyoid movement timing in clinical settings. Hand-held manometry equipment for tongue-pressure measurement is much more accessible than videofluoroscopy, endoscopy or ultrasound in clinical practice.
In swallowing, both the tongue and the hyoid bone move first in superior-anterior direction, and subsequently in an inferior-posterior direction (Ishida, Palmer and Hiiemae, 2002; Kim and McCullough, 2008; Martin, 1991; Perlman, Vandaele and Otterbacher, 1995; Steele and van Lieshout, 2004, 2009). The onset of superior hyoid movement is conventionally considered to be the onset boundary event of the pharyngeal swallow (Lof and Robbins, 1990), and typically lags movement of the bolus head past the posterior nasal spine by 0.2–0.5 seconds in healthy liquid swallows (Leonard and McKenzie, 2006). Although the temporal relationship between tongue movement and hyoid movement has been described in previous studies (Stone and Shawker, 1986), the relative timing between tongue-palate pressures and hyoid movement has not, to our knowledge, been previously described. Ultrasound is a noninvasive technique, through which clinicians and researchers can easily observe hyoid movement in swallowing. Readers are referred to the recent technical review by Chi-Fishman (2005) for a more detailed description of this technology. The hyoid bone generates a characteristic dark shadow on a sagittal ultrasound image, which appears as a line, running upward from the posterior aspect of the geniohyoid muscle and intersecting the posterior surface of the tongue. This shadow moves from the posterior (left) to anterior (right) side of the oral cavity on the image during swallowing, as illustrated in Figures 1a and 1b.
Figure 1.
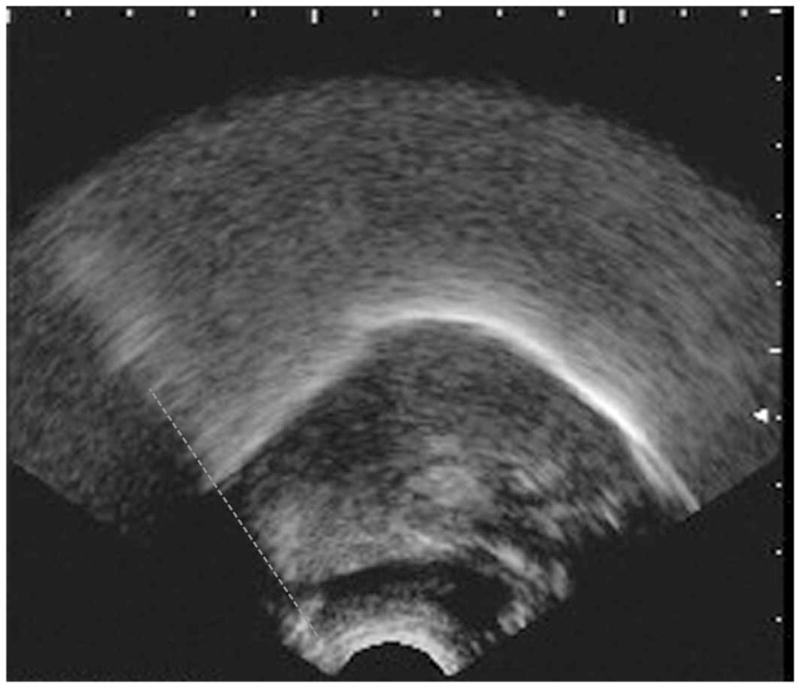
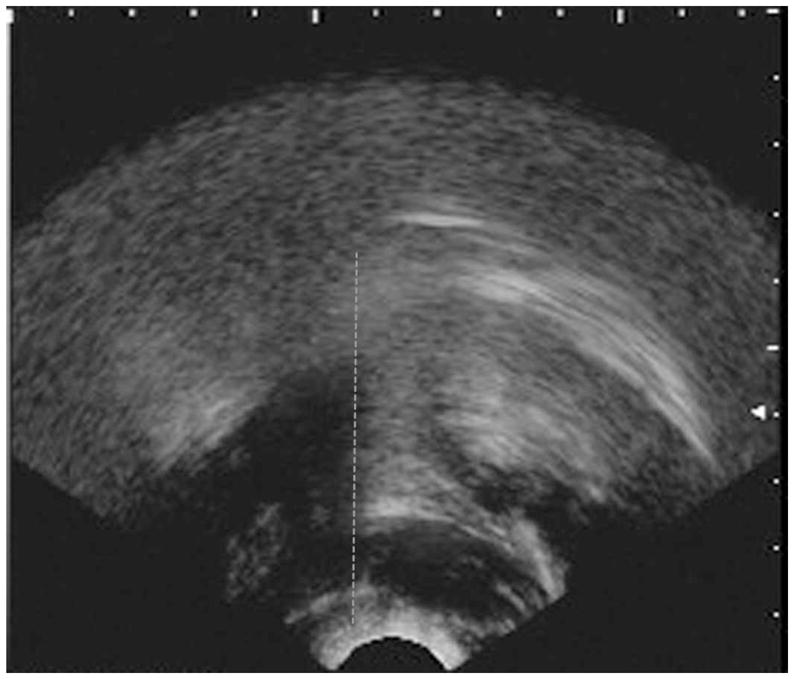
Figure 1a. An example of a B-mode midsagittal ultrasound image. The image is oriented with the front of the mouth to the right hand side of the picture. The hyoid shadow, highlighted with a dashed grey line, is shown on the left hand side of the image, and represents the hyoid at rest (corresponding to indices H1 and H3 in our study).
Figure 1b. An example of a B-mode midsagittal ultrasound image showing peak anterior displacement of the hyoid shadow (highlighted with a dashed grey line). This image corresponds to index H2 in this study.
One of the first studies to use ultrasound to explore the relationship between tongue and hyoid movement in swallowing was reported by Stone and Shawker (1986). They used midsagittal B-mode ultrasound imaging and also fixed metal pellets to the tongue (34–45 mm behind the tongue tip) to track both durational and spatial characteristics of tongue movement during 20cc water swallows in 6 healthy women. Hyoid events were captured based on anterior movement of the hyoid shadow on the ultrasound image, while tongue movement events were based on tracking of the metal pellets, allowing a comparison between the onsets, peaks and offsets of movement of the tongue and the hyoid. They reported that maximum tongue height was achieved, on average, 0.4 seconds after the onset of hyoid movement and 0.10 seconds prior to the arrival of the hyoid shadow at its most anterior position on the ultrasound image. Both the tongue and hyoid were described to show a plateau-like phase at maximum height (reflected to be the interval of tongue-palate contact), and that beginning of tongue-palate descent lagged the onset of hyoid descent by an average of 0.2 seconds.
Chi-Fishman and Sonies (2002) also used midsagittal B-mode ultrasound to study hyoid movement in swallowing, exploring the impact of liquid bolus viscosity on temporal aspects of hyoid shadow displacement. They failed to find significant differences in hyoid movement durations between thin and nectar-thick liquids, but longer durations were observed with spoon-thick liquids in 31 healthy adults (16 male). The relationship between hyoid kinematics and tongue events was not described. Recently, a study by Steele, Bailey and Molfenter (2010) examined the effects of bolus viscosity on tongue-pressure patterns, and identified a systematic increase in the durations of both the rise (i.e. onset-to-peak) and release-phases of tongue-palate pressure between water and nectar-thick liquids. Their study did not examine hyoid events.
We undertook a study using concurrent submental B-mode ultrasound and intra-oral manometry to explore the timing relationship between tongue pressure registration and hyoid movement in healthy swallowing. Based on the logic that tongue-palate pressures are required to transport the bolus head past the posterior nasal spine, and evidence from Leonard and McKenzie (2006) that this event usually precedes the onset of hyoid movement, we hypothesized that hyoid movement would lag tongue-palate pressures. Further, we hypothesized that these timing relationships would reflect primary anatomical connections and would, therefore, not be affected by liquid bolus viscosity, nor by participant gender.
Methods
Participants
Twenty healthy young adults (10 male, 10 female) between 20 and 39 years participated as volunteers in the study (mean age: 27.5 years, standard deviation = 5.13 years). Their eligibility was determined through completion of a medical history data form, an evaluation of oral motor function, and a swallowing screening performed by a registered speech-language pathologist. None of the participants demonstrated any signs of dysphagia, and none reported any history of neurological or gastrointestinal disorders. The study received human subjects approval from the institutional review boards of the Toronto Rehabilitation Institute and the University of Toronto.
Data Collection
Tongue-palate pressures were collected using the tongue-bulb array of the Swallowing Signals Lab on the KayPentax Digital Swallowing Workstation (DSW). This system allows one to identify the timing of the tongue pressure wave at three locations across the palate. A soft silicon strip housing 3 air-filled pressure bulbs (each approximately 5mm in diameter and height) was fixed to the anterior, mid and posterior palate in midline (8mm inter-bulb distance) using a small amount of surgical glue (Iso-Dent [Isobutyl Cyanoacrylate], Ellman International Inc., Oceanside, New York, USA). Pressure data were acquired at a sampling rate of 250Hz using an upper recording limit of 750 mm Hg.
Time-linked mid-sagittal B-mode ultrasound images were collected at 29.97 frames/second using a GE Logiq alpha 100MP ultrasound scanner (General Electric Medical Systems, WI 53201) with a E72 6.5 MHz microconvex array transducer. These images were fed into the video channel of the DSW for concurrent signal registration. A specially designed head and transducer support, the Comfortable Head Anchor for Sonographic Examinations (CHASE), was used to stabilize the participant’s head throughout data collection, ensuring a stable and fixed depth and angle of the transducer in relation to the head.
Participants were asked to perform a variety of reiterated swallowing and tongue-pressure tasks in randomly-ordered sequences (5 task repetitions per sequence); only the data for the swallows will be discussed in this manuscript. At the beginning of the data collection session, a sequence of 5 reiterated maximum isometric anterior tongue-palate presses was performed to establish a pressure amplitude reference scale for subsequent data normalization. For the swallowing tasks, participants were asked to execute five comfortably-sized discrete swallows of liquid from a 125 cc cup at a comfortable rate, removing the cup from the lips between sips. The stimuli were water and commercially pre-thickened nectar-thick apple juice (“Resource” by Nestlé Nutrition, Higland Park, Michigan, USA). Sip-size was not controlled, nor measured, however previous research from our lab has established that participants typically take sips of 5–10 ml under these conditions (Bennett, Van Lieshout, Pelletier and Steele, 2009).
Data Processing
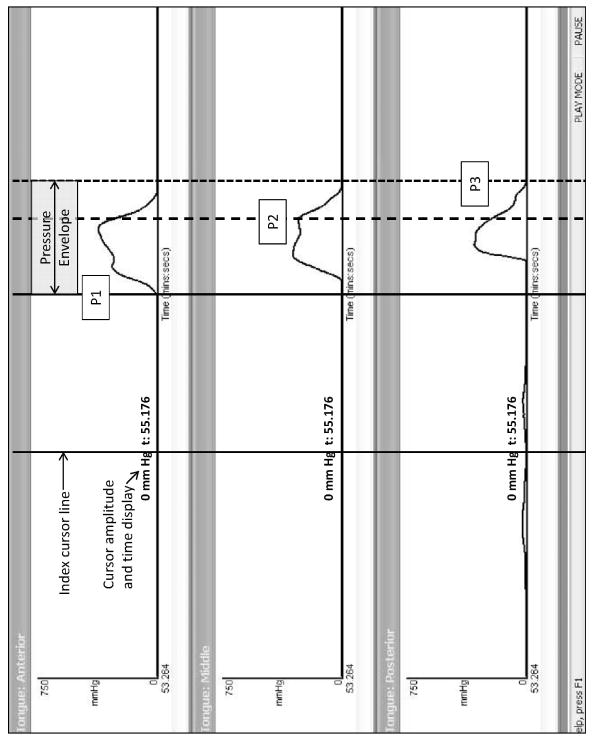
Following data collection, the tongue-pressure signals were arranged on a computer monitor so that all three signals were visible, together with the ultrasound image. Two trained research assistants used the cursor event-indexing function of the KayPentax DSW software to identify the onsets, peaks and offsets of pressure at each bulb. The timing of each event was then recorded, based on the timecode of the signal, which is displayed along the x-axis of the image, and recorded at the bottom of each cursor line. These events were then integrated across all three bulbs to identify the onset, peak and offset of the tongue-pressure envelope (the term we will use to indicate the overall pressure event across all three bulb locations, as follows (see Figure 2):
Figure 2.
Illustration of tongue pressure waveforms collected at the anterior, mid and posterior palate using the KayPentax Swallowing Signals Lab 3 tongue bulb array. An example of a cursor for the time index feature of the software is shown on the left (solid black line), with amplitude and time of the cursor point identified at the bottom. The pressure waveforms are indexed to show the duration of the tongue pressure envelope as well asnt the temporal location of events within that envelope: onset of the first tongue-palate pressure increase across all three bulb locations, (P1), the terminal peak pressure registered across all bulb locations (P2) and the terminal pressure offset across all three bulbs (P3). These events were indexed during liquid swallows
P1: First tongue-palate pressure onset (i.e., upwards departure from resting baseline) across the 3 pressure bulbs;
P2: Terminal peak tongue-palate pressure (i.e. the beginning of the downwards signal deflection towards baseline) across the 3 pressure bulbs;
P3: Terminal tongue-palate pressure offset (i.e. final arrival back at baseline) across the 3 pressure bulbs.
The ultrasound recordings were similarly reviewed frame-by-frame to identify the temporal boundaries of hyoid shadow displacement as follows:
H1: First video frame showing anterior movement of the hyoid shadow during a swallow.
H2: Final video frame prior to posterior movement of the hyoid shadow during a swallow.
H3: First video frame showing arrival of the hyoid shadow back at a stable rest position after the swallow.
Figure 1a provides an example of the kinds of ultrasound images that would be seen for events H1 and H3, showing the hyoid shadow at its minimum (left-most) position. Figure 1b provides an example corresponding to event H2, showing full excursion of the hyoid shadow to its anterior-most position on the image.
All event indices were entered into a spreadsheet and converted into milliseconds to enable the calculation of three timing difference measures, using P1 (the onset of tongue-palate pressures) as time zero:
T1: Hyoid onset lag (i.e. P1-H1)
T2: Hyoid descent lag (i.e. P2-H2)
T3: Hyoid offset lag (i.e. (P3-H3).
Analysis
In order to collect a full set of timing-difference measures for a particular swallow, it was necessary for the hyoid shadow to be clearly visible on all ultrasound image frames and also that a complete set of tongue-pressure waveform data for all three sensors was available. Due to image and signal quality concerns, a number of swallows were excluded. In total, data for 54 water swallows and 64 nectar-thick juice swallows from 15 participants (6 female) were available for analysis. Due to the imbalance in data across gender and the fact that there was no a priori expectation that tongue-pressure and hyoid timing would differ according to this factor, the data were pooled across gender for the statistical analysis.
The first step in the analysis was to divide the data into those swallows in which hyoid movement events lagged the corresponding tongue-pressure event, and those in which the reverse pattern of anticipatory hyoid movement was observed, and to express the associated timing difference as an absolute difference in milliseconds. Chi-square statistics were used to examine the relative proportion of swallows in which tongue-pressure events anticipated the associated hyoid movement events across stimulus type. Descriptive statistics for timing difference were then calculated for each parameter (onset lag; descent lag; offset lag). The time-difference data were determined not to follow a normal distribution, so a natural log transform was used prior to subsequent analysis. A mixed model analysis of variance (ANOVA) with repeated measures and factors of leading structure (tongue; hyoid) and stimulus (water; nectar) was conducted for each parameter.
Results
Means and 95% confidence intervals for absolute timing differences are shown by leading structure and parameter in Table 1. It is noteworthy that variation in leading structure was observed in this dataset for all parameters. In terms of onset patterns, hyoid movement was observed to lag the onset of tongue-palate pressures in 63.2% of swallows, and this pattern did not differ significantly between stimuli (χ2 = 0.516; df = 1, 114; p = 0.47). With respect to timing differences in the beginning of descent, hyoid movement was observed to lag the beginning of tongue-palate pressure release in 71.6% of cases, and again did not differ across stimuli (χ2 = 1.337; df = 1, 116; p = 0.25). Similarly, the termination of hyoid descent lagged the offset of tongue-palate pressures in the majority (72.2%) of cases, with no difference in pattern across stimuli (χ2 = 0.249; df = 1, 115; p = 0.62).
Table 1.
Descriptive Statistics for tongue-pressure and hyoid movement timing relationships in healthy liquid swallowing (in milliseconds)
| Measure | Leading Structure | Stimulus | Mean (ms) | 95% Confidence Interval (ms) | |
|---|---|---|---|---|---|
| Lower boundary | Upper boundary | ||||
| Onset Lag | Tongue (63%) | Water | 404 | 300 | 509 |
| Nectar | 605 | 267 | 943 | ||
| Hyoid (37%) | Water | 503 | 87 | 920 | |
| Nectar | 463 | 236 | 691 | ||
| Descent Lag | Tongue (72%) | Water | 286 | 238 | 334 |
| Nectar | 522 | 201 | 844 | ||
| Hyoid (28%) | Water | 844 | 272 | 1417 | |
| Nectar | 606 | 407 | 804 | ||
| Offset Lag | Tongue (72%) | Water | 350 | 286 | 413 |
| Nectar | 629 | 324 | 934 | ||
| Hyoid (28%) | Water | 851 | 341 | 1361 | |
| Nectar | 749 | 586 | 913 | ||
The ANOVAs failed to identify any statistically significant differences in onset lag durations between the tongue-lead and hyoid-lead data, or between stimuli at a p-value of < 0.05. There were no significant leading-structure by stimulus interactions for onset lag measures. For both the descent-lag and off-set lag parameters, a statistically significant main effect of leading structure was observed. Specifically, significantly greater lag times were observed when hyoid descent anticipated the beginning of tongue-palate pressure release [F(1, 75.87) = 21.01, p = 0.000], and when hyoid offset anticipated tongue-palate pressure offset [F(1, 66.49) = 22.09, p = 0.000]. Cohen’s d effect-size calculations on the non log-transform data for these parameters identified these differences to be 0.38 for descent lag and 0.25 for offset lag; both of these values are considered to show weak effects (Kotrlik and Williams, 2003).
Discussion
This study failed to find a systematic lag between tongue-pressure timing and hyoid movement in healthy swallowing. These data corroborate previous findings that the sequence of events in swallowing between tongue events (tongue-movement, tongue-palate pressure generation, bolus movement past the posterior nasal spine) and hyoid movement is not invariant (Kendall, Leonard and McKenzie, 2003; Kendall, McKenzie, Leonard, Goncalves and Walker, 2000). In the current study, hyoid events were observed to anticipate the corresponding tongue-pressure events in approximately 25% of cases, with no particular pattern across stimuli. Anticipatory hyoid movement (prior to arrival of the bolus head in the upper pharynx) has been described before as a putatively protective event (i.e., earlier onset of airway closure), and it would be reasonable to expect that this phenomenon might be more common with thin liquids than with thickened liquids, but this was not found to be the case in this study of healthy young adults.
There are some inherent limitations to using non-invasive technologies to examine tongue and hyoid events in swallowing. When co-registering these signals, there is a difference in the temporal resolution (a sampling rate of 250 Hz for the tongue-pressure waveforms vs. frames of 33 milliseconds in length for the ultrasound images), which introduces a margin of error into the subsequent timing comparisons. Determining when the shadow of the hyoid has reached or ended its maximum position is a subjective decision during frame-by-frame ultrasound review. Furthermore, it is sometimes difficult to determine the visual boundaries of the hyoid shadow itself, and this may introduce variation into timing measures, even when measures are taken to limit procedural variations in transducer depth and angle during data collection. Adding to this limitation is the possibility that signal registration lags may occur with the input of video signals from the ultrasound equipment into the KayPentax DSW. We have investigated the signal registration lag of the ultrasound equipment in comparison to an oscillogram on speech production tasks, and have determined that the accuracy of the image timing may include a delay of up to 3 frames (100 milliseconds). Thus, the results reported in this manuscript should be interpreted with caution given the possibility that this delay may have influenced both the categorization of data according to leading structure, and the magnitude of the observed timing relationships. However, it can safely be accepted that wherever our data show a sequence of hyoid movement (from the ultrasound images) prior to the registration of tongue-pressures, that the hyoid was indeed the leading structure.
The indexing of events in the tongue-pressure waveform can also involve some measurement error due to pixel resolution issues when using a computer cursor to mark events displayed on a computer monitor. The resolution of concurrent tongue-palate manometry and B-mode ultrasound imaging does not permit a more fine-grained analysis that might determine whether voluntary increases in tongue-palate pressure, for example those that might occur at moments during the maximum tongue-height plateau, might facilitate greater hyoid excursion. This possibility would be most interesting to measure in patients with dysphagia for whom hyolaryngeal excursion might not always be adequate to achieve the associated functional goals of airway protection and upper esophageal sphincter opening (Jacob, Kahrilas, Logemann, Shah and Ha, 1989; Logemann et al., 1992).
Our data set did not contain a large enough sample of balanced data from participants of both genders to permit an analysis of differences between genders. We have no a priori reason to expect that tongue-pressure and hyoid movement timing relationships would differ between the sexes, although it is possible that the lower anatomical position of the larynx at rest in males might influence the timing requirements for hyolaryngeal excursion onset relative to bolus transport. This is a question for future study.
It should also be noted that the swallows in the present study were spontaneous swallows rather than command swallows. Daniels and colleagues have shown that the use of a command swallow paradigm results in a higher bolus position at swallow onset (Daniels, Schroeder, DeGeorge, Corey and Rosenbek, 2007). It would be reasonable to assume that the relative timing of tongue-palate pressures and hyoid movement might differ between command and spontaneous swallows. Stone and Shawker (1986) did not report whether their participants performed command or spontaneous swallows, whereas command swallows were used in the study by Chi-Fishman and Sonies (2002) and in the videofluoroscopic study of hyoid movement and bolus transit latencies reported by Leonard and McKenzie (2006).
Conclusions
We had expected that tongue-pressure onset would anticipate hyoid movement onset as a rule, on the basis that mechanical stabilization of the tongue against the palate would be likely to result in an increase in tongue-palate pressures and would serve to facilitate hyoid excursion. The observed variations in sequencing challenge this assumption and mean that one cannot assume that tongue-pressure resistance training will engage hyolaryngeal movement. Therefore, the current data do not provide evidence to support the use of tongue-pressure resistance training as an intervention for reduced hyolaryngeal excursion in patients with dysphagia.
Similarly, we did not see an invariant pattern in the termination of maximum tongue-palate pressure and maximum hyoid excursion. Consequently, although event timing-differences fall within ½ second, on average, in the current data set, we must conclude that tongue-palate pressure generation and hyoid movement are, in fact, separate phenomena in the swallowing sequence. This also means that non-invasive measures of tongue-pressure timing cannot be used reliably as proxy measures of hyoid movement timing.
Acknowledgments
This research was supported by operating and career award grants from the Canadian Institutes of Health Research (grants 69521, 82668, 84534, and 83888). Additional funding support was provided by the Toronto Rehabilitation Institute and an Ontario Ministry of Research and Innovation Early Researcher Award to Dr. Steele. The authors acknowledge the support of the Toronto Rehabilitation Institute, which receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-term Care in Ontario. The views expressed do not necessarily reflect those of the Ministry. The authors gratefully acknowledge assistance provided by Rebecca Cliffe, Sonja Molfenter, Erin Yeates, Anna Ammoury, Ashley Waito, Melanie Moore and Stephanie Ko during data collection and processing.
References
- BENNETT JW, VAN LIESHOUT PH, PELLETIER CA, STEELE CM. Sip-Sizing Behaviors in Natural Drinking Conditions Compared to Instructed Experimental Conditions. Dysphagia. 2009;24(2):152–158. doi: 10.1007/s00455-008-9183-y. [DOI] [PubMed] [Google Scholar]
- CHI-FISHMAN G, SONIES BC. Effects of systematic bolus viscosity and volume changes on hyoid movement kinematics. Dysphagia. 2002;17:278–287. doi: 10.1007/s00455-002-0070-7. [DOI] [PubMed] [Google Scholar]
- CHI-FISHMAN G, STONE M, MCCALL GN. Lingual action in normal sequential swallowing. Journal of Speech, Language and Hearing Research. 1998;41:771–785. doi: 10.1044/jslhr.4104.771. [DOI] [PubMed] [Google Scholar]
- DANIELS SK, SCHROEDER MF, DEGEORGE PC, COREY DM, ROSENBEK JC. Effects of verbal cue on bolus flow during swallowing. American Journal of Speech-Language Pathology. 2007;16:140–147. doi: 10.1044/1058-0360(2007/018). [DOI] [PubMed] [Google Scholar]
- HIIEMAE KM, PALMER JB, MEDICIS SW, HEGENER J, JACKSON BS, LIEBERMAN DE. Hyoid and tongue surface movements in speaking and eating. Archives of Oral Biology. 2002;47:11–27. doi: 10.1016/s0003-9969(01)00092-9. [DOI] [PubMed] [Google Scholar]
- HIIEMAE KM, PALMER JB. Tongue movements in feeding and speech. Critical Reviews in Oral Biology and Medicine. 2003;14:413–429. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- ISHIDA R, PALMER JB, HIIEMAE KM. Hyoid motion during swallowing: Factors affecting forward and upward displacement. Dysphagia. 2002;17:262–272. doi: 10.1007/s00455-002-0064-5. [DOI] [PubMed] [Google Scholar]
- JACOB P, KAHRILAS PJ, LOGEMANN JA, SHAH V, HA T. Upper esophageal sphincter opening and modulation during swallowing. Gastroenterology. 1989;97:1469–1478. doi: 10.1016/0016-5085(89)90391-0. [DOI] [PubMed] [Google Scholar]
- JEAN A. Brain stem control of swallowing: Neuronal network and cellular mechanisms. Physiological Reviews. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- KENDALL KA, LEONARD RJ, MCKENZIE SW. Sequence variability during hypopharyngeal bolus transit. Dysphagia. 2003;18:85–91. doi: 10.1007/s00455-002-0086-z. [DOI] [PubMed] [Google Scholar]
- KENDALL KA, MCKENZIE S, LEONARD RJ, GONCALVES MI, WALKER A. Timing of events in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:74–83. doi: 10.1007/s004550010004. [DOI] [PubMed] [Google Scholar]
- KIM Y, MCCULLOUGH GH. Maximal hyoid displacement in normal swallowing. Dysphagia. 2008;23:274–279. doi: 10.1007/s00455-007-9135-y. [DOI] [PubMed] [Google Scholar]
- KOTRLIK JW, WILLIAMS HA. The incorporation of effect size in informaton technology, learning, and performance research. Information Technology, Learning, and Performance Journal. 2003;21:1–7. [Google Scholar]
- LEONARD R, MCKENZIE S. Hyoid-bolus transit latencies in normal swallow. Dysphagia. 2006;21:183–190. doi: 10.1007/s00455-006-9025-8. [DOI] [PubMed] [Google Scholar]
- LOF GL, ROBBINS J. Test-retest variability in normal swallowing. Dysphagia. 1990;4:236–242. doi: 10.1007/BF02407271. [DOI] [PubMed] [Google Scholar]
- LOGEMANN JA, KAHRILAS PJ, CHENG J, PAULOSKI BR, GIBBONS PJ, RADEMAKER AW, LIN S. Closure mechanisms of laryngeal vestibule during swallow. American Journal of Physiology. 1992;262:G338–G344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- MARTIN RE. Doctoral dissertation. Madison, WI: University of Wisconsin-Madison; 1991. A comparison of lingual movement in swallowing and speech production. [Google Scholar]
- ONO T, HORI K, NOKUBI T. Pattern of tongue-pressure on hard palate during swallowing. Dysphagia. 2004;19:259–264. doi: 10.1007/s00455-004-0010-9. [DOI] [PubMed] [Google Scholar]
- PEARSON WG, Jr, LANGMORE SE, ZUMWALT AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2010 doi: 10.1007/s00455-010-9315-z. Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERLMAN AL, VANDAELE DJ, OTTERBACHER MS. Quantitative assessment of hyoid bone displacement from video images during swallowing. Journal of Speech and Hearing Research. 1995;38:579–585. doi: 10.1044/jshr.3803.579. [DOI] [PubMed] [Google Scholar]
- SAWCZUK A, MOSIER KM. Neural control of tongue movement with respect to respiration and swallowing. Critical Reviews in Oral Biology and Medicine. 2001;12:18–37. doi: 10.1177/10454411010120010101. [DOI] [PubMed] [Google Scholar]
- STEELE CM, BAILEY GL, MOLFENTER SM. Tongue-pressure modulation during swallowing: Water vs. nectar-thick liquids. Journal of Speech, Language & Hearing Research. 2010;53(2):273–283. doi: 10.1044/1092-4388(2009/09-0076). [DOI] [PubMed] [Google Scholar]
- STEELE CM, VAN LIESHOUT PH. Use of electromagnetic midsagittal articulography in the study of swallowing. Journal of Speech, Language & Hearing Research. 2004;47:342–352. doi: 10.1044/1092-4388(2004/027). [DOI] [PubMed] [Google Scholar]
- STEELE CM, VAN LIESHOUT PHHM. Tongue movements during water swallowing in healthy young and older adults. Journal of Speech, Language and Hearing Research. 2009;52:1255–1267. doi: 10.1044/1092-4388(2009/08-0131). [DOI] [PubMed] [Google Scholar]
- STONE M, SHAWKER TH. An ultrasound examination of tongue movement during swallowing. Dysphagia. 1986;1:78–83. doi: 10.1007/BF02407118. [DOI] [PubMed] [Google Scholar]
- YEATES EM, STEELE CM, PELLETIER CA. Tongue pressure and submental surface electromyography measures during non-effortful and effortful saliva swallows in healthy women. American Journal of Speech-Language Pathology. 2010;19:1–8. doi: 10.1044/1058-0360(2010/09-0040). [DOI] [PubMed] [Google Scholar]
- YOUMANS SR, STIERWALT JA. Measures of tongue function related to normal swallowing. Dysphagia. 2006;21:102–111. doi: 10.1007/s00455-006-9013-z. [DOI] [PubMed] [Google Scholar]