Abstract
The increasing use of microwave devices over recent years has meant the bioeffects of microwave exposure have been widely investigated and reported. However the exact biological fate of bone marrow MSCs (BM-MSCs) after microwave radiation remains unknown. In this study, the potential cytotoxicity on MSC proliferation, apoptosis, cell cycle, and in vitro differentiation were assayed following 2.856 GHz microwave exposure at a specific absorption rate (SAR) of 4 W/kg. Importantly, our findings indicated no significant changes in cell viability, cell division and apoptosis after microwave treatment. Furthermore, we detected no significant effects on the differentiation ability of these cells in vitro, with the exception of reduction in mRNA expression levels of osteopontin (OPN) and osteocalcin (OCN). These findings suggest that microwave treatment at a SAR of 4 W/kg has undefined adverse effects on BM-MSCs. However, the reduced-expression of proteins related to osteogenic differentiation suggests that microwave can the influence at the mRNA expression genetic level.
Introduction
Mesenchymal stem cells (MSCs) are multipotent cells that can be induced to differentiate into a variety of mesenchymal tissues, including bone, cartilage, fat, bone marrow stroma, and muscle [1–3]. Despite being widely used in cell-based therapy and tissue engineering, the fate of MSCs after microwave exposure is largely unknown. Microwaves exist at a rate of oscillation of an electromagnetic field in the range of approximately 300 MHz to 300 GHz. They are considered to be a non-ionizing electromagnetic field and are typically present in the environment through radar, radio/TV communications, mobile-phone base stations, occupational use, and medical applications [4–6]. Therefore, the influence of 2.856 GHz microwaves at the cellular and molecular level is a critical area of research, which may provide insights into the appearance of common genotoxic effects that are yet to be resolved.
Biophysical stimulations such as pulsed electromagnetic field have been extensively employed in clinical settings to accelerate and finalize the healing process of a fresh fracture, and to enhance the spontaneous repair capability of bone tissue [7–9]. Several reports have indicated that pulsed electromagnetic field may play a key role in affecting the differentiation ability of mesenchymal stem cells (MSCs) [10–12]. However, to the best of our knowledge, a systemic study to evaluate the potential effects of pulsed 2.856 GHz microwave on MSCs in vitro has not been performed. Generally, radiofrequency/microwave radiation is classified as an environmental pollutant that can be harmful to human health [13]. Although it may not directly cause DNA damage (strand deterioration), several adverse effects on multiple targets after microwave exposure have been reported [5,14,15]. Our previous findings have revealed that non-ionizing radiation electromagnetic pulses (EMP) are indeed unable to induce oxidative stress but reduce the generation of free radicals in rat liver mitochondria [16]. In 2011, the International Agency for Research on Cancer (IARC) classified radiofrequency electromagnetic fields as “possibly carcinogenic to humans” (Group 2B) [13]. This has often been misinterpreted as indicating that some measure of risk has been observed. To date, research has suggested that the possible adverse effects on human cannot be conclusively ruled out based on the available data [13,17,18]. However, strong evidence has been shown in recent studies that the long-term usage of mobile and cordless phones is correlated with cancer risk [19–23]. In this study, MSCs isolated from C57BL/6 mice bone and bone marrow were treated with pulsed 2.856 GHz microwave with a SAR level of 4 W/kg. The SAR was chosen according to the American National Standard Institute (ANSI) standards for safe exposure levels to microwave radiation [24]. Consequently, the potential cytotoxicity on MSCs proliferation, apoptosis, cell cycle, and in vitro differentiation were assayed.
Materials and Methods
Mice
Male C57BL/6 mice (age 2.5 weeks; permission number: SCXK-2007-004) were provided by the Laboratory Animal Center, Academy of Military Medical Sciences, Beijing, China. Six mice were housed in accordance with institutional animal care policies with access to water and food under the standard laboratory procedures. All experiments were performed under protocols approved by the Academy of Military Medical Sciences Animal Care and Use Committee and from the approval of our ethics committee.
Cells
Primary murine MSCs derived from murine bone and bone marrow were isolated and cultured as described previously [25]. Briefly, the mice were sacrificed by cervical dislocation and their bilateral femurs and tibias were retrieved and ground under sterile conditions. After digestion with collagenase II and centrifugation, the bone pieces and pellets were seeded and grown in minimal essential medium (MEM, Gibco) consisting of 4 mM L-glutamine, 100 U/mL penicillin, 100 U/mL streptomycin, and 10% fetal bovine serum (FBS). The medium was changed 72 h after seeding and then subsequently every 3 days. When the cells coated approximately 70% of the bottom of the bottle, they were digested with 0.25% trypsin, diluted and then subcultured. The cells were cultured in a humidified atmosphere of 5% CO2 at 37°C.
Experimental groups
For detection of MSCs proliferation, apoptosis and cell cycle, the MSCs from a single mouse were divided into three groups: (i) sham, (ii) microwave exposure and (iii) positive control which was irradiated with 2.0 Gy 60Co γ-ray. Each group contained at least three samples or Petri dishes from three different mice. The MSCs isolated from one mouse were divided into two groups: (i) sham and (ii) microwave exposure for detection of in vitro differentiation and mRNA expression for OCN and OPN, each group contained at least three samples from another three mice.
Pulsed microwave exposure and γ-ray radiation
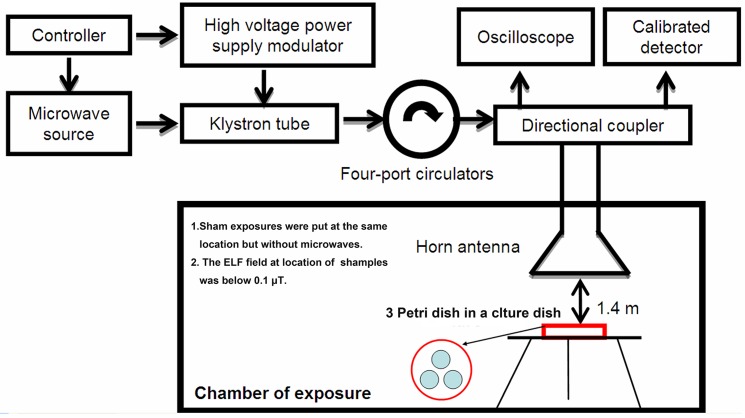
A previously described microwave exposure system was used in this study [26]. In brief, 2.856 GHz pulsed microwaves were generated and transmitted by a microwave source and rectangular waveguide, and microwave energy was transmitted to an electromagnetic shield chamber by an A16-dB standard-gain horn antenna for MSCs exposure. The distance from the antenna to the top of the culture dish was 1.4 m (Fig. 1). The microwave pulses were delivered at 50 pulses per second (pps), with a pulse width of 500 ns. The peak field power densities were tested with a calibrated detector and the oscilloscope was set to 200 W/cm2. The average field power densities were calculated as 5 mW/cm2, and the exposure time was set to 6 min. The relative dielectric constant of the culture medium was 76.4, and the conductivity was 2.5 S/m. The density of the medium was equal to that of water. The SAR was calculated to be 4 W/kg, based on the finite difference time domain (FDTD) method using the following formula: SAR = σE2/ ρ(W/kg) [27,28]. The sham exposure group was handled and processed in parallel to the exposure groups but without exposure to microwaves. The ELF background fields at the location of sham and microwave exposures were below 0.1 μT detected with ELF fields strength measurement system (Holaday Industrial Ins. HI-3604), the static magnetic fields were mainly came from the natural static magnetic fields of the earth. At the same time, the positive controls were irradiated with 60Co γ-ray in the radiation center of Beijing Institute of Radiation Medicine with a dose rate of 1.0 Gy/min for 2.0 min.
Fig 1. Schematic diagram of the microwave exposure system which mainly comprises the microwave source, klystron tube, four-port circulators, directional coupler, and horn antenna.
Monitoring temperature
The real-time variation of the culture medium temperature was monitored using an optical fiber temperature probe (interval time: 1.0 s, resolution: 0.1°C) and a M3300 optical fiber thermometer (Luxtron Co., Santa Clara, CA., USA). The geometry of the temperature measurement is displayed in Fig. 2B. The diameter of the temperature probe was 0.5 mm, and the distance from the probe to bottom of culture dish was 0.5 mm.
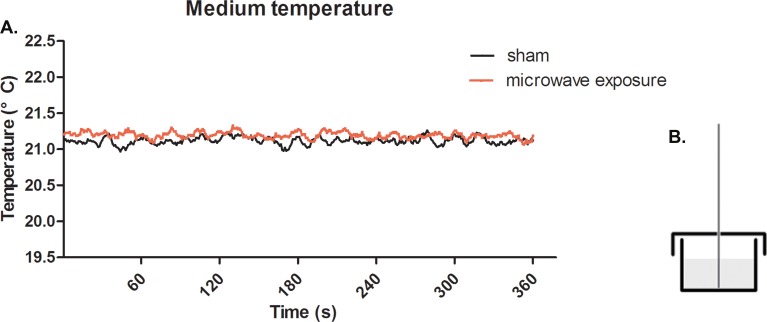
Fig 2. Real-time temperature detection of medium under 2.856 GHz microwave exposure at SAR of 4 W/kg.
(A) Solid red line represents the temperature changes under microwave exposure and the solid black line represents the sham group in parallel to the exposure group (in the absence of microwave radiation). (B) Schematic diagram describing the geometry of temperature measurements.
MTT assay
Briefly, 100 μL BM-MSC cells (4×103 per well) were seeded in 96-well plates immediately after microwave exposure. The microwave and sham exposure groups were cultured on the same plate, with four plates in total. Cell proliferation was detected at days 1, 2, 4, and 6 after exposure. Then, 20 μL MTT (5 mg/mL dissolved in PBS) was added to each well and incubated at 37°C for 4 h. Subsequently, the supernatant was completely removed and 150 μL DMSO was added to the solution, followed by vigorous shaking for 10 min. Absorbance was detected at 490 nm with ELLASA (Multiskan MK3, Thermo Co. USA), where the OD490nm represented the activity of cell viability.
Flow cytometry
MSCs were collected at 6 h after microwave exposure and washed twice with PBS. Apoptosis and cell cycle were assayed according to the manufacturer’s instructions using commercially available colorimetric kits (Nanjing KeyGEN Biotech. Co. Ltd., China). Data were collected on a FACS Cytomics FC-500 (Beckman Coulter) and analyzed using CXP 2.1 software (Beckman Coulter).
In vitro differentiation
For in vitro differentiation, cells were induced with osteogenic induction media containing 0.1 μM dexamethasone, 50 μM ascorbate-2 phosphate,and 10 mM glycerophosphate (Sigma). To induce adipogenic differentiation, cells were cultured in adipogenic induction media containing 1 μM dexamethasone, 200 μM indomethacin, 0.5 μM 3-isobutyl-1-methyl-xanthine, and 10 μg/mL insulin (Sigma). Von Kossa and Oil-Red-O staining were performed as described previously to characterize osteoblastss and adipocytes [25,29]. To induce chondrogenic differentiation, a commercial chondrogenesis differentiation kit was employed according to the manufacturer’s protocols (Gibco).
Differentiated chondrogenic pellet histopathology
H&E staining was performed to analyze chondrocyte differentiation. The differentiated chondrogenic pellets were fixed in 10% (v/v) neutral formalin, embedded in paraffin and sectioned into 5-μm slices. After dewaxing, the sections were stained with hematoxylin for 5 min and eosin for 60 s. Further microscopic examinations were performed after the slices were mounted.
Real-time PCR
Total RNA was extracted with Trizol (Sigma) and reverse-transcribed into cDNA using a reverse transcriptase kit (Takara). cDNA was used as the template for real-time PCR (StepOne Real-Time PCR system, Applied Biosystems. Inc., USA) with SYBR-Green reagent (Applied Biosystems. Inc.) to determine specific gene expression. Primer sequences were as follows: mouse β-actin, 5′-GGCCCAGAGCAAGAGAGGTA-3′ (forward) and 5′-CATGTCGTCCCAGTTGGTAACA-3′ (reverse); mouse OPN, 5′-AGCAAGAAACTCTTCCAAGCAA-3′ (forward) and 5′-GTGAGATTCGTCAGATTCATCCG-3′ (reverse); mouse OCN 5′-CTGACCTCACAGATCCCAAGC-3′ (forward) and 5′-TGGTCTGATAGCTCGTCACAAG -3′ (reverse). The PCR reaction conditions were 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 15 s, and 72°C for 40 s during the holding and cycling stages, and finally 95°C for 15 s, 60°C for 1 min, and 95°C for 15s during the melt curve stage.
Statistical analysis
The experiments were blind-designed with experiment grouping, detections and statistical analysis performed by different authors. Data are presented as the mean ± SD. Statistical differences were assessed using the unpaired two-tailed Student’s t-test and one-way analysis of variance (ANOVA). Differences at P < 0.05 were considered significant. Statistical power was estimated and calculated using software SAS 9.1.3.
Results
Microwave exposure effect on medium temperature
Microwave energy was emitted by an A16-dB standard-gain horn antenna to an electromagnetic shield chamber (dimensions, 7×6.5×4 m; detailed description provided in a previous report [26]). The temperature and humidity of the chamber was controlled throughout the measurements. The cell samples were placed under the antenna with a working distance of 1.4 m. Upon examination, we found no apparent changes in temperature when the samples were exposed to microwaves. In the case of the sham exposure groups, the temperature was found to remain at approximately 21°C within a 6 min period (Fig. 2A). The results indicated that the microwave at SAR of 4 W/kg had no effect on the medium temperature, indicating that there were non-thermal effects when the SAR settings below 4 W/kg.
MSC proliferation viability unaffected by microwave exposure
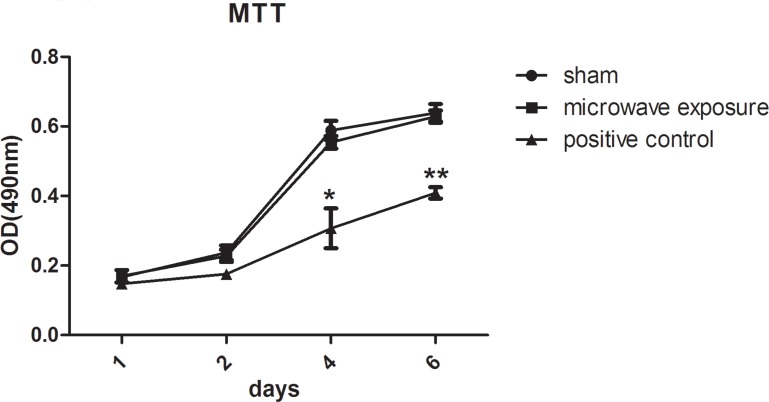
MTT assays were conducted at days 1, 2, 4, and 6 after microwave exposure. MSC proliferation was found to increase significantly within 6 days in sham and exposure groups, the OD490nm values in the sham and exposure groups increased from 0.167 ± 0.01 to 0.639 ± 0.025 (n = 3) and from 0.169 ± 0.017 to 0.628 ± 0.017 (n = 3), respectively, resulting in good levels of cell viability (Fig. 3). There were no obvious differences between sham and exposure groups with P value of 0.84 (statistical power = 0.052) and 0.33 (statistical power = 0.07). Moreover, MSC physiological conditions presented no abnormality after exposure compared to the sham group, as observed using optical microscopy. However, cell viability decreased significantly compared to sham group at day 4 and day 6 with the OD490nm values of 0.307 ± 0.058 (n = 3, P = 0.023, statistical power >0.999) and 0.409 ± 0.016 (n = 3, P = 0.001, statistical power >0.999) after γ-ray radiation (Fig. 3). The MTT assay results indicated that 2.856 GHz pulsed microwave treatment at SAR of 4 W/kg did not influence the MSC viability and proliferation characteristics.
Fig 3. MSC proliferation detected with MTT assays after 2.856 GHz microwave exposure at SAR of 4 W/kg (n = 3, each group contained three cell samples from three different mice).
Solid line with black squares represent the microwave exposure group, solid line with points represent the sham group in parallel to the exposure group in the absence of microwave radiation, and the solid line with black triangles represent the positive control irradiated with 2.0 Gy 60Co γ-ray. Compared with sham group, cell viability decreased significantly at day 4 and day 6 with the OD490nm values of 0.307 ± 0.058 (n = 3, P = 0.023, statistical power >0.999) and 0.409 ± 0.016 (n = 3, P = 0.001, statistical power >0.999) after γ-ray radiation. However, there were no significant differences in cell viability between sham and microwave exposures.
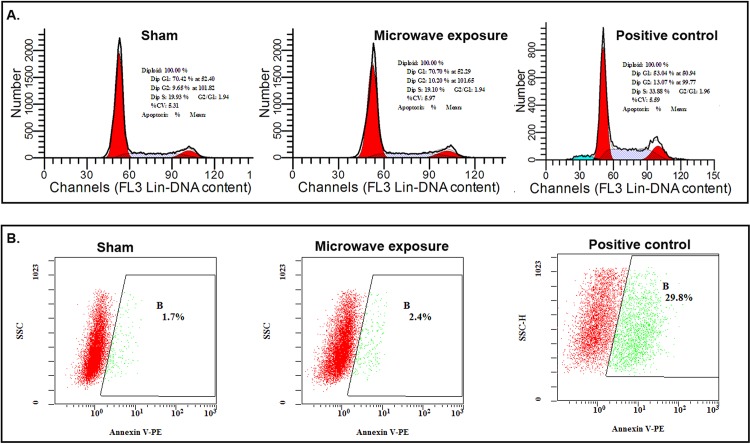
Effect of pulsed microwave on MSC apoptosis
Apoptosis and cell cycle were detected at 6 h after exposure. Representative results are shown in Fig. 4. The cell cycle results showed that cells from the both sham and exposure groups were predominantly located in the G1 stage, exhibiting good levels of viability (Fig. 4A): sham exposure, 70.42% cells at 52.4 and exposure, 70.7% at 52.29 (Fig. 4A). In the G2 phase, 9.65% of cells in the sham exposure group were detected at 101.8 and 10.20% of those in the exposure group at 101.65 (Fig. 4A). Overall, 19.93% cells of the sham group were in the S phase compared to 19.10% of the exposure group (Fig. 4A). The results indicated that the 2.856 GHz pulsed microwave treatment at SAR of 4 W/kg was not able to induce MSCs apoptosis. However, in the γ-irradiated group 53.04% cells were at 50.94 and 13.07% of those at 99.77 in the G1 and G2 phase respectively, 33.88% cells were at S stage indicating the decreased cell viability. The results were further confirmed using an annexin V-PE apoptosis detection kit (Nanjing KeyGEN Biotech. Co. Ltd., China). In this case, the apoptosis rate of the sham and exposure groups were estimated at 2.67% ± 0.55% (n = 3) and 3.07% ± 0.49% (n = 3), respectively, displaying minimal variation after microwave exposure without obvious statistical differences with P value of 0.55 (statistical power = 0.113). However, 60Co γ-ray radiation obviously induced apoptosis with the value of 28.4% ± 3.89% compared with sham group (n = 3, P = 0.009, statistical power >0.999). Representative results are shown in Fig. 4B.
Fig 4. Cell cycle and apoptosis of MSCs detected with flow cytometry after 2.856 GHz microwave exposure at SAR of 4 W/kg.
(A) Cell cycle detected with PI. The representative results of sham, microwave exposure and the positive control were shown in the upper panel. (B) Apoptosis detected with annexin V-PE. The representative results of sham, microwave exposure and the positive control were shown in the lower panel.
In vitro differentiation of MSCs and osteogenic protein mRNA expression
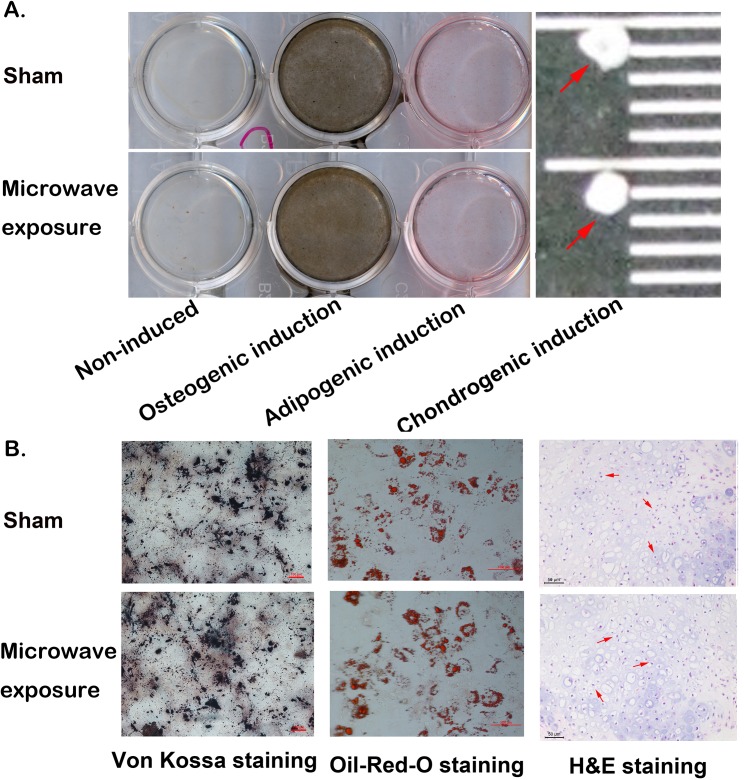
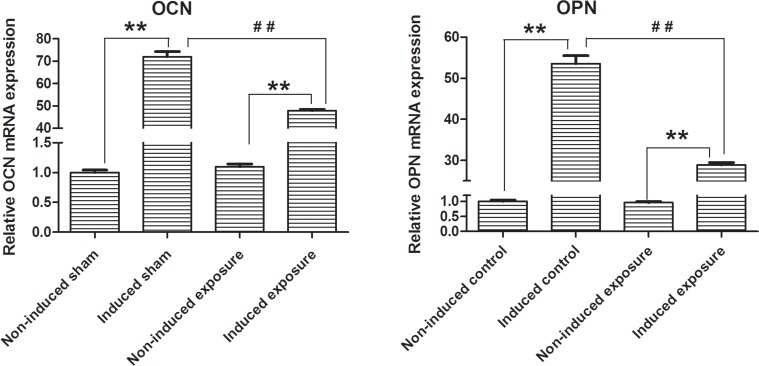
MSCs were induced with different induction media immediately after microwave exposure. Osteoblasts, adipogenic, and chondrogenic cells were differentiated from MSCs in both the sham and microwave exposure groups. However, we found no significant differences between the two groups (Fig. 5). Von Kossa and Oil-Red-O staining demonstrated that high amounts of calcium and fat were deposited and secreted after induction across the two groups, and there were no obvious differences in average optical density between two groups assayed with software LeicaV3.4 (Fig. 5B). The average optical densities of Von Kossa staining in sham and exposure groups were 52.83 ± 4.65 (n = 3) and 51.54 ± 2.50 (n = 3), respectively. For Oil-Red-O staining, average optical densities in both groups were 20.51 ± 1.65 (n = 3) and 21.17 ± 2.60 (n = 3), respectively. There were no statistical differences in osteogenic and adipogenic differentiations between two groups with P values of 0.74 (statistical power = 0.063) and 0.81 (statistical power = 0.060). Concurrently, there were no distinct changes in the size of induced chondrogenic pellets (Fig. 5A). Furthermore, H&E staining showed that chondrogenic cells in both cases were arranged evenly as shown by the arrows in Fig. 5B. The relative osteogenic proteins, OCN and OPN were detected using qPCR. Compared with non-induced groups, OCN and OPN mRNA levels were both significantly increased after induction (n = 3, P < 0.01). Interestingly, these results indicated that the induced mRNA expression levels of OPN and OCN were both significantly reduced after microwave exposure compared with the induced sham groups. The relative induced mRNA expression level of OCN and OPN decreased significantly from 71.94 ± 2.31 and 53.52 ± 1.97 to 47.89 ± 0.49 and 28.90 ± 0.57 after exposure, respectively. (n = 3, P < 0.01, statistical power >0.999) (Fig. 6).
Fig 5. MSCs in vitro differentiation after 2.856 GHz microwave exposure at SAR of 4 W/kg.
(A) Osteogenic induction and adipogenic induction were detected with Von Kossa and Oil-Red-O staining. The induced chondrogenic pellets were placed onto a black ruler as indicated by the arrows. (B) Microscopic examination of induced osteoplasts (left panel), and adipocyted (middle panel) with Von Kossa and Oil-Red-O staining (scale bar = 100 μm), and chondrocytes (right panel) with H&E staining (scale bar = 50 μm).
Fig 6. Relative OPN and OCN mRNA expression detected with Q-PCR after 2.856 GHz microwave exposure at SAR of 4 W/kg.
(left panel) OCN; (right panel) OPN. Compared with the non-induced groups, mRNA levels of OCN and OPN were both significantly increased after induction (**, n = 3, P < 0.01, statistical power >0.999, each group contained three samples from three different mice). OPN and OCN mRNA expression levels were both significantly reduced after microwave exposure compared with the sham group (##, n = 3, P < 0.01, statistical power >0.999, each group contained three samples from three different mice).
Discussion
Our results indicate that BM-MSCs exposed to 2.856 GHz microwave radiation at a SAR of 4 W/kg displayed no significant changes in cell viability, apoptosis, and in vitro differentiation, with the exception of reduced levels of mRNA expression for OCN and OPN.
With the ever-increasing application of microwaves, public concerns about their possible health impact have been raised. Several studies have shown that microwaves cause various biological effects depending upon their field strengths, frequencies, waveforms, modulation and durations of exposure [30,31]. Such microwave-induced damage is known to lead to death in single cell organisms, inhibit cell proliferation, cause DNA damage and alter gene expression [32–35]. However, no obvious adverse effects after the exposure of MSCs to microwaves were observed in this study.
We observed no changes in medium temperature, which suggested a non-thermal effect on MSCs after microwave exposure. Our results indicated that microwave exposure had no effect on MSC proliferation, apoptosis, and differentiation. Nevertheless, the expression levels of OCN and OPN mRNA were reduced after exposure, which suggests that microwaves may influence MSCs at the level of transcription.
Microwaves from mobile phones may inhibit the formation of 53BP1 foci in human primary fibroblasts, MSCs, and lymphocytes, which indicates a possible links to cancer risk [36]. However,the pre-exposure of mice to 900-MHz radiofrequency fields showed that adaptive responses reduce the level of hematopoietic damage caused by ionizing radiation [37–39]. The differentiation of MSCs into chondrocytes could be enhanced by millimeter waves [40]; however the fact that 2.856 GHz microwaves had no effects on in vitro differentiation capabilities may have been the result of different wave types and irradiation conditions. In addition, microwaves may alter cell morphology, disrupt cell division [41], and influence cell membrane permeability [42], resulting in either the degeneration, apoptosis or necrosis of cells at different stages. Recent work on chromosomes and DNA as targets for resonance interaction between living cells and microwaves [43] had also suggested that exposure to microwave radiation can damage the gene structure [44]. Meanwhile, numerous experimental evidences have reported that radiofrequency radiation does not induce genetic effects [45,46].
Thus, identifying and evaluating the biological effects of microwave has been a complex and controversial process because the mechanisms by which microwave exert their effects remain poorly characterized. It is critical that the key targets and mechanisms of pulsed microwaves should be characterized in the near future.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Stappenbeck TS, Miyoshi H (2009) The role of stromal stem cells in tissue regeneration and wound repair. Science 324: 1666–1669. 10.1126/science.1172687 [DOI] [PubMed] [Google Scholar]
- 2. Knight MN, Hankenson KD (2013) Mesenchymal Stem Cells in Bone Regeneration. Adv Wound Care (New Rochelle) 2: 306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Das M, Sundell IB, Koka PS (2013) Adult mesenchymal stem cells and their potency in the cell-based therapy. J Stem Cells 8: 1–16. [PubMed] [Google Scholar]
- 4. Kesari KK, Siddiqui MH, Meena R, Verma HN, Kumar S (2013) Cell phone radiation exposure on brain and associated biological systems. Indian J Exp Biol 51: 187–200. [PubMed] [Google Scholar]
- 5. Szmigielski S (2013) Reaction of the immune system to low-level RF/MW exposures. Sci Total Environ 454–455: 393–400. 10.1016/j.scitotenv.2013.11.054 [DOI] [PubMed] [Google Scholar]
- 6. Cucurachi S, Tamis WL, Vijver MG, Peijnenburg WJ, Bolte JF, et al. (2013) A review of the ecological effects of radiofrequency electromagnetic fields (RF-EMF). Environ Int 51: 116–140. 10.1016/j.envint.2012.10.009 [DOI] [PubMed] [Google Scholar]
- 7. Massari L, Caruso G, Sollazzo V, Setti S (2009) Pulsed electromagnetic fields and low intensity pulsed ultrasound in bone tissue. Clin Cases Miner Bone Metab 6: 149–154. [PMC free article] [PubMed] [Google Scholar]
- 8. Midura RJ, Ibiwoye MO, Powell KA, Sakai Y, Doehring T, et al. (2005) Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J Orthop Res 23: 1035–1046. [DOI] [PubMed] [Google Scholar]
- 9. Walker NA, Denegar CR, Preische J (2007) Low-intensity pulsed ultrasound and pulsed electromagnetic field in the treatment of tibial fractures: a systematic review. J Athl Train 42: 530–535. [PMC free article] [PubMed] [Google Scholar]
- 10. Ceccarelli G, Bloise N, Mantelli M, Gastaldi G, Fassina L, et al. (2013) A comparative analysis of the in vitro effects of pulsed electromagnetic field treatment on osteogenic differentiation of two different mesenchymal cell lineages. Biores Open Access 2: 283–294. 10.1089/biores.2013.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Esposito M, Lucariello A, Costanzo C, Fiumarella A, Giannini A, et al. (2013) Differentiation of human umbilical cord-derived mesenchymal stem cells, WJ-MSCs, into chondrogenic cells in the presence of pulsed electromagnetic fields. In Vivo 27: 495–500. [PubMed] [Google Scholar]
- 12. Teven CM, Greives M, Natale RB, Su Y, Luo Q, et al. (2012) Differentiation of osteoprogenitor cells is induced by high-frequency pulsed electromagnetic fields. J Craniofac Surg 23: 586–593. 10.1097/SCS.0b013e31824cd6de [DOI] [PubMed] [Google Scholar]
- 13. Baan R, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. (2011) Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol 12: 624–626. [DOI] [PubMed] [Google Scholar]
- 14. Kesari KK, Kumar S, Nirala J, Siddiqui MH, Behari J (2013) Biophysical evaluation of radiofrequency electromagnetic field effects on male reproductive pattern. Cell Biochem Biophys 65: 85–96. 10.1007/s12013-012-9414-6 [DOI] [PubMed] [Google Scholar]
- 15. Ghanbari M, Mortazavi SB, Khavanin A, Khazaei M (2013) The Effects of Cell Phone Waves (900 MHz-GSM Band) on Sperm Parameters and Total Antioxidant Capacity in Rats. Int J Fertil Steril 7: 21–28. [PMC free article] [PubMed] [Google Scholar]
- 16. Wang C, Zhou H, Peng R, Wang L, Su Z, et al. (2013) Electromagnetic pulse reduces free radical generation in rat liver mitochondria in vitro. Free Radic Res 47: 276–282. 10.3109/10715762.2013.768342 [DOI] [PubMed] [Google Scholar]
- 17. Carlberg M, Soderqvist F, Hansson Mild K, Hardell L (2013) Meningioma patients diagnosed 2007–2009 and the association with use of mobile and cordless phones: a case—control study. Environ Health 12: 60 10.1186/1476-069X-12-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Vocht F, Hannam K, Buchan I (2013) Environmental risk factors for cancers of the brain and nervous system: the use of ecological data to generate hypotheses. Occup Environ Med 70: 349–356. 10.1136/oemed-2012-100954 [DOI] [PubMed] [Google Scholar]
- 19. Coureau G, Bouvier G, Lebailly P, Fabbro-Peray P, Gruber A, et al. (2014) Mobile phone use and brain tumours in the CERENAT case-control study. Occup Environ Med 71: 514–522. 10.1136/oemed-2013-101754 [DOI] [PubMed] [Google Scholar]
- 20. Hardell L, Carlberg M (2013) Use of mobile and cordless phones and survival of patients with glioma. Neuroepidemiology 40: 101–108. 10.1159/000341905 [DOI] [PubMed] [Google Scholar]
- 21. Hardell L, Carlberg M, Hansson Mild K (2013) Use of mobile phones and cordless phones is associated with increased risk for glioma and acoustic neuroma. Pathophysiology 20: 85–110. 10.1016/j.pathophys.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 22. Hardell L, Carlberg M, Soderqvist F, Mild KH (2013) Pooled analysis of case-control studies on acoustic neuroma diagnosed 1997–2003 and 2007–2009 and use of mobile and cordless phones. Int J Oncol 43: 1036–1044. 10.3892/ijo.2013.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hardell L, Carlberg M, Soderqvist F, Mild KH (2013) Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol 43: 1833–1845. 10.3892/ijo.2013.2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin JC (2003) Safety standards for human exposure to radio frequency radiation and their biological rationale. Microwave Magazine, IEEE 4: 22–26. [Google Scholar]
- 25. Yang YM, Li H, Zhang L, Dang RJ, Li P, et al. (2013) A new method for isolating and culturing mouse bone marrow mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 21: 1563–1567. 10.7534/j.issn.1009-2137.2013.06.037 [DOI] [PubMed] [Google Scholar]
- 26. Wang H, Peng R, Zhou H, Wang S, Gao Y, et al. (2013) Impairment of long-term potentiation induction is essential for the disruption of spatial memory after microwave exposure. Int J Radiat Biol 89: 1100–1107. 10.3109/09553002.2013.817701 [DOI] [PubMed] [Google Scholar]
- 27. Esmekaya MA, Seyhan N, Omeroglu S (2010) Pulse modulated 900 MHz radiation induces hypothyroidism and apoptosis in thyroid cells: a light, electron microscopy and immunohistochemical study. Int J Radiat Biol 86: 1106–1116. 10.3109/09553002.2010.502960 [DOI] [PubMed] [Google Scholar]
- 28. Celuch M, Gwarek WK (2007) Properties of the FDTD method relevant to the analysis of microwave power problems. J Microw Power Electromagn Energy 41: 62–80. [DOI] [PubMed] [Google Scholar]
- 29. Wang XY, Lan Y, He WY, Zhang L, Yao HY, et al. (2008) Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood 111: 2436–2443. [DOI] [PubMed] [Google Scholar]
- 30. Kubinyi G, Thuroczy G, Bakos J, Boloni E, Sinay H, et al. (1996) Effect of continuous-wave and amplitude-modulated 2.45 GHz microwave radiation on the liver and brain aminoacyl-transfer RNA synthetases of in utero exposed mice. Bioelectromagnetics 17: 497–503. [DOI] [PubMed] [Google Scholar]
- 31. IARC (2013) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Non-ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields Lyon, France,. IARC Press; 102: 1–406. [PMC free article] [PubMed] [Google Scholar]
- 32. Aksoy U, Sahin S, Ozkoc S, Ergor G (2005) The effect of electromagnetic waves on the growth of Entamoeba histolytica and Entamoeba dispar. Saudi Med J 26: 1388–1390. [PubMed] [Google Scholar]
- 33. Cleary SF, Du Z, Cao G, Liu LM, McCrady C (1996) Effect of isothermal radiofrequency radiation on cytolytic T lymphocytes. FASEB J 10: 913–919. [DOI] [PubMed] [Google Scholar]
- 34. Diem E, Schwarz C, Adlkofer F, Jahn O, Rudiger H (2005) Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res 583: 178–183. [DOI] [PubMed] [Google Scholar]
- 35. Belyaev IY, Koch CB, Terenius O, Roxström-Lindquist K, Malmgren LO, et al. (2006) Exposure of rat brain to 915 MHz GSM microwaves induces changes in gene expression but not double stranded DNA breaks or effects on chromatin conformation,. Bioelectromagnetics 27: 295–306. [DOI] [PubMed] [Google Scholar]
- 36. Belyaev I, Markova E, Malmgren L (2010) Microwaves from Mobile Phones Inhibit 53BP1 Focus Formation in Human Stem Cells More Strongly Than in Differentiated Cells: Possible Mechanistic Link to Cancer Risk. Environ Health Perspect 118: 394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cao Y, Xu Q, Jin ZD, Zhou Z, Nie JH, et al. (2011) Induction of adaptive response: pre-exposure of mice to 900 MHz radiofrequency fields reduces hematopoietic damage caused by subsequent exposure to ionising radiation. Int J Radiat Biol 87: 720–728. 10.3109/09553002.2010.550981 [DOI] [PubMed] [Google Scholar]
- 38. Cao Y, Zhang W, Lu MX, Xu Q, Meng QQ, et al. (2009) 900-MHz microwave radiation enhances gamma-ray adverse effects on SHG44 cells. J Toxicol Environ Health A 72: 727–732. 10.1080/15287390902841466 [DOI] [PubMed] [Google Scholar]
- 39. Cao Y, Xu Q, Jin ZD, Zhang J, Lu MX, et al. (2010) Effects of 900-MHz microwave radiation on gamma-ray-induced damage to mouse hematopoietic system. J Toxicol Environ Health A 73: 507–513. 10.1080/15287390903523451 [DOI] [PubMed] [Google Scholar]
- 40. Wu GW, Liu XX, Wu MX, Zhao JY, Chen WL, et al. (2009) Experimental study of millimeter wave-induced differentiation of bone marrow mesenchymal stem cells into chondrocytes. Int J Mol Med 23: 461–467. [DOI] [PubMed] [Google Scholar]
- 41. Velizarov S, Raskmark P, Kwee S (1999) The effects of radiofrequency fields on cell proliferation are non-thermal. Bioelectrochem Bioenerg 48: 177–180. [DOI] [PubMed] [Google Scholar]
- 42. Pakhomov AG, Akyel Y, Pakhomova ON, Stuck BE, Murphy MR (1998) Current state and implications of research on biological effects of millimeter waves: a review of the literature. Bioelectromagnetics 19: 393–413. [DOI] [PubMed] [Google Scholar]
- 43. Belyaev IY, Alipov YD, Polunin VA, Shcheglov VS (1993) Evidence for dependence of resonant frequency of millimeter wave interaction with Escherichia coli K12 cells on haploid genome length. Electro Magnetobiol 12: 39–49. 8030305 [Google Scholar]
- 44. Tice RR, Hook GG, Donner M, McRee DI, Guy AW (2002) Genotoxicity of radiofrequency signals. I. Investigation of DNA damage and micronuclei induction in cultured human blood cells. Bioelectromagnetics 23: 113–126. [DOI] [PubMed] [Google Scholar]
- 45. Vijayalaxmi Leal BZ, Szilagyi M, Prihoda TJ, Meltz ML (2000) Primary DNA damage in human blood lymphocytes exposed in vitro to 2450 MHz radiofrequency radiation. Radiat Res 153: 479–486. [DOI] [PubMed] [Google Scholar]
- 46. McNamee JP, Bellier PV, Gajda GB, Lavallee BF, Marro L, et al. (2003) No evidence for genotoxic effects from 24 h exposure of human leukocytes to 1.9 GHz radiofrequency fields. Radiat Res 159: 693–697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.