Abstract
Methamphetamine and related compounds are now the second most commonly used illicit substance worldwide, after cannabis. Reports of methamphetamine‐associated cardiomyopathy (MAC) are increasing, but MAC has not been well reviewed. This analysis of MAC will provide an overview of the pharmacology of methamphetamine, historical perspective and epidemiology, a review of case and clinical studies, and a summary of the proposed mechanisms for MAC. Clinically, many questions remain, including the appropriate therapeutic interventions for MAC, the incidence and prevalence of cardiac pathology in methamphetamine users, risk factors for developing MAC, and prognosis of these patients. In conclusion, recognition of the significance of MAC among physicians and other medical caregivers is important given the growing use of methamphetamine and related stimulants worldwide.
Introduction
After cannabis, methamphetamine and related compounds have become the most widely abused illicit drug worldwide.1 A myriad of clinical complications have been associated with methamphetamine use. Although methamphetamine‐associated cardiomyopathy (MAC) is increasingly being reported, this has not been well studied. In 2010, heart failure ranked as the third most common reason for hospitalization in the United States among adults,2 and registry data have shown that >5% of patients hospitalized for heart failure reported abusing stimulants, including methamphetamine.3 This article will review (1) the pharmacology of methamphetamine, (2) historical perspective and epidemiology, (3) case and clinical studies, (4) proposed mechanisms of MAC, and (5) treatment.
Pharmacology
Amphetamine is a synthetic derivative of phenethylamine, a natural amine that is biosynthesized from phenylalanine.4 The addition of an extra methyl group to amphetamine yields methamphetamine (Figure 1), which has increased lipid solubility and crosses the blood–brain barrier more readily, thereby increasing the stimulant properties on the central nervous system.
Figure 1.
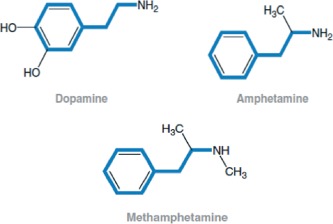
Chemical structures of methamphetamine and related compounds.11
There are 2 isomeric forms of methamphetamine, a dextro‐isomer and a levo‐isomer.5 The dextro‐isomer is the abused drug, as it is the central nervous system stimulant and is five times more biologically active, while the levo‐isomer is used in over‐the‐counter nasal decongestants.6 The most popular route of administration is smoking of crystal methamphetamine; injection and insufflation are other popular routes.7
Depending on route of administration and urine pH, methamphetamine is excreted primarily in urine, with approximately 30% to 50% of a dose excreted as the parent drug and up to 10% as dextroamphetamine.8, 9 The half‐life of the drug is approximately 9 to 12 hours, and this appears to be independent of the route of administration.4 Initial drug screening typically uses immunoassay to detect the presence of methamphetamine of amphetamine in urine. False positives can occur, and further testing can be obtained using techniques such as gas chromatography–mass spectrometry or chiral chromatography.10
Though structurally very similar to catecholamines (Figure 1), methamphetamine exerts its sympathomimetic effects indirectly by causing increased release of dopamine, norepinephrine, epinephrine, and serotonin into the synapse.11 It does not have direct sympathomimetic properties. Various mechanisms have been described, such as methamphetamine entering the presynaptic neurons via both transporters and passive diffusion to cause release of catecholamines into the cytosol and eventually into the synapse.11 Also, reuptake transporters for these neurotransmitters are blocked by methamphetamine, causing increased neuronal activity.
Clinically desirable effects of methamphetamine use may include increased alertness, euphoria, energy, and decreased appetite.12 Dopamine appears to be the major neurotransmitter affected by methamphetamine abuse, with dopaminergic neurons involved in the mesolimbic and mesocortical pathways playing a key role in reward, pleasure, and mood.13
Methamphetamine is more potent and longer lasting than cocaine, which only blocks the reuptake of catecholamines.5 When smoked, the sense of euphoria can last for hours as opposed to minutes with cocaine. Also, because it is less expensive, methamphetamine is becoming a progressively more attractive drug for abuse.
Historical Perspective
Nagai Nagayoshi first isolated ephedrine from the Chinese shrub Ephedra distachya in 1885.14 Ephedra is a genus with several species, including E. sinica (ma huang), which has been used in Chinese traditional medicine for thousands of years for treatment of asthma and hay fever.15 Nagayoshi then synthesized methamphetamine from ephedrine in 1893.14
Methamphetamine has been prescribed for a variety of clinical conditions. The US Food and Drug Administration approved methamphetamine for treatment of narcolepsy, depression, alcoholism, and hay fever in 1944 and for the treatment of obesity in 1947.16 Methamphetamine was widely used by both Allied and Axis forces in World War II as a stimulant to decrease fatigue and heighten alertness.14, 16 In the United States in 1967, methamphetamine reached a peak of 31 million prescriptions. Currently it is approved for obesity and attention deficit hyperactivity disorder.17
Epidemiology of Methamphetamine Abuse
According to the 2011 National Survey on Drug Use and Health, illicit‐drug use had risen to nearly its highest level in 10 years in the United States, with 8.7% of Americans age ≥12 years, or approximately 22.5 million people, saying they had used illicit substances in the month prior to the survey.18 The number of current methamphetamine users rose to 439,000. Historically, methamphetamine abuse was most common in Hawaii and on the West Coast, but there has been a clear spread eastward (Figure 2).19 The mean age at first use in 2011 was only 17.8 years.18
Figure 2.
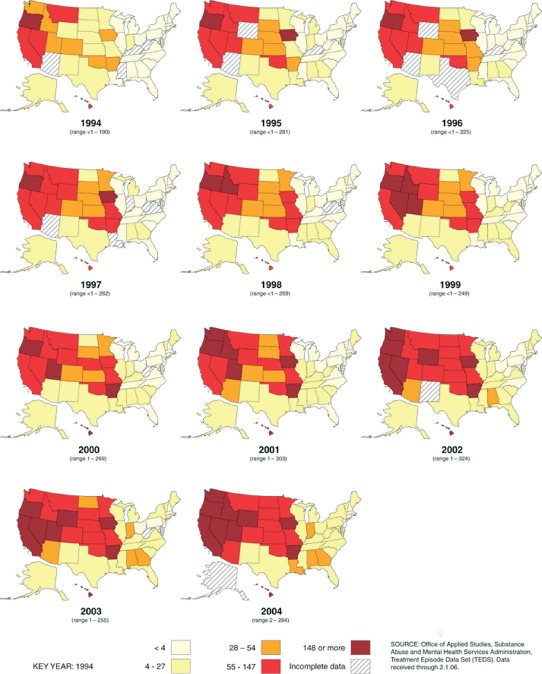
Primary methamphetamine/amphetamine admission rates by state,1994–2004 (per 100 000 population age ≥12).19
Methamphetamine and Cardiac Pathology
Methamphetamine has been linked to various cardiac pathologies. Hypertension and tachycardia appear to increase with increasing doses of methamphetamine due to adrenergic stimulation.20 Other pathologies described include arrhythmias; vasospasm; accelerated atherosclerosis; acute coronary syndrome; sudden cardiac death; coronary, carotid, and aortic dissections; and circulatory collapse, as well as cardiomyopathy.
Case Reports and Clinical Studies
Reports of death related to centrally stimulating amines such as amphetamine were noted as early as the 1940s.21 Kalant et al described the linkage between methamphetamine abuse and death from left ventricular (LV) failure in 2 persons in 1975.22 In 1979, Rajs described the pathologic findings of cardiac‐chamber enlargement, LV hypertrophy, hemorrhage, fibrosis, and contraction‐band necrosis in 14 subjects who had used centrally acting amines such as amphetamine or methamphetamine.23
Multiple case reports from postmortem studies then followed, and in 1989, Jacobs was the first to describe an association of methamphetamine use with dilated cardiomyopathy in a living patient.24 A 48‐year‐old woman was hospitalized for pulmonary edema. Her echocardiogram showed a dilated, poorly contracting left ventricle, and subsequent cardiac catheterization revealed global LV hypokinesis with a left ventricular ejection fraction (LVEF) of 35% and normal coronary arteries. The patient had abused methamphetamine pills for weight loss; after discontinuation of this drug, her LV systolic dysfunction resolved. Also in 1989, Nestor described a case of a 28‐year‐old woman hospitalized with pulmonary edema and dilated cardiomyopathy who admitted to smoking crystal methamphetamine. This was the first association between smokable methamphetamine and cardiac dysfunction. The patient was documented to have no cardiac ischemia or infarct on a thallium treadmill stress test. Numerous cases of MAC have since been reported.
Case series have also been published on MAC. Wijetunga et al performed a retrospective analysis on patients discharged from a tertiary‐care hospital with the diagnosis of cardiomyopathy over a 4‐year time period.25 More than 1600 patients were identified, of whom 120 had a diagnosis of substance abuse as well. After excluding those with coronary artery disease, alcohol or cocaine abuse, or other potential etiologies of cardiomyopathy, 21 subjects remained who were methamphetamine users. Nineteen underwent echocardiography, which revealed dilated LV chamber size with globally depressed LV systolic function to varying degrees in most patients.
Yeo et al performed a case–control study looking at patients age <45 years who were discharged from a tertiary‐care hospital with diagnosis of either congestive heart failure or cardiomyopathy.26 Controls were age‐matched, hospitalized patients who had an echocardiogram with normal LVEF of ≥55% and no wall‐motion abnormalities. Methamphetamine users had a 3.7‐fold increased odds ratio of congestive heart failure or cardiomyopathy as compared with controls.
Ito et al looked retrospectively at patients age <45 years who were hospitalized for either cardiomyopathy or heart failure.27 After exclusion of coronary artery disease or valvular heart disease, patients were divided into 2 groups, one that used methamphetamine and another that did not. The group that used methamphetamine had, on echocardiography, higher LV volumes and lower LVEFs than nonusers.
As previously reported, MAC appears to be potentially reversible upon cessation of methamphetamine use. Anecdotal experiences have also noted remarkable improvement in cardiac function in patients with MAC who have discontinued drug use. In one case report, cardiac magnetic resonance imaging was performed in a patient with severe MAC, demonstrating no delayed gadolinium enhancement to suggest any significant fibrosis.28 The patient discontinued methamphetamine use and was placed on medical therapy, including a β‐blocker and angiotensin‐converting enzyme inhibitor. Her LVEF improved from 37% to 64% after 6 months. The timing of recovery of LV systolic function, and when meaningful recovery is no longer possible, remain unknown, however.
Proposed Mechanisms of Methamphetamine‐Associated Cardiomyopathy
The mechanisms underlying cardiomyopathy in methamphetamine use are most likely multifactorial. Proposed etiologies for cardiac injury include catecholamine excess, coronary vasospasm and ischemia, increases in reactive oxygen species (ROS), mitochondrial injury, changes in myocardial metabolism, and direct toxic effects.29, 30 Pathologically, ventricular hypertrophy and dilation, fibrosis, and contraction‐band necrosis commonly have been found.29
Catecholamine excess with associated coronary vasospasm has been postulated to be a cause of MAC. Chen reported on a case of a 19‐year‐old male who abused methamphetamine presenting with chest pain and was found to have inferolateral ST elevations on electrocardiogram.31 He underwent emergent cardiac catheterization, which revealed no evidence of significant epicardial coronary stenosis; however, there was Thrombolysis In Myocardial Infarction grade 1 flow in all major epicardial vessels with myocardial blush grade of 0, suggestive of global coronary microvascular vasospasm. Hong et al described a patient who abused methamphetamine and died of cardiogenic shock.32 Postmortem examination revealed diffuse transmural myocardial ischemia and focal areas of infarction. The coronary arteries, however, were free of obstructive lesions.
MAC also has been ascribed to increases in ROS. Rats treated with methamphetamine injections were found to have both increased ROS and LV dilation with systolic dysfunction.30 Mitochondrial injury has been proposed to be another, and perhaps not mutually exclusive, mechanism for MAC. Kaiho demonstrated mitochondrial changes and myoglobin loss in rats undergoing intraperitoneal injection of methamphetamine.33 The loss of myoglobin was demonstrated in the rat ventricle, and myocardial cells with myoglobin loss demonstrated marked mitochondrial swelling. Disruption of oxidative phosphorylation associated with myoglobin loss was proposed as a cause of cardiomyopathy with methamphetamine use.
Impairment of cardiac function with methamphetamine use may also be related to a hyperadrenergic state and a reversible stress‐induced cardiomyopathy. Srikanth et al described a 42‐year‐old methamphetamine user who was found to have transient LV dysfunction and wall‐motion abnormalities consistent with a stress‐induced cardiomyopathy.34 An index ventriculogram showed apical ballooning consistent with a takotsubo process. An echocardiogram performed 3 days later demonstrated significant improvement in LV function. Catecholamine excess, in this case produced by methamphetamine, has been postulated to be the cause for stress‐induced cardiomyopathy. Patients with a hyperadrenergic state such as from pheochromocytoma causing a reversible cardiomyopathy have been well described.35 Also, Abraham et al reported 9 patients who developed transient stress‐induced cardiomyopathy after intravenous administration of catecholamines such as epinephrine or dobutamine.36 Another link between methamphetamine use, catecholamine surge, and stress is the resultant cardiac‐contraction band necrosis that can be seen in all 3 settings.37
MAC may be confounded by polysubstance abuse, particularly alcohol and cocaine use. Mendelson et al showed that the concurrent administration of alcohol with methamphetamine increased the rate‐pressure product as compared with methamphetamine use alone.38 This increase in workload may have a synergistic deleterious effect on the process of cardiomyopathy. Scant data exist on the concomitant use of cocaine and methamphetamine.39 One study did find that patients who by survey abused methamphetamine alone, without history of alcohol or cocaine abuse, did have dilated cardiomyopathy.40 Fleury et al studied predictors of cardiovascular response such as increased heart rate and blood pressure to methamphetamine administration.41 Recent alcohol use was found to be a predictor, as was route of administration. Intravenous drug use had higher peak changes in diastolic blood pressure vs those who smoked methamphetamine. Also, female methamphetamine abusers were found to have lower diastolic and systolic pressures at baseline than males, which may have a protective effect.
Treatment
With withdrawal of adrenergic stress, resolution of LV systolic dysfunction may be seen as noted in patients with stress‐induced cardiomyopathies, pheochromocytomas, and MAC. The potential for early reversibility of MAC has significant medical and social implications. Theoretically, as methamphetamine use induces a hyperadrenergic state, there may be potential preferential cardiac remodeling with β‐blockers compared with other standard therapies; but this remains to be established, and caution should be used with respect to β‐blocker therapy in active methamphetamine use.42 Blockade of the renin‐angiotensin system is also recommended for patients with reduced LV systolic function.43
Anti‐methamphetamine monoclonal antibodies have been used in rats with reduction in methamphetamine‐induced locomotor activity as well as hypertension and tachycardia.44 Also, aripiprazole, a partial dopamine and serotonin agonist, in humans appeared to attenuate some of the stimulant effects of methamphetamine, both in the central nervous and cardiovascular systems.45
Conclusion
Methamphetamine and related substances are now among the most abused drugs worldwide. There is a growing body of evidence that methamphetamine abuse is associated with cardiomyopathy, although the mechanism for cardiac dysfunction is still unclear. Evidence points toward contributions from drug‐induced vasospasm and ischemia, direct toxicity of methamphetamine, as well as deleterious effects of excess catecholamines on cardiomyocytes.
Clinically, many uncertainties remain, including the appropriate therapeutic interventions not only for MAC itself, but also for methamphetamine abuse in general. There appears to be an unclear window during which reversibility of cardiac dysfunction can occur with cessation of methamphetamine. Important areas for future research include finding the incidence and prevalence of cardiac pathology in methamphetamine abusers and risk factors for and prognosis of MAC. Future studies will need to adjust for concomitant drug use, including cocaine and alcohol. Recognition of MAC among medical caregivers is important given the growing use of methamphetamine and related stimulants.
Acknowledgments
Kristine Oki and Kristi Ching helped with this article, as did Dr. David Fergusson. Marlene Oishi, Tina Takamoto, and Christin Lozano assisted with finding many of the resources and journals.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
References
- 1. United Nations Office on Drugs and Crime . World Drug Report 2010 http://www.unodc.org/documents/wdr/WDR_2010/World_Drug_Report_2010_lo‐res.pdf.
- 2.US Department of Health and Human Services, Healthcare Cost and Utilization Project, Agency for Healthcare Research and Quality. Statistics for US community hospital stays, principal diagnosis based on CCS (Clinical Classifications Software), 2010. http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=C4D88AA0629A6D0E&Form=DispTab&JS=Y&Action=%3E%3ENext%3E%3E&__InDispTab=Yes&_Results=Print&SortOpt=.
- 3. Diercks DB, Fonarow GC, Kirk JD, et al; ADHERE Scientific Advisory Committee and Investigators. Illicit stimulant use in a United States heart failure population presenting to the emergency department (from the Acute Decompensated Heart Failure National Registry Emergency Module). Am J Cardiol. 2008;102:1216–1219. [DOI] [PubMed] [Google Scholar]
- 4. Schep LJ, Slaughter RJ, Beasley DM. The clinical toxicology of metamfetamine. Clin Toxicol (Phila). 2010;48:675–694. [DOI] [PubMed] [Google Scholar]
- 5. Cho AK. Ice: a new dosage form of an old drug. Science. 1990;249:631–634. [DOI] [PubMed] [Google Scholar]
- 6. Gal J. Amphetamines in nasal inhalers. J Toxicol Clin Toxicol. 1982;19:517–518. [Google Scholar]
- 7.Drug and Alcohol Services Administration System, Substance Abuse and Mental Health Services Administration. The DASIS Report: Smoked Methamphetamine/Amphetamines, 1992–2002 http://www.samhsa.gov/data/2k4/MethSmoked/MethSmoked.htm. Published January 7, 2005.
- 8. Cook CE, Jeffcoat AR, Hill JM, et al. Pharmacokinetics of methamphetamine self‐administered to human subjects by smoking S‐(+)‐methamphetamine hydrochloride. Drug Metab Dispos. 1993;21:717–723. [PubMed] [Google Scholar]
- 9. Kim I, Oyler JM, Moolchan ET, et al. Urinary pharmacokinetics of methamphetamine and its metabolite, amphetamine following controlled oral administration to humans. Ther Drug Monit. 2004;26:664–672. [DOI] [PubMed] [Google Scholar]
- 10. Valentine JL, Middleton R. GC‐MS identification of sympathomimetic amine drugs in urine: rapid methodology applicable for emergency clinical toxicology. J Anal Toxicol. 2000;24:211–222. [DOI] [PubMed] [Google Scholar]
- 11. Fleckenstein AE, Volz TJ, Riddle EL, et al. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. [DOI] [PubMed] [Google Scholar]
- 12. Busto U, Bendayan R, Sellers EM. Clinical pharmacokinetics of non‐opiate abused drugs. Clin Pharmacokinet. 1989;16:1–26. [DOI] [PubMed] [Google Scholar]
- 13. Katzung BG, ed. Basic and Clinical Pharmacology. 8th ed. New York, NY: Lange Medical Books/McGraw‐Hill; 2001. [Google Scholar]
- 14. Suwaki H, Fukui S, Konuma K. Methamphetamine abuse in Japan: its 45‐year history and the current situation In: Klee H, ed. Amphetamine Misuse: International Perspectives on Current Trends. Amsterdam: Harwood Academic Publishers; 1997. [Google Scholar]
- 15. Bruneton J. Toxic Plants Dangerous to Humans and Animals. Paris: Intercept‐Lavoisier; 1999. [Google Scholar]
- 16. Grinspoon L, Hedblom P. The Speed Culture: Amphetamine Use and Abuse in America. Cambridge, MA: Harvard University Press; 1975. [Google Scholar]
- 17. Anglin MD, Burke C, Perrochet B, et al. History of the methamphetamine problem. J Psychoactive Drugs. 2000;32:137–141. [DOI] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings Rockville, MD: Substance Abuse and Mental Health Services Administration; 2012. http://www.samhsa.gov/data/NSDUH/2k11Results/NSDUHresults2011.htm#2.3.
- 19.Drug and Alcohol Services Administration System, Substance Abuse and Mental Health Services Administration. The DASIS Report, Chapter 2: Trends in Substance Abuse Treatment Admissions, 1994–2004 http://wwwdasis.samhsa.gov/teds04/TEDSAd2k4Chp2.htm.
- 20. Hart CL, Gunderson EW, Perez A, et al. Acute physiological and behavioral effects of intranasal methamphetamine in humans. Neuropsychopharmacology. 2008;33:1847–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gericke O. Suicide by ingestion of amphetamine sulfate. JAMA. 1945;128:1098–1099. [Google Scholar]
- 22. Kalant H, Kalant OJ. Death in amphetamine users: causes and rates. Can Med Assoc J. 1975;112:299–304. [PMC free article] [PubMed] [Google Scholar]
- 23. Rajs J, Falconer B. Cardiac lesions in intravenous drug addicts. Forensic Sci Int. 1979;13:193–209. [DOI] [PubMed] [Google Scholar]
- 24. Jacobs LJ. Reversible dilated cardiomyopathy induced by methamphetamine. Clin Cardiol. 1989;12:725–727. [DOI] [PubMed] [Google Scholar]
- 25. Wijetunga M, Seto T, Lindsay J, et al. Crystal methamphetamine‐associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41:981–986. [DOI] [PubMed] [Google Scholar]
- 26. Yeo KK, Wijetunga M, Ito H, et al. The association of methamphetamine use and cardiomyopathy in young patients. Am J Med. 2007;120:165–171. [DOI] [PubMed] [Google Scholar]
- 27. Ito H, Yeo KK, Wijetunga M, et al. A comparison of echocardiographic findings in young adults with cardiomyopathy: with and without a history of methamphetamine abuse. Clin Cardiol. 2009;32:E18–E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopez JE, Yeo K, Caputo G, et al. Recovery of methamphetamine associated cardiomyopathy predicted by late gadolinium enhanced cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaye S, McKetin R; National Drug and Alcohol Research Centre (Australia). Cardiotoxicity Associated With Methamphetamine Use and Signs of Cardiovascular Pathology Among Methamphetamine Users. Sydney: NDARC; 2005. [Google Scholar]
- 30. Lord KC, Shenouda SK, McIlwain E, et al. Oxidative stress contributes to methamphetamine‐induced left ventricular dysfunction. Cardiovasc Res. 2010;87:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen JP. Methamphetamine‐associated acute myocardial infarction and cardiogenic shock with normal coronary arteries: refractory global coronary microvascular spasm. J Invasive Cardiol. 2007;19:E89–E92. [PubMed] [Google Scholar]
- 32. Hong R, Matsuyama E, Nur K. Cardiomyopathy associated with the smoking of crystal methamphetamine. JAMA. 1991;265:1152–1154. [PubMed] [Google Scholar]
- 33. Kaiho M, Ishiyama I. Morphological study of acute myocardial lesions experimentally induced by methamphetamine. Nihon Hoigaku Zasshi. 1989;43:460–468. [PubMed] [Google Scholar]
- 34. Srikanth S, Barua R, Ambrose J. Methamphetamine‐associated acute left ventricular dysfunction: a variant of stress‐induced cardiomyopathy. Cardiology. 2008;109:188–192. [DOI] [PubMed] [Google Scholar]
- 35. Elian D, Harpaz D, Sucher E, et al. Reversible catecholamine‐induced cardiomyopathy presenting as acute pulmonary edema in a patient with pheochromocytoma. Cardiology. 1993;83:118–120. [DOI] [PubMed] [Google Scholar]
- 36. Abraham J, Mudd JO, Kapur NK, et al. Stress cardiomyopathy after intravenous administration of catecholamines and beta‐receptor agonists. J Am Coll Cardiol. 2009;53:1320–1325. [DOI] [PubMed] [Google Scholar]
- 37. Samuels MA. The brain‐heart connection. Circulation. 2007;116:77–84. [DOI] [PubMed] [Google Scholar]
- 38. Mendelson J, Jones RT, Upton R, et al. Methamphetamine and ethanol interactions in humans. Clin Pharmacol Ther. 1995;57:559–568. [DOI] [PubMed] [Google Scholar]
- 39. Mooney ME, Herin DV, Schmitz JM, et al. Effects of oral methamphetamine on cocaine use: a randomized, double‐blind, placebo‐controlled trial. Drug Alcohol Depend. 2009;101:34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Won SK, Parikh N, Buchthal S, et al. Methamphetamine‐associated cardiomyopathy is associated with impaired myocardial energy usage via 31phosphorus magnetic resonance spectroscopy. Circulation. 2013;127:AP014. [Google Scholar]
- 41. Fleury G, De La Garza R 2nd, Mahoney JJ 3rd, et al. Predictors of cardiovascular response to methamphetamine administration in methamphetamine‐dependent individuals. Am J Addict. 2008;17:103–110. [DOI] [PubMed] [Google Scholar]
- 42. Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST‐elevation myocardial infarction—executive summary. J Am Coll Cardiol. 2007;50:652–726. [Google Scholar]
- 43. Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. [DOI] [PubMed] [Google Scholar]
- 44. Gentry WB, Laurenzana EM, Williams DK, et al. Safety and efficiency of an anti‐(+)‐methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)‐methamphetamine in rats. Int Immunopharmacol. 2006;6:968–977. [DOI] [PubMed] [Google Scholar]
- 45. Sevak RJ, Vansickel AR, Stoops WW, et al. Discriminative‐stimulus, subject‐rated, and physiological effects of methamphetamine in humans pretreated with aripiprazole. J Clin Psychopharmacol. 2011;31:470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]


