Abstract
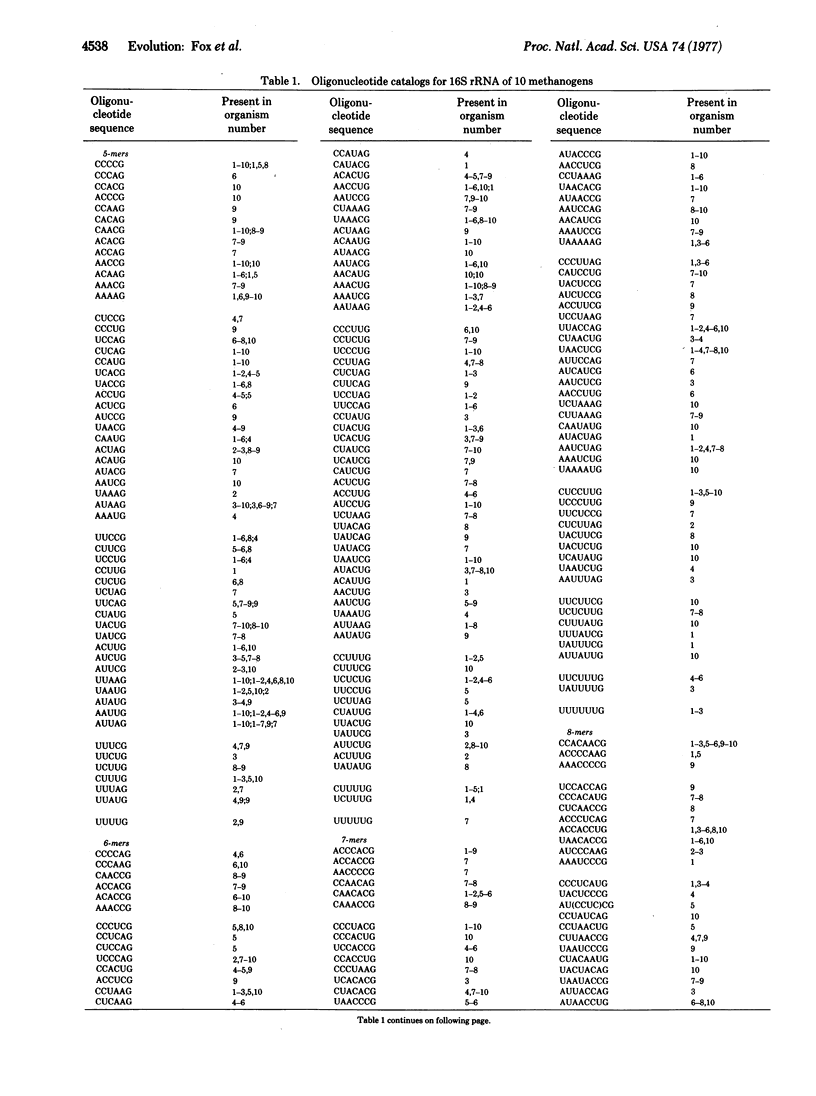
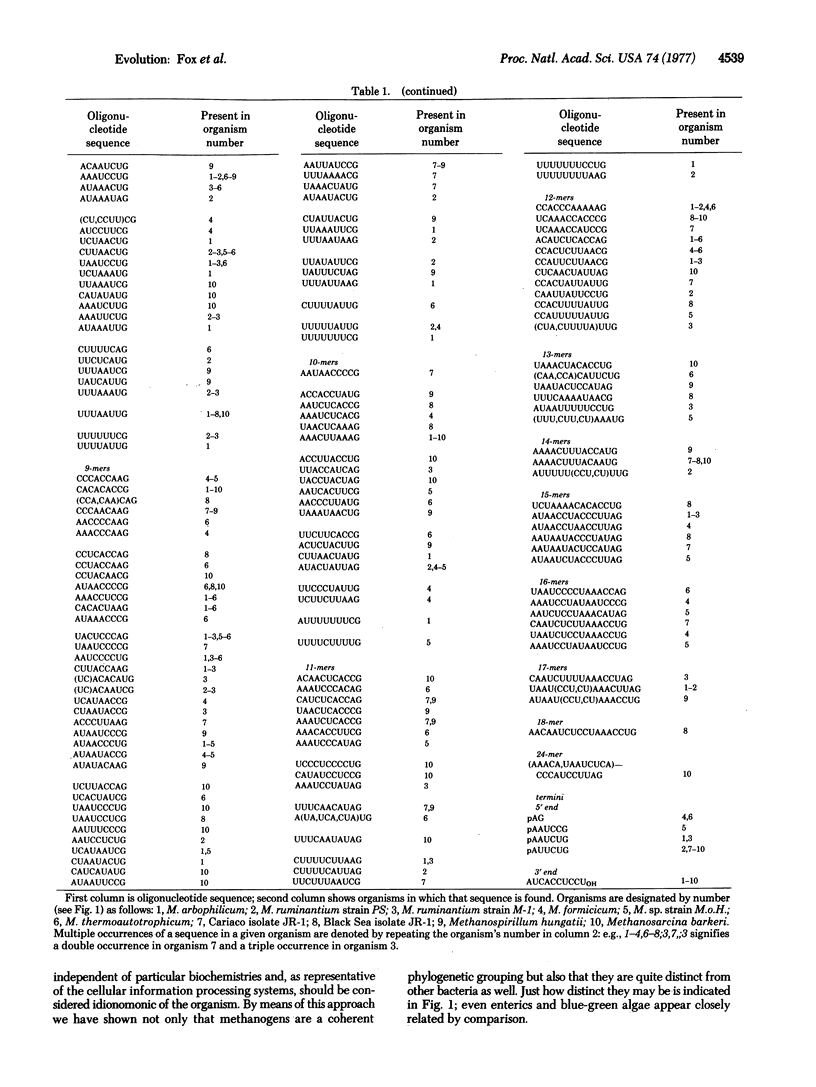
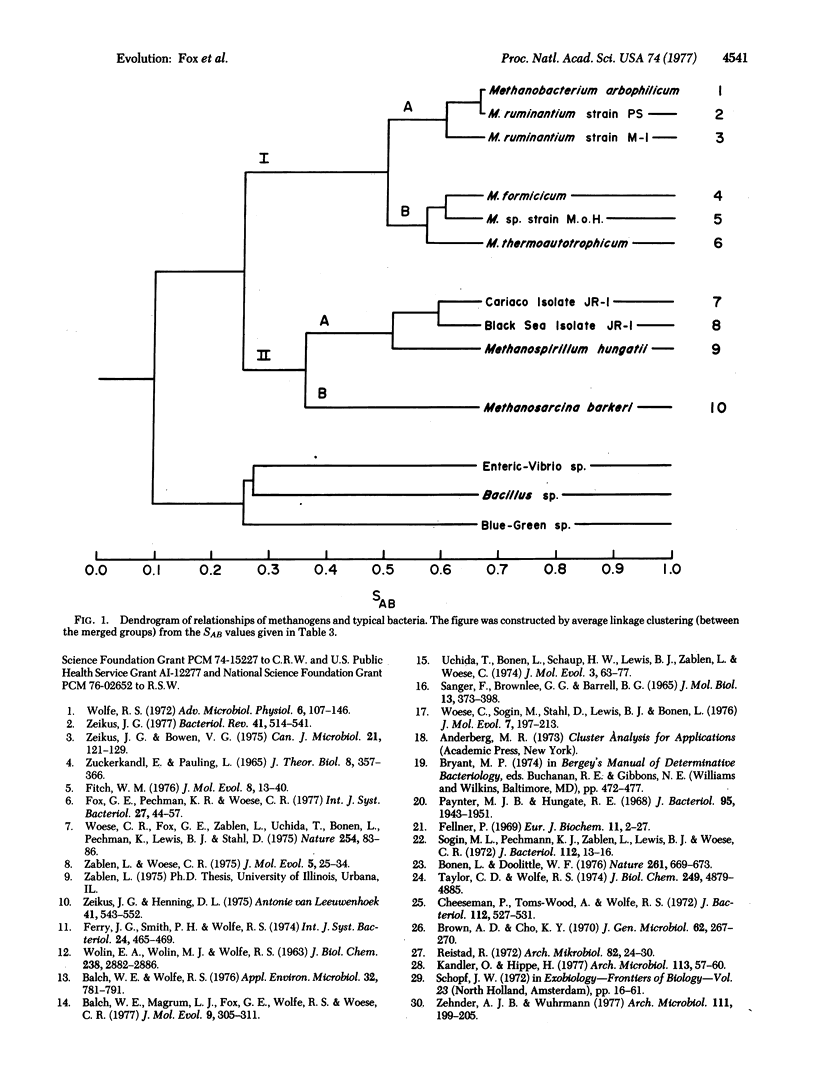
The 16S ribosomal RNAs from 10 species of methanogenic bacteria have been characterized in terms of the oligonucleotides produced by T1 RNase digestion. Comparative analysis of these data reveals the methanogens to constitute a distinct phylogenetic group containing two major divisions. These organisms appear to be only distantly related to typical bacteria.
Keywords: comparative oligonucleotide cataloging, phylogeny, molecular evolution
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Magrum L. J., Fox G. E., Wolfe R. S., Woese C. R. An ancient divergence among the bacteria. J Mol Evol. 1977 Aug 5;9(4):305–311. doi: 10.1007/BF01796092. [DOI] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F. Partial sequences of 16S rRNA and the phylogeny of blue-green algae and chloroplasts. Nature. 1976 Jun 24;261(5562):669–673. doi: 10.1038/261669a0. [DOI] [PubMed] [Google Scholar]
- Brown A. D., Cho K. Y. The walls of the extremely halophilic cocci: gram-positive bacteria lacking muramic acid. J Gen Microbiol. 1970 Aug;62(2):267–270. doi: 10.1099/00221287-62-2-267. [DOI] [PubMed] [Google Scholar]
- Cheeseman P., Toms-Wood A., Wolfe R. S. Isolation and properties of a fluorescent compound, factor 420 , from Methanobacterium strain M.o.H. J Bacteriol. 1972 Oct;112(1):527–531. doi: 10.1128/jb.112.1.527-531.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch W. M. The molecular evolution of cytochrome c in eukaryotes. J Mol Evol. 1976 Jun 23;8(1):13–40. doi: 10.1007/BF01738880. [DOI] [PubMed] [Google Scholar]
- Kandler O., Hippe H. Lack of peptidoglycan in the cell walls of Methanosarcina barkeri. Arch Microbiol. 1977 May 13;113(1-2):57–60. doi: 10.1007/BF00428580. [DOI] [PubMed] [Google Scholar]
- Paynter M. J., Hungate R. E. Characterization of Methanobacterium mobilis, sp. n., isolated from the bovine rumen. J Bacteriol. 1968 May;95(5):1943–1951. doi: 10.1128/jb.95.5.1943-1951.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reistad R. Cell wall of an extremely halophilic coccus. Investigation of ninhydrin-positive compounds. Arch Mikrobiol. 1972;82(1):24–30. doi: 10.1007/BF00424926. [DOI] [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Pechman K. J., Zablen L., Lewis B. J., Woese C. R. Observations on the post-transcriptionally modified nucleotides in the 16S ribosomal ribonucleic acid. J Bacteriol. 1972 Oct;112(1):13–16. doi: 10.1128/jb.112.1.13-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- Uchida T., Bonen L., Schaup H. W., Lewis B. J., Zablen L., Woese C. The use of ribonuclease U2 in RNA sequence determination. Some corrections in the catalog of oligomers produced by ribonuclease T1 digestion of Escherichia coli 16S ribosomal RNA. J Mol Evol. 1974 Feb 28;3(1):63–77. doi: 10.1007/BF01795977. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Woese C. R., Fox G. E., Zablen L., Uchida T., Bonen L., Pechman K., Lewis B. J., Stahl D. Conservation of primary structure in 16S ribosomal RNA. Nature. 1975 Mar 6;254(5495):83–86. doi: 10.1038/254083a0. [DOI] [PubMed] [Google Scholar]
- Woese C., Sogin M., Stahl D., Lewis B. J., Bonen L. A comparison of the 16S ribosomal RNAs from mesophilic and thermophilic bacilli: some modifications in the Sanger method for RNA sequencing. J Mol Evol. 1976 Apr 9;7(3):197–213. doi: 10.1007/BF01731489. [DOI] [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- Zablen L., Woese C. R. Procaryote phylogeny IV: concerning the phylogenetic status of a photosynthetic bacterium. J Mol Evol. 1975 Jun 9;5(1):25–34. doi: 10.1007/BF01732011. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Bowen V. G. Comparative ultrastructure of methanogenic bacteria. Can J Microbiol. 1975 Feb;21(2):121–129. doi: 10.1139/m75-019. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Henning D. L. Methanobacterium arbophilicum sp.nov. An obligate anaerobe isolated from wetwood of living trees. Antonie Van Leeuwenhoek. 1975;41(4):543–552. doi: 10.1007/BF02565096. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerkandl E., Pauling L. Molecules as documents of evolutionary history. J Theor Biol. 1965 Mar;8(2):357–366. doi: 10.1016/0022-5193(65)90083-4. [DOI] [PubMed] [Google Scholar]


