Abstract
Introduction
Mycobacterium ulcerans (M. ulcerans) is a necrotizing skin infection endemic to the Bellarine Peninsula, Australia. Current treatment recommendations include 8 weeks of combination antibiotics, with adjuvant surgery if necessary. However, antibiotic toxicity often results in early treatment cessation and local experience suggests that shorter antibiotic courses may be effective with concurrent surgery. We report the outcomes of patients in the Barwon Health M. ulcerans cohort who received shorter courses of antibiotic therapy than 8 weeks.
Methodology / Principal findings
A retrospective analysis was performed of all M. ulcerans infections treated at Barwon Health from March 1, 1998 to July 31, 2013. Sixty-two patients, with a median age of 65 years, received < 56 days of antibiotics and 51 (82%) of these patients underwent concurrent surgical excision. Most received a two-drug regimen of rifampicin combined with either ciprofloxacin or clarithromycin for a median 29 days (IQR 21–41days). Cessation rates were 55% for adverse events and 36% based on clinician decision. The overall success rate was 95% (98% with concurrent surgery; 82% with antibiotics alone) with a 50% success rate for those who received < 14 days of antibiotics increasing to 94% if they received 14–27 days and 100% for 28–55 days (p<0.01). A 100% success rate was seen for concurrent surgery and 14–27 days of antibiotics versus 67% for concurrent surgery and < 14 days of antibiotics (p = 0.12). No previously identified risk factors for treatment failure with surgery alone were associated with reduced treatment success rates with < 56 days of antibiotics.
Conclusion
In selected patients, antibiotic treatment durations for M. ulcerans shorter than the current WHO recommended 8 weeks duration may be associated with successful outcomes.
Author Summary
Buruli ulcer is a necrotizing skin and subcutaneous tissue infection caused by Mycobacterium ulcerans and is the third most common mycobacterial infection, behind tuberculosis and leprosy, world-wide. In recent years, the World Health Organisation has modified its guidelines for M. ulcerans treatment, moving from predominantly surgical to predominantly medical based management. It now recommends the combination of oral rifampicin and intramuscular streptomycin for a period of eight weeks as first-line therapy, with surgery as adjunctive therapy if necessary. The Barwon Health experience from south-eastern Australia has demonstrated that the entirely oral combination of rifampicin with either ciprofloxacin or clarithromycin for eight weeks can be an effective treatment option. However, these antibiotics are often toxic leading to early cessation, especially in the elderly. In addition, clinicians have been using a shorter duration of therapy for smaller lesions that have also been surgically managed. This study reviews our experience treating M. ulcerans with antibiotic durations of less than 8 weeks and demonstrates that successful outcomes can be achieved in selected patients, with success rates influenced by the duration of treatment and the use of surgical excision. This finding needs confirmation in further studies, but could have significant benefits in terms of reducing toxicity and improving adherence associated with Buruli ulcer antibiotic treatment.
Introduction
Mycobacterium ulcerans (M. ulcerans) infection, or Buruli ulcer, is a necrotizing infection of the skin and subcutaneous tissue and is the third most common mycobacterial infection in immunocompetant people after tuberculosis and leprosy. It has been reported in 33 predominantly subtropical and tropical countries, with west and central Africa being the worst affected regions [1]. In Australia endemic foci of infection are found in tropical Far North Queensland and temperate regions of southern Victoria [2].
Up until 2004, the World Health Organisation (WHO) recommended wide surgical excision as treatment for M. ulcerans lesions, with no role for antibiotics which were thought to be ineffective [3]. However surgical treatment was often difficult to access in resource-limited settings [4], was limited by high recurrence rates [5, 6] and in severe cases caused significant cosmetic morbidity and increased costs [7, 8]. Mounting clinical evidence of the effectiveness of antibiotics [9–14] has resulted in a paradigm shift to a predominantly medical approach. The latest WHO recommendations are for eight weeks combination antibiotic therapy with intramuscular streptomycin and oral rifampicin. Surgical intervention is recommended only to hasten healing of more extensive ulcers, if antibiotics are contraindicated or not tolerated, or at a patient’s request. In addition, if surgery is required, an initial four weeks of antibiotics prior to surgery is recommended [2, 15].
Clinicians at Barwon Health began using antibiotics to treat M. ulcerans infections from the Bellarine Peninsula in 1998 [5]. Since this time the proportion of patients receiving antibiotics has increased, resulting in fewer recurrences and permitting more conservative, and thus less reconstructive, surgery [12]. Oral antibiotics have subsequently reduced hospitalisations and the cost of treatment [7]. Our current treatment practice for the majority of M. ulcerans lesions is an oral combination of rifampicin with either ciprofloxacin or clarithromycin for eight weeks. Surgery is used for debriding necrotic wounds or closing large tissue defects to increase the rate of wound healing, for patients unable or unwilling to take antibiotics, and for those preferring the more rapid healing of small lesions that surgical excision and direct closure enables compared with the often prolonged healing of lesions treated with antibiotics alone [2].
However, these protracted antibiotic treatment regimens are not without toxicity, and 16–33% of patients, often elderly, cease them early due to side effects [12, 13]. In addition, shorter courses of adjunctive antibiotics (4–6 weeks) have been offered for smaller lesions treated with surgical excision and direct closure based on previous experience where cure was obtained for 100% of 21 patients who received between 12 and 30 days of antibiotics combined with surgical excision [12].
The aim of this study was to review the experience and outcomes of patients in the Barwon Health M. ulcerans observational cohort who received a shorter course of antibiotic therapy than the standard recommended duration of 8 weeks.
Methods
A retrospective analysis was performed of all confirmed M. ulcerans infections treated at Barwon Health from March 1, 1998 to July 31, 2013. Data was collected prospectively using Epi-info 6 (CDC Atlanta, USA). Definitions used for the study can be found in Table 1.
Table 1. Parameter definitions used during analysis.
| Item | Definition |
|---|---|
| M. ulcerans case | Lesion clinically consistent with M. ulcerans PLUS |
| - Positive M. ulcerans PCR or culture* from a swab, biopsy or excised lesion | |
| - Histopathology of an excised lesion showing a necrotic ulcer with the presence of acid-fast bacilli consistent with acute M. ulcerans infection | |
| Surgical definitions | - Major surgery: closure of an excised lesion using a split skin or full thickness skin graft or vascularised tissue flap |
| - Positive margins: histology of excised tissue showing granulomatous inflammation or necrotic tissue extending to the tissue margins | |
| Long-course antibiotics | Duration of antibiotics ≥ 56 days |
| Short-course antibiotics | Duration of antibiotics < 56 days |
| Treatment success | Healing of the entire lesion without recurrence 12 months after the antibiotic treatment course had been completed. |
| Treatment failure | A recurrent M. ulcerans lesion within 12 months of treatment completion that was either a) culture positive for M. ulcerans or b) had histopathology showing a necrotic ulcer with the presence of acid-fast bacilli consistent with acute M. ulcerans and did not show evidence of a paradoxical reaction |
*Mycobacterial cultures were performed using Lowenstein–Jensen media and incubated for 12 weeks
Patients who had received antibiotics for M. ulcerans were included in the study. Treatment duration was separated into ≥ 56 days (long-course therapy), and < 56 days (short-course therapy). Patients were followed for 12 months after the cessation of treatment. Drug dosages for adults included rifampicin 10 mg/kg/day (up to a maximum of 600 mg daily), ciprofloxacin 500 mg twice daily, moxifloxacin 400 mg once daily, clarithromycin 500 mg twice daily and ethambutol 15 mg/kg/day. A complication of medical therapy was defined as an adverse event attributed to an antibiotic that required its cessation. Immune suppression was defined as current treatment with immunosuppressive medication (eg. prednisolone) or active malignancy. The position of a M. ulcerans lesion was described as distal if it was on or below the elbow or knee.
Surgical specimens were sent to the Victorian Infectious Diseases Reference Laboratory (VIDRL) for mycobacterial culture using Lowenstein-Jensen media and a 12-week incubation period.
Data was analysed using STATA 13 (StataCorp, Texas, USA). The main variable assessed was treatment outcome and defined as success or failure as outlined in Table 1. Proportions were compared using 2x2 tables and the Chi-squared and Fisher’s exact test. Median values were compared using the Mann-Whitney test.
Ethics
This is an observational, retrospective cohort study with analysis performed on anonymised data. Verbal patient consent, or consent from a parent or guardian in the case of a minor, was gained for collection of data and noted in the patient medical record. Barwon Health’s Human Research and Ethic Committee have approved this analysis.
Results
Demographics
From March 1, 1998 to July 31, 2013 there were 252 patients diagnosed with M. ulcerans infection at Barwon Health. Forty-one patients who were not treated with antibiotics were excluded from this analysis, two were excluded due to unrelated deaths during follow-up and two were lost to follow-up. Therefore 207 patients were included; 110 (53%) were male and the median age was 59 years (IQR 36–75 years). Sixty-two (30%) patients received less than 56 days of antibiotic treatment (short-course therapy) whilst 145 (70%) patients had 56 days or more (long-course therapy) (Table 2).
Table 2. Baseline characteristics of patients receiving short-course (< 56 day) or long-course (≥ 56 days) antibiotics for M. ulcerans infection.
| Short-course (n = 62) | Long-course (n = 145) | p value | |
|---|---|---|---|
| Sex | |||
| Male | 25 (40%) | 85 (59%) | p = 0.02 |
| Female | 37 (60%) | 60 (41%) | |
| Age | |||
| < 60 years | 24 (39%) | 80 (55%) | p = 0.03 |
| ≥ 60 years | 38 (61%) | 65 (45%) | |
| Immune suppression | |||
| No | 54 (87%) | 137 (94%) | p = 0.07 |
| Yes | 8 (13%) | 8 (6%) | |
| Diabetes | |||
| No | 54 (87%) | 133 (92%) | p = 0.30 |
| Yes | 8 (13%) | 12 (8%) | |
| Type of lesion | |||
| Ulcerative | 56 (90%) | 121 (83%) | p = 0.07 |
| Nodular | 5 (8%) | 6 (4%) | |
| Plaque | 0 (0%) | 2 (1%) | |
| Oedematous | 1 (2%) | 16 (11%) | |
| WHO category | |||
| 1 | 57 (92%) | 113 (77%) | p = 0.003 |
| 2 | 0 (0%) | 24 (17%) | |
| 3 | 5 @ (8%) | 9 (6%) | |
| Lesion Position | |||
| Proximal | 10 (16%) | 13 (9%) | p = 0.13 |
| Distal | 52 (84%) | 133 (91%) | |
| Duration of symptoms prior to diagnosis* | |||
| ≤ 75 days | 46 (78%) | 110 (77%) | p = 0.92 |
| > 75 days | 13 (22%) | 32 (23%) | |
| Positive margins # | |||
| No | 23 (48%) | 15 (19%) | p<0.001 |
| Yes | 25 (52%) | 65 (81%) | |
| Major surgery & | |||
| No | 25 (49%) | 25 (30%) | p = 0.03 |
| Yes | 26 (51%) | 57 (70%) | |
@ 1 patient with an oedematous lesion, 3 patients with 2 lesions and 1 patient with 13 lesions
*n = 200 (missing data in 7 patients)
#n = 128
&n = 133
Comparisons of patient characteristics for short (n = 62) and long-course (n = 145) antibiotic groups
Those receiving short-course antibiotics were more likely to be female, ≥ 60 years of age, have WHO category 1 lesions, and were less likely to have had major surgery or have positive margins (Table 2). However there were no significant differences in the proportions that were diabetic, the type or site of lesions, or the duration of symptoms prior to diagnosis (Table 2).
Short-course treatment cohort (n = 62)
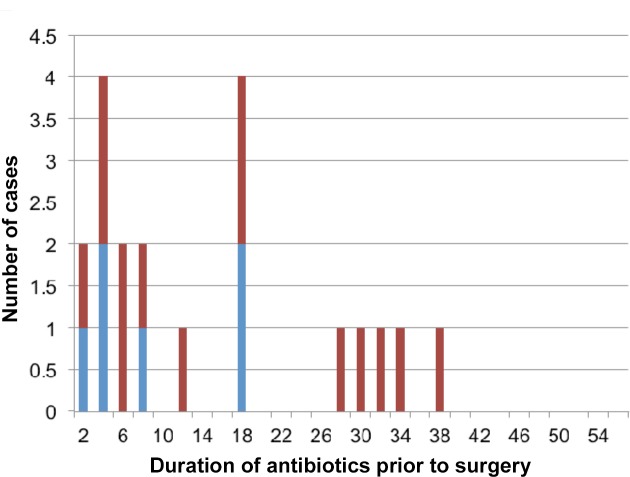
The median age of the patients was 65 years (range 2–94 years) and their lesion was present for a median of 42 days prior to diagnosis (IQR, 24–70 days). Fifty-one (82%) patients had their lesions surgically excised, with the defect directly closed in 25 (49%) cases, and closed with split skin grafts in 21 (41%), a vascularised tissue flap in 4 (8%) and both a split skin graft and a vascularised tissue flap in 1 case (2%). Thirty-nine (76%) of those who had surgery had previously commenced antibiotics for a median of 8 days (IQR, 4–18 days). Twenty of these patients had specimens sent for M. ulcerans culture and their median duration of antibiotics prior to surgery was 12 days (IQR 5–28 days). Six of these patients (30%) had positive mycobacterial cultures; 4 had received 8 days or less of antibiotics, one had received 17 days and one 18 days of antibiotics prior to surgery (Fig. 1).
Fig 1. Red column-Negative tissue culture.
Blue column-Positive tissue culture.
The regimens used were predominantly rifampicin-based in combination with ciprofloxacin in 41 (66%), clarithromycin in 12 (19%), clarithromycin/ethambutol in 3 (5%) and moxifloxacin in 2 (3%) patients. Other combinations included clarithromycin with either ethambutol in 2 patients (3%) or ciprofloxacin (1 patient; 2%) and clarithromycin alone in one patient (2%). The median duration of treatment was 29 days (IQR 21–41 days). Cessation secondary to side effects, often attributed to more than one of the drugs in a combination, occurred in 34 (55%) patients. The most common side effects included nausea, vomiting, diarrhoea, hepatitis, rash, joint aches and acute renal failure. In twenty-two (36%) patients the clinician’s decision to cease treatment was based on presumed adequate treatment when combined with surgical management of the lesions. Two patients ceased due to interactions with concomitant medications or patient’s wishes in each of the surgically treated and antibiotic only groups.
Overall treatment results (n = 62)
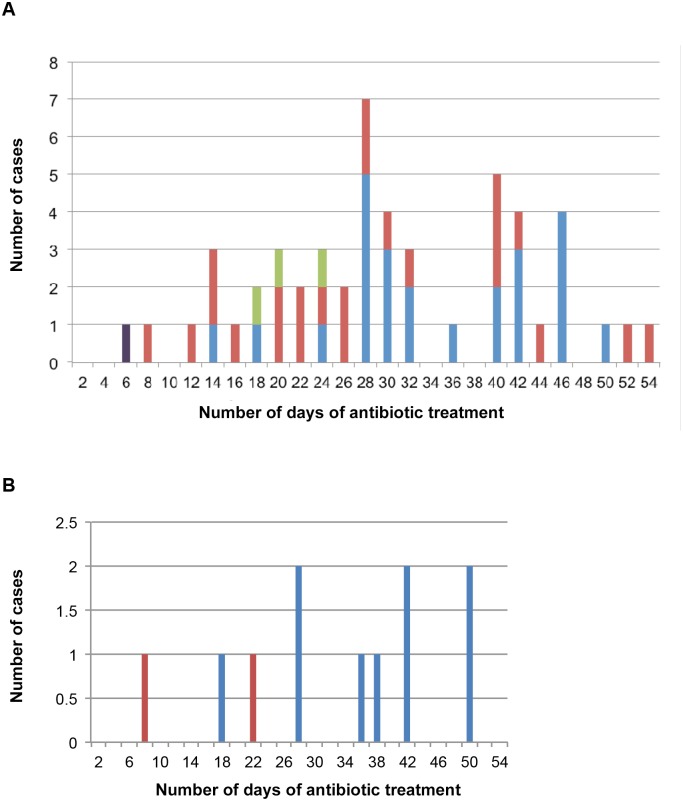
Fifty-nine of 62 (95%) short-course patients experienced treatment success, including the 5 WHO category 3 patients. Details of the three patients who failed treatment are listed in Table 3. This compares with a 99% success rate (144/145) for patients who received long-course treatment. Outcomes according to the duration of antibiotic treatment are presented in Table 4 and Fig. 2. Overall, those who received less than 14 days of therapy had a 50% success rate which increased to 94% if they received 14–27 days of treatment and 100% for 28–55 days treatment (p<0.01; Table 4, Fig. 2A). The success rate for those receiving < 28 days compared to ≥ 28 days was reduced (86% versus 100%, p = 0.04).
Table 3. Characteristics of patients who failed short-course antibiotic treatment for M. ulcerans infection.
| Year | Age/Sex | Co-morbid | Lesion | Sx | Abx | Days | Cessation reason | Result |
|---|---|---|---|---|---|---|---|---|
| 2001 | 79F | Diabetes | Distal leg ulcer | No | ClarithromycinEthambutol | 7 | Nausea | Local recurrence |
| 2002 | 73F | Diabetes I/S^ | Distal wrist nodule | No | Rifampicin Clarithromycin Ethambutol | 21 | Hepatitis | Local recurrence |
| 2006 | 94F | Nil noted | Distal leg ulcer | Yes* | Rifampicin Ciprofloxacin | 6 | Nausea / diarrhoea | Local and distal (ankle) recurrence |
Sx = surgery; Abx = antibiotics
^Immunosuppressed on prednisolone for eczema
*positive surgical margin
Table 4. Proportion of patients achieving treatment success according to antibiotic treatment duration for M. ulcerans infection.
| Days of antibiotic treatment | p value | ||||
|---|---|---|---|---|---|
| 1–13 | 14–27 | 28–41 | 42–56 | ||
| Overall (n = 62) | |||||
| Treatment success | 2 (50%) | 17 (94%) | 28 (100%) | 12 (100%) | 0.001 |
| Treatment failure | 2 (50%) | 1 (6%) | 0 (0%) | 0 (0%) | |
| Surgery + antibiotics (n = 51) | |||||
| Treatment success | 2 (67%) | 16 (100%) | 23 (100%) | 9 (100%) | 0.001 |
| Treatment failure | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Antibiotics alone (n = 11) | |||||
| Treatment success | 0 (0%) | 1 (50%) | 5 (100%) | 3 (100%) | 0.05 |
| Treatment failure | 1 (100%) | 1 (50%) | 0 (0%) | 0 (0%) | |
Fig 2. A) Purple column-Positive margin / treatment failure.
Green column-Unknown margin / treatment success. Red column -Negative margin / treatment success. Blue column-Positive margin / treatment success. B) Red column-Treatment failure. Blue column-Treatment success.
Surgery plus antibiotics (n = 51)
There was a 98% (50/51 cases) treatment success rate in those receiving antibiotics and surgery (Table 4, Fig. 2). Cure rates were significantly increased in those who received longer durations of therapy (p<0.001). All 23 patients treated with antibiotics and surgery for 14–28 days achieved treatment success, in comparison to 67% (2 patients) if they received antibiotics for less than 14 days (p = 0.12). When only cases with positive margins were included the cure rates remained higher for treatment durations of 14–28 days compared with < 14 days (8/8 patients versus 0/1 patients, p = 0.11).
Antibiotics alone (n = 11)
Although the numbers are small, there was an 82% (9/11 cases) success rate for those treated with antibiotics alone. Cure rates were significantly increased in those who received a duration of therapy greater than 28 days (p = 0.05). Two of three patients receiving antibiotics alone for less than 28 days failed treatment, whilst the 6 patients receiving 28–42 days of treatment were cured (p = 0.08; Table 4; Fig. 2B).
Success rates with respect to known risk factors for recurrence with surgery alone
There were no significant differences in recurrence rates for those patients with positive margins, age ≥ 60 years, duration of symptoms > 75 days, immunosuppression or distal lesions (Table 5).
Table 5. Proportion of patients achieving treatment success for M. ulcerans infection in high-risk categories.
| Parameter | No per group | No treatment success (%) | p value |
|---|---|---|---|
| Positive surgical margins | 25 | 24 (96) | 0.33 |
| Negative surgical margins | 23 | 23 (100) | |
| Age < 60 years | 24 | 24 (100) | 0.28 |
| Age ≥ 60 years | 38 | 35 (92) | |
| Duration of symptoms ≤ 75 days | 46 | 44 (96) | 0.53 |
| Duration of symptoms > 75 days | 13 | 12 (92) | |
| Immunosuppressed | 6 | 5 (83) | 0.27 |
| Not immunosuppressed | 56 | 54 (96) | |
| Distal lesion | 52 | 49 (94) | 1.0 |
| Proximal lesion | 10 | 10 (100) |
Discussion
Our study suggests that an antibiotic treatment course for M. ulcerans infections of less than the current WHO recommended of 8 weeks duration can be associated, in selected patients, with good outcomes with a success rate of 95% overall and 98% in patients who received combined medical and surgical therapy. However the effectiveness of short-course therapy was influenced by the duration of antibiotics. Success rates of 100% were achieved after at least 28 days of antibiotics but this reduced to 94% if 14–27 days of antibiotics were used and only 50% if less than 14 days of antibiotics were used (p = 0.001).
Surgery may have also influenced success rates with improved results in those who received antibiotics plus surgery compared to antibiotics alone. Although the numbers were small in the group treated with antibiotics alone (11 patients), it may be that shorter antibiotic treatment courses are effective when there has been a reduction of bacterial load by surgery. This finding needs to be further investigated in studies involving larger patient numbers.
These outcomes with increased patient numbers build on our previous findings from this cohort of 100% treatment success in 21 patients receiving 12–30 days of antibiotics combined with surgical excision [12]. The overall success rate of 98% in this study is similar to that reported from our Barwon Health cohort treated with antibiotics alone for at least 56 days (36 patients; 97% success rate)[13] or at least 60 days of antibiotics plus surgery (44 patients; 100% success rate) [12], and in the long-course group reported in this study (99%). This suggests that in our observational cohorts similar success rates are possible for short-course treatment compared to when antibiotics have been used for at least 56 days.
These success rates are also comparable to studies from African cohorts using 56 days of antibiotics. In a randomised trial in Ghana, 8 weeks of rifampicin and streptomycin or 4 weeks of rifampicin and streptomycin followed by 4 weeks of oral rifampicin and clarithromycin reported no recurrences after 12 months in 147 patients [9]. In Benin an 8-week combination of clarithromycin and rifampicin in 30 patients, with half undergoing surgery, resulted in no treatment failures [10]. In a separate study in Ghana, 8 weeks of rifampicin and streptomycin resulted in no recurrences at one year in 158 patients, of whom only 8 had adjunctive surgical treatment [11]. Finally, in an observational trial in Ghana involving 43 patients with lesions less than 15cm, using 2 weeks of rifampicin and streptomycin followed by 6 weeks of rifampicin and clarithromycin, 93% had completely healed their lesions and there were no recurrences out to one year [14].
Supportive evidence for the effectiveness of short-course treatment is provided by the inability in our study to culture M. ulcerans from surgical specimens taken after more than 18 days of antibiotics (Fig. 1). Further Australian experience reported by Gordon and colleagues [16] showed that excisional samples from 2 patients were culture negative after 4 or 6 weeks of rifampicin combined with moxifloxacin or clarithromycin. Similar findings have also been reported from a WHO-sponsored study of 21 African patients treated with oral rifampicin and intramuscular streptomycin where M. ulcerans could not be cultured from 11 excised lesions after 4 weeks of antibiotic treatment [17]. Furthermore, all samples except one were culture negative after 4 weeks of rifampicin and clarithromycin in 9 patients in Benin [10].
Of note however, in a recent study by Phillips and colleagues [14], where patients were treated with 2 weeks of rifampicin and streptomycin followed by 6 weeks or oral rifampicin and clarithromycin, 3 out of 7 lesions were culture positive 12 weeks after initiation of treatment and 4 weeks after cessation of therapy, albeit at a lower bacterial loads. However despite this all the lesions healed and there were no recurrences at 12 months. Furthermore, Nienhuis and colleagues showed that 3 of 5 lesions healed without surgical debridement after being culture positive post treatment with 4 weeks of standard therapy followed by 4 weeks of rifampicin and clarithromycin [9]. This suggests that sterilization of tissue cultures during antibiotic treatment may not be required to achieve successful treatment outcomes and this further supports the potential for antibiotic treatment durations to be shortened. Spontaneous healing of lesions without treatment has also been reported [18] and it may be possible that once antibiotics +/- surgery have reduced the bacterial load the immune system can sterilize any persisting infection.
In our study, the majority of patients in the short-course group were selected because of antibiotic side-effects requiring a cessation in treatment. If severe side-effects relate to increased serum antibiotic levels compared to those not experiencing such reactions, it is possible that this may have contributed to enhanced microbiological efficacy and improved rates of cure with a reduced antibiotic course.
A limitation of this study is its observational design where patients were not randomised into short and long-course treatment groups. Therefore there were differences in baseline characteristics between the groups that may have influenced outcomes. Firstly there was a higher proportion of patients with WHO category 1 lesions in the short-course group. If smaller lesions with a lower bacterial load require less antibiotic treatment this may have positively influenced outcomes in this group. Secondly the short-course group had a lower proportion of patients with positive margins which may favour improved outcomes as they are associated with a higher rate of failure in the absence of antibiotic treatment [19]. Conversely the short-course group had a higher proportion of patients with immunosuppression and age > 60 years, which have been associated with an increased rate of surgical treatment failure in the absence of antibiotics and may have negatively affected outcomes in this group [19]. Of note in our study, none of the factors associated with treatment failure for surgery alone were associated with treatment failure with the use of short-course compared to long-course antibiotic therapy (Table 5). Furthermore the majority of patients received fluoroquinolone antibiotics in combination with rifampicin and results therefore may not be generalisable to patients not receiving fluoroquinolone-containing regimens. Finally, the lack of randomization also means that there may have been other unmeasured confounders that could have influenced outcomes.
Our study represents a selected subset group of patients from south-eastern Australia. The results may not be generalisable to all M. ulcerans lesions and other settings such as Africa where a higher proportion of patients present late with advanced disease (WHO category 2 and 3) [11], cohorts are younger, the proportion of ulcerative lesions is less, and there is an incidence of HIV co-infection [20]. Nevertheless, based on our findings, we advocate for further research into the effectiveness of shorter antibiotic regimens for the treatment of M. ulcerans, including prospective randomised trials comparing them against standard 8-week treatment regimens. Shorter regimens could potentially include 4 weeks of antibiotics for surgically-excised lesions and 6 weeks of antibiotics if they are used alone. If successful, this could have significant benefits in terms of reducing toxicity and improving adherence associated with M. ulcerans antibiotic treatment.
Conclusions
In selected patients, antibiotic treatment durations for M. ulcerans shorter than the current WHO recommended 8 weeks duration may be associated with successful outcomes. Success may be influenced by the duration of treatment and the use of surgical excision.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.World Health Organisation. Buruli ulcer country information. In. http://www.who.int/buruli/country/en/.
- 2. O’Brien DP, Jenkin GA, Buntine JA, Steffen CM, McDonald A, et al. (2014) Treatment and prevention of Mycobacterium ulcerans infection (Buruli ulcer) in Australia: Guideline update. Medical Journal of Australia 200: 267–270. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organisation. Provisional guidance on the role of specific antibiotics in the management of Mycobacterium ulcerans disease. In. Geneva; 2004. pp. 1–31. [Google Scholar]
- 4. Buruli ulcer: progress report, 2004–2008 (2008). Wkly Epidemiol Rec 83: 145–154. [PubMed] [Google Scholar]
- 5. O’Brien DP, Hughes AJ, Cheng AC, Henry MJ, Callan P, et al. (2007) Outcomes for Mycobacterium ulcerans infection with combined surgery and antibiotic therapy: findings from a south-eastern Australian case series. Med J Aust 186: 58–61. [DOI] [PubMed] [Google Scholar]
- 6. Kibadi K, Mputu-Yamba JB, Mokassa B, Panda M, Muyembe-Tamfum JJ. (2009) [Relapse after surgical treatment of mycobacterium ulcerans infection (buruli ulcer): study of risk factors in 84 patients in the Democratic Republic of the Congo]. Med Trop (Mars) 69: 471–474. [PubMed] [Google Scholar]
- 7. Pak J, O’Brien DP, Quek TY, Athan E. (2012) Treatment costs of Mycobacterium ulcerans in the antibiotic era. International Health 2012,4:123–127. 10.1016/j.inhe.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 8. Asiedu K, Etuaful S. (1998) Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am J Trop Med Hyg 59: 1015–1022. [DOI] [PubMed] [Google Scholar]
- 9. Nienhuis WA, Stienstra Y, Thompson WA, Awuah PC, Abass KM, et al. (2010) Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375: 664–672. 10.1016/S0140-6736(09)61962-0 [DOI] [PubMed] [Google Scholar]
- 10. Chauty A, Ardant MF, Marsollier L, Pluschke G, Landier J, et al. (2011) Oral treatment for Mycobacterium ulcerans infection: results from a pilot study in Benin. Clin Infect Dis 52: 94–96. 10.1093/cid/ciq072 [DOI] [PubMed] [Google Scholar]
- 11. Sarfo FS, Phillips R, Asiedu K, Ampadu E, Bobi N, et al. (2010) Clinical efficacy of combination of rifampin and streptomycin for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 54: 3678–3685. 10.1128/AAC.00299-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Brien DP, McDonald A, Callan P, Robson M, Friedman ND, et al. (2012) Successful outcomes with oral fluoroquinolones combined with rifampicin in the treatment of Mycobacterium ulcerans: an observational cohort study. PLoS Negl Trop Dis 6: e1473 10.1371/journal.pntd.0001473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friedman ND, Athan E, Hughes A, Khajehnoori M, McDonald A, et al. (2013) Mycobacterium ulcerans disease: Experience with primary oral medical therapy in an Australian cohort. PLOS Negl Trop Dis 7: e2315 10.1371/journal.pntd.0002315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phillips RO, Sarfo FS, Abass MK, Abotsi J, Wilson T, et al. (2014) Clinical and bacteriological efficacy of rifampin-streptomycin combination for two weeks followed by rifampin and clarithromycin for six weeks for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother 58: 1161–1166. 10.1128/AAC.02165-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organisation. Treatment of Mycobacterium ulcerans disease (Buruli ulcer): guidance for health workers. Geneva, Switzerland; 2012. [Google Scholar]
- 16. Gordon CL, Buntine JA, Hayman JA, Lavender CJ, Fyfe JAM et al. (2010) All-oral antibiotic treatment for Buruli ulcer: A report of four patients. PLOS Negl Trop Dis 4: e770 10.1371/journal.pntd.0000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Etuaful S, Carbonnelle B, Grosset J, Lucas S, Horsfield C, et al. (2005) Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother 49: 3182–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gordon CL, Buntine JA, Hayman JA, Lavender CJ, Fyfe JA, et al. (2011) Spontaneous clearance of Mycobacterium ulcerans in a case of Buruli ulcer. PLoS Negl Trop Dis 5: e1290 10.1371/journal.pntd.0001290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O’Brien DP, Walton A, Hughes AJ, Friedman ND, McDonald A, et al. (2013) Risk factors for recurrent Mycobacterium ulcerans disease after exclusive surgical treatment in an Australian cohort. Med J Aust 198: 436–439. [DOI] [PubMed] [Google Scholar]
- 20.Christinet V, Rossel L, Serafini M, Delhumeau C, Odermatt P, et al. (2014) Impact of HIV on the Severity of Buruli Ulcer Disease: Results from a Retrospective Study in Cameroon. Open Forum Infectious Diseases (in press). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.