Abstract
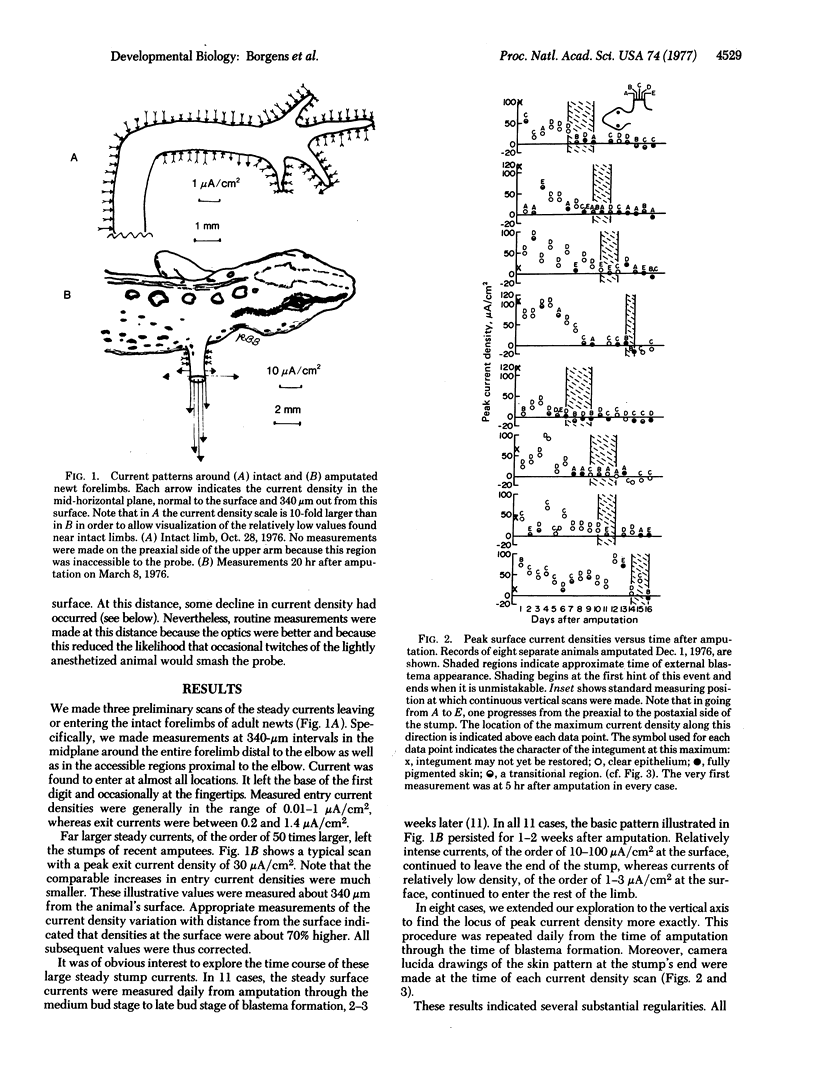
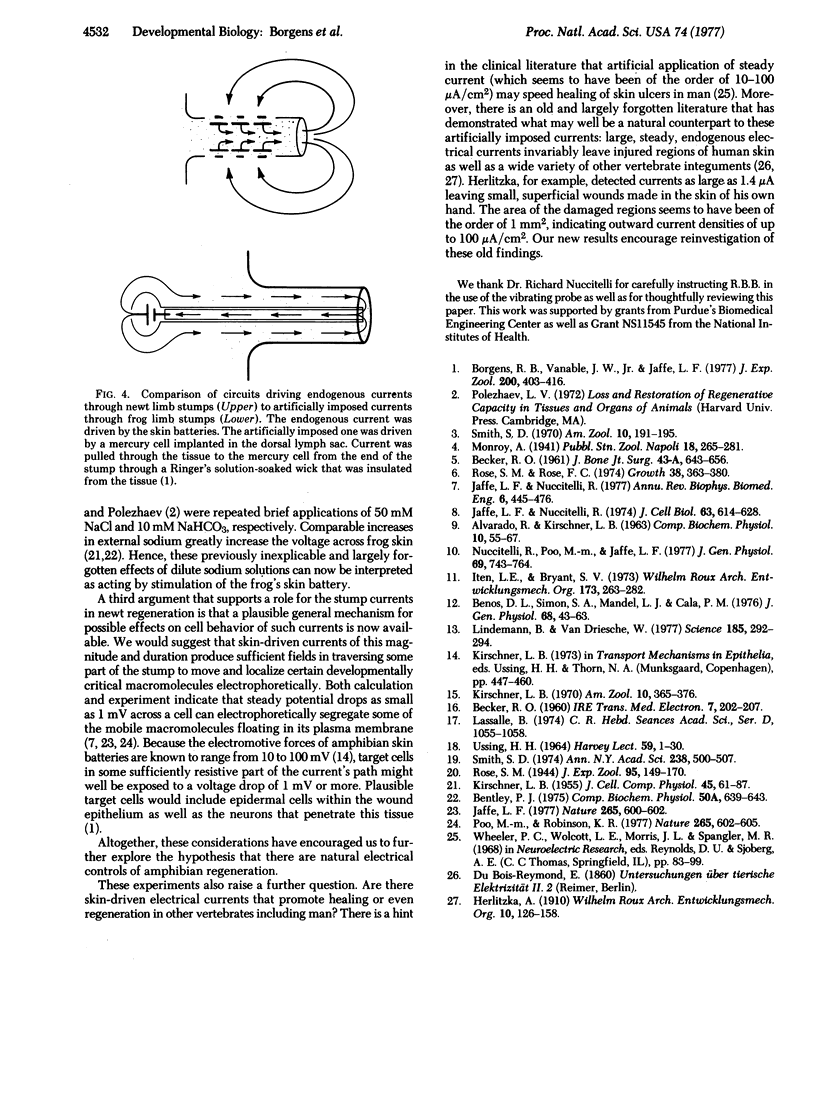
Electrical currents near regenerating newt limbs were measured with a recently developed vibrating probe. Steady currents with local surface densities of 10 to 100 muA/cm2 or more leave the end of the stump during the first 5-10 days after amputation and are balanced by currents with densities of only 1-3 muA/cm2 that enter the intact skin around the stump. They are immediately dependent upon the entry of sodium ions into this skin and are therefore inferred to be skin-driven. The outward currents are comparable in direction, density, duration, and position to artificially imposed currents previously found sufficient to induce significant regeneration of amputated adult frog limbs. This comparison suggests that the endogenous stump currents play some causal role in initiating regeneration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVARADO R. H., KIRSCHNER L. B. OSMOTIC AND IONIC REGULATION IN AMBYSTOMA TIGRINUM. Comp Biochem Physiol. 1963 Sep;10:55–67. doi: 10.1016/0010-406x(63)90102-6. [DOI] [PubMed] [Google Scholar]
- BECKER R. O. The bioelectric factors in amphibian-limb regeneration. J Bone Joint Surg Am. 1961 Jul;43-A:643–656. [PubMed] [Google Scholar]
- BECKER R. O. The bioelectric field pattern in the salamander and its simulation by an electronic analog. IRE Trans Med Electron. 1960 Jul;ME-7:202–207. doi: 10.1109/iret-me.1960.5008048. [DOI] [PubMed] [Google Scholar]
- Benos D. J., Simon S. A., Mandel L. J., Cala P. M. Effect of amiloride and some of its analogues of cation transport in isolated frog skin and thin lipid membranes. J Gen Physiol. 1976 Jul;68(1):43–63. doi: 10.1085/jgp.68.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley P. J. The electrical P.D. across the integument of some neotenous urodele amphibians. Comp Biochem Physiol A Comp Physiol. 1975 Apr 1;50(4):639–643. doi: 10.1016/0300-9629(75)90119-x. [DOI] [PubMed] [Google Scholar]
- Borgens R. B., Vanable J. W., Jr, Jaffe L. F. Bioelectricity and regeneration. I. Initiation of frog limb regeneration by minute currents. J Exp Zool. 1977 Jun;200(3):403–416. doi: 10.1002/jez.1402000310. [DOI] [PubMed] [Google Scholar]
- Hoffman R. A. The epiphyseal complex in fish and reptiles. Am Zool. 1970 May;10(2):191–199. doi: 10.1093/icb/10.2.191. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. Electrophoresis along cell membranes. Nature. 1977 Feb 17;265(5595):600–602. doi: 10.1038/265600a0. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. An ultrasensitive vibrating probe for measuring steady extracellular currents. J Cell Biol. 1974 Nov;63(2 Pt 1):614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- KIRSCHNER L. B. On the mechanism of active sodium transport across the frog skin. J Cell Physiol. 1955 Feb;45(1):61–87. doi: 10.1002/jcp.1030450106. [DOI] [PubMed] [Google Scholar]
- Kirschner L. B. The study of NaCl transport in aquatic animals. Am Zool. 1970 Aug;10(3):365–376. doi: 10.1093/icb/10.3.365. [DOI] [PubMed] [Google Scholar]
- Lindemann B., Van Driessche W. Sodium-specific membrane channels of frog skin are pores: current fluctuations reveal high turnover. Science. 1977 Jan 21;195(4275):292–294. doi: 10.1126/science.299785. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R., Poo M. M., Jaffe L. F. Relations between ameboid movement and membrane-controlled electrical currents. J Gen Physiol. 1977 Jun;69(6):743–763. doi: 10.1085/jgp.69.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M., Robinson K. R. Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature. 1977 Feb 17;265(5595):602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- Rose S. M., Rose F. C. Electrical studies on normally regenerating, on x-rayed, and on denervated limb stumps of Triturus. Growth. 1974 Sep;38(3):363–380. [PubMed] [Google Scholar]
- Smith S. D. Effects of electrode placement on stimulation of adult frog limb regeneration. Ann N Y Acad Sci. 1974;238:500–507. doi: 10.1111/j.1749-6632.1974.tb26816.x. [DOI] [PubMed] [Google Scholar]
- Ussing H. H. Transport of electrolytes and water across epithelia. Harvey Lect. 1965;59:1–30. [PubMed] [Google Scholar]


