Abstract
Background: Opioid utilization for acute pain has been associated with numerous adverse events, potentially resulting in longer inpatient stays and increased costs.
Objective: To examine the effect of intravenous (IV) acetaminophen administered intraoperatively on postoperative opioid consumption in adult subjects who underwent hip or knee replacement.
Methods: This retrospective cohort study evaluated postoperative opioid consumption in 176 randomly selected adult subjects who underwent hip or knee replacement at Duke University Hospital (DUH). Eighty-eight subjects received a single, intraoperative, 1 g dose of IV acetaminophen. The other subjects did not receive any IV acetaminophen. This study evaluated mean opioid consumption (in oral morphine equivalents) during the 24-hour postoperative period in the 2 groups. Other endpoints included length of stay in the postanesthesia care unit (PACU), incidence of oversedation, need for acute opioid reversal, and adjunctive analgesic utilization.
Results: Subjects who were given a single dose of intraoperative acetaminophen received an average of 149.3 mg of oral morphine equivalents during the 24 hours following surgery compared to 147.2 mg in participants who were not exposed to IV acetaminophen (P = .904). The difference in average length of PACU stay between the IV acetaminophen group (163 minutes) and those subjects not exposed to IV acetaminophen (169 minutes) was not statistically significant (P = .588). No subjects in the study experienced oversedation or required acute opioid reversal.
Conclusion: There was not a statistically significant difference in postoperative opioid consumption between patients receiving and not receiving IV acetaminophen intraoperatively.
Key Words: analgesia, intravenous acetaminophen, orthopedic surgery
Acute postoperative pain management continues to be a difficult issue for health care providers and their patients. It has previously been estimated that up to 80% of patients experience acute pain after surgery, with 86% of those patients reporting moderate, severe, or extreme pain.1 Unrelieved or inadequately managed postoperative pain can adversely impact both patients and health care institutions. Patients with inadequately managed acute postoperative pain are at increased risk of developing chronic pain.2 Additionally, immunosuppression from unrelieved pain delays wound healing, slows recovery, and increases risk of infection.3 Another patient-related consequence of intense postoperative pain is delayed ambulation, which increases risk of thromboembolism and delays discharge. Traditionally, the consequences of undertreating acute postoperative pain for hospitals have been extended length of stay, increased risk of readmission, and increased cost of care.3
Although postoperative pain management has always been a challenge for health care institutions, the need to meet this challenge has been further amplified in recent years. In 2001, The Joint Commission (TJC) developed standards requiring hospitals to focus on appropriate pain management, monitoring, and education.4 Even more recently, patient satisfaction surveys related to inpatient stays are being reported via Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS).5 This 27-question survey includes 2 questions specifically inquiring about pain management during the inpatient stay. Under the Affordable Care Act of 2010, the Centers for Medicare and Medicaid (CMS) have established hospital reimbursement based on HCAHPS scores. This policy took effect at the start of fiscal year 2013 (all patient discharges beginning October 1, 2012).6 Although there have always been negative repercussions for hospitals for undermanaging postoperative pain, these recent measures from TJC and CMS further incentivize hospitals to appropriately treat patients who experience acute postoperative pain.
Traditionally, opioid analgesics have served as the foundation for the management of moderate-to-severe acute postoperative pain.7 However, their utilization is associated with a myriad of adverse effects, including pruritus, gastrointestinal effects (nausea, vomiting, constipation), central nervous system effects (somnolence, dizziness, oversedation), and respiratory depression.8 There have been multiple reports linking increased opioid utilization to a greater likelihood of suffering opioid-related adverse events.9-11 Previous literature has associated opioidrelated adverse events with increased length of stay and hospitalization costs in a postoperative patient population.9
The potential consequences of over-reliance on opioids is one of the reasons why recent guidelines published by the American Society of Anesthesiologists (ASA) on acute perioperative pain management advocate for a multimodal approach to acute analgesia (targeting different mechanisms of postoperative pain) whenever possible.12 The rationale for utilization of nonopioid adjunctive analgesics in conjunction with opioids for acute surgical pain is to maximize patient pain control while avoiding excessive opioid consumption in the postoperative period. Potential nonopioid agents to be utilized in this setting include nonsteroidal anti-infl ammatory drugs (NSAIDs), cyclooxygenase-2 (COX-2)–selective medications, regional blockade with local anesthetics, pregabalin, gabapentin, or acetaminophen. Furthermore, ASA members recommend that patients receive an around-the-clock regimen of NSAIDs, COX-2 medications, or acetaminophen. In addition to the ASA guidelines, other sources have advocated for a multimodal approach to postoperative analgesia.13,14
Intravenous (IV) acetaminophen is one of the medications utilized in a multimodal analgesia regimen.15 Acetaminophen has long been available in the United States as an oral or rectal formulation. In November 2010, the US Food and Drug Administration (FDA) approved an IV formulation for the reduction of fever, management of mild-to-moderate pain, and management of moderate-to-severe pain with adjunctive opioid analgesics. IV acetaminophen reaches a 70% higher maximum concentration compared to the same dose of oral acetaminophen.16 In addition to having a higher maximum concentration (Cmax) than oral acetaminophen, the IV dosage form reaches its Cmax more quickly than its oral counterpart. However, overall exposure as measured by total area under the concentration time curve (AUC) is very similar to the oral formulation. A study published by Singla and colleagues17 in 2012 showed that a single dose of 1 g IV acetaminophen achieved earlier and higher plasma and cerebrospinal fl uid levels than equivalent doses of both oral and rectal acetaminophen in a single dose study.
Two pivotal studies were conducted to assess the impact of IV acetaminophen on postoperative pain.18,19 Sinatra and colleagues18 compared IV acetaminophen 1 g every 6 hours to placebo in adults who underwent total hip or knee replacement. In this study, subjects who received IV acetaminophen reported decreased pain intensity at 6 (P < .05) and 24 hours (P < .01). Additionally, subjects in the IV acetaminophen group consumed significantly less IV morphine at 6 (-46%) and 24 (-33%) hours after randomization. Wininger and colleagues19 evaluated the use of IV acetaminophen (1 g every 6 hours or 650 mg every 4 hours for 24 hours) versus placebo in adults who underwent abdominal laparoscopic surgery. Patients receiving either of the IV acetaminophen regimens experienced decreased pain intensity (P < .007 for 1 g every 6 hours; P < .019 for 650 mg every 4 hours). However, there were also no differences noted in opioid consumption during 0 to 12 hours after randomization or 12 to 24 hours after randomization. These are the 2 procedure-related pain studies cited in the prescribing information for IV acetaminophen, but it should be noted that these studies did not randomize subjects until the morning following procedural completion and they do not address the intraoperative or immediate postoperative periods.
Duke University Hospital (DUH) added IV acetaminophen to formulary in March 2011, with restrictions associated with its use. These restrictions included limitation of the agent to patients unable to tolerate an oral diet and placement of an automatic stop time of 24 hours on each order. A medication use evaluation (MUE) conducted after IV acetaminophen was added to formulary revealed that it was most frequently utilized in the orthopedic surgical population. Furthermore, the MUE showed that many patients were receiving a single dose intraoperatively before transitioning to oral nonopioid adjunctive analgesics. The purpose of IV acetaminophen administration intraoperatively at DUH was to maximize the pain-reducing potential of the medication based on its route of administration, with the patient experiencing the benefit upon arrival in the postanesthesia care unit (PACU). It is policy at DUH that the patient must have a pain score of 4/10 or less before leaving the PACU, so the use of IV acetaminophen prior to surgical completion may result in patients being eligible to leave the PACU more quickly.
Because clinical trials investigated the use of IV acetaminophen postoperatively, the intraoperative use of IV acetaminophen was investigated based on historical DUH records. The main objectives of this study were to assess changes in postoperative opioid consumption, length of PACU stay, incidence of oversedation, and the need for acute opioid reversal in adults who underwent hip or knee replacement and received IV acetaminophen intraoperatively.
Methods
Study Design and Inclusion/Exclusion Criteria
This study was designed as a retrospective, observational cohort, and received approval from the institutional review board at DUH. Patients were eligible for inclusion in this retrospective, observational cohort study if they were 18 years of age or older and underwent hip or knee replacement at DUH from July 1, 2010 through March 31, 2012. Patients who received IV acetaminophen associated with the procedure were eligible for inclusion if they received a single, 1 g dose within 60 minutes prior to surgery completion. Patients were excluded from the study if they received an IV NSAID or IV ketamine during the intraoperative period. Patients receiving a dose of IV acetaminophen other than 1 g, or those who were administered additional IV acetaminophen doses during the 24-hour postoperative period, were excluded.
Patient Identification and Selection
Patients were identified for potential study inclusion based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. Duke Enterprise Data Unified Content Explorer (DEDUCE) database queries identified adults at DUH who underwent hip or knee replacement (ICD-9-CM codes 81.51, 81.52, and 81.54) during the prespecified time period and adults who underwent either procedure and received IV acetaminophen during their admission. The DEDUCE database is a repository of clinical information for patients treated at DUH. Other information obtained from the DEDUCE database query were patient gender, age, race, and admitting service. For patients who received IV acetaminophen during their admission for hip or knee replacement, the Innovian Perioperative Care database was used to determine whether or not a single 1 g dose was infused within 1 hour prior to time of surgery completion. The Innovian Perioperative Care database stores procedure-related and medication information from pre anesthesia induction until the patient leaves the PACU. For each patient included in the study, demographic information (age, gender, weight, race, procedure type) was recorded from the historical patient chart and electronic medication administration record (MAR). A patient was considered “opioid-tolerant” if his or her list of outpatient medications upon admission to DUH for hip or knee replacement revealed daily opioid use of 60 mg or more of oral morphine equivalents. Data collected from each patient encounter were entered into a computerized database (Excel, Microsoft Corporation, Redmond, WA).
Randomization and Treatment Arms
This study contained 2 treatment arms: subjects who received a single dose of IV acetaminophen within 1 hour prior to hip or knee replacement completion, and subjects who did not receive IV acetaminophen during their index admission for hip or knee replacement. All associated patient encounters from the DEDUCE database queries were entered into a random number generator to determine the order of evaluation. Using the order provided by the random number generator, patients were subsequently reviewed at random applying study exclusionary criteria to determine eligibility for inclusion into the final study analysis.
Endpoints
For each patient included into the final study analysis, the historical patient chart, electronic MAR, and Innovian were used to document endpoint information. The primary endpoint was opioid consumption in oral morphine equivalents during the 24 hours immediately following completion of hip or knee replacement in adults at DUH. All opioid usage was documented in the 24 hours immediately following procedure completion (as noted by Innovian). All of the opioids were subsequently converted into oral morphine equivalents, with each dose being rounded to the nearest 0.1 mg. Nearly all of the conversions were performed using the National Comprehensive Cancer Network Adult Cancer Pain Guidelines single-dose opioid equivalency chart.20 Conversions from epidural hydromorphone relied on a previously reported comparison from the literature.21 Many subjects utilized opioid patientcontrolled analgesia (PCA); in some cases, utilization crossed the 24-hour time frame after surgery. In these cases, the proportion of total PCA time within the 24-hour postoperative study period was used to calculate PCA utilization for these subjects. For example, a patient may have received PCA morphine for a total of 40 hours after hip or knee replacement, but only the first 20 hours were during the 24-hour postoperative study period. If that was the case, and the patient used a total of 50 mg of PCA morphine over the 40-hour period, then 25 mg of PCA morphine were recorded as the opioid usage and subsequently converted to oral morphine equivalents. Once all of the opioid doses were converted to oral morphine equivalents, the total opioid requirements were added up and the mean 24-hour postoperative opioid consumption was compared between the 2 groups. A post hoc analysis was conducted to determine whether there was a difference in opioid consumption based on type of procedure (hip or knee replacement) completed.
Secondary endpoints for this study included time in minutes spent in the PACU, percentage of subjects in each group utilizing adjunctive analgesics during the 24-hour postoperative period, percentage of subjects with a Richmond Agitation and Sedation Scale (RASS) score less than or equal to -4, and percentage of subjects requiring naloxone bolus administration. To assess length of PACU stay, Innovian was used to record the time of entrance to and exit from the PACU for each patient included in the study. These times were then used to calculate length of PACU stay for each subject. To assess adjunctive analgesic use, subjects were categorized as to whether or not they received certain nonopioid analgesics during the 24 hours following surgery. Post hoc analyses were conducted to assess potential differences in utilization based on type of procedure performed. To assess oversedation, subjects were documented as either having or not having a RASS score of less than or equal to -4 (corresponding with heavy sedation) during the study period.22 To document need for acute opioid reversal, subjects were recorded as either requiring or not requiring a bolus administration of naloxone during the study period.
Power and Statistical Analysis
A power analysis using a previous study examining postoperative opioid utilization in adults who underwent hip or knee replacement23 indicated that 87 subjects in each group were needed to detect a 30% difference in 24-hour oral morphine usage, assuming 80% power and an a priori 2-sided significance level of .05. An unpaired, 2-tailed t test was used to assess differences in mean opioid consumption and time in the PACU between the group of subjects who received intraoperative IV acetaminophen and those subjects not exposed to IV acetaminophen. Descriptive statistics (means, standard deviations, medians, and ranges) were also recorded for both of these endpoints. A post hoc, unpaired, 2-tailed t test was used to assess the difference in mean opioid consumption between subjects who underwent hip or knee replacement, regardless of whether IV acetaminophen was administered. A Fisher’s exact test was used to evaluate differences in adjunctive analgesic use between the 2 groups, with a post hoc Fisher’s exact test conducted to evaluate differences in adjunctive analgesic utilization based on type of procedure (hip or knee replacement). The percentages of subjects in each group who experienced a RASS score of less than or equal to -4 or required naloxone bolus administration during the postoperative study period were documented in both groups. To compare demographics of the 2 subject groups, an unpaired, 2-tailed t test was used for continuous variables and a Fisher’s exact test was used for categorical data. Any statistical analyses were performed using SAS, version 9.3 (SAS Institute, Cary, NC).
Results
Patient Demographics and Characteristics
A total of 3,698 adult patients underwent hip or knee replacement at DUH from July 2010 through March 2012 (Figure 1). After applying a random number generator to these patient encounters, 431 patients were randomly evaluated by applying the study exclusion criteria. Of the 431 patients evaluated, a total of 255 patients were excluded from the final study population (see Figure 1 for reasons). The 2 primary reasons for study exclusion were administration of IV acetaminophen outside of the 1 hour immediately prior to surgery completion (n = 155) and administration of intraoperative ketamine (n = 65). In the final study population of 176 subjects, 88 were in the group that received intraoperative IV acetaminophen and 88 were in the group that did not receive IV acetaminophen (Figure 1). The number of subjects in the final study population was more than what was required to adequately meet power in both groups.
Figure 1.
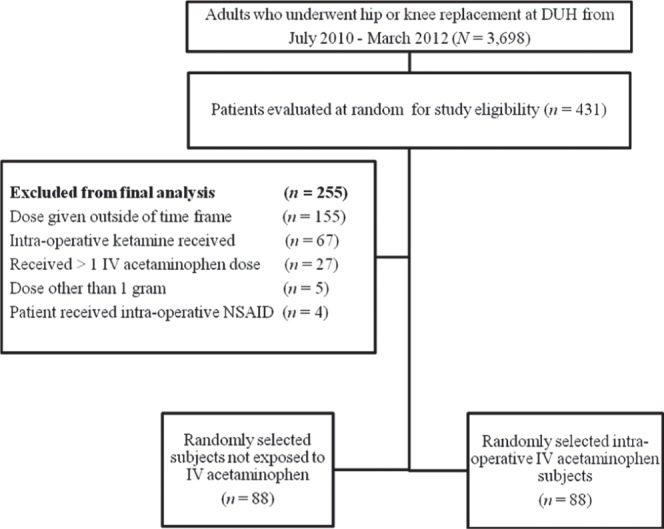
Selection of patients for study inclusion. DUH = Duke University Hospital; IV = intravenous; NSAID = nonsteroidal anti-inflammatory drug.
Patient demographics and characteristics are listed in Table 1. Randomly selecting patients for evaluation using the random number generator produced groups that were similar with regard to age, gender, weight, and race. Only 3 patients in the IV acetaminophen group and 1 patient in the nonexposed group met the prespecified “opioid-tolerant” criteria (using ≥60 mg of oral morphine equivalents daily prior to hip or knee replacement). The greatest disparity between the 2 patient groups was in the type of procedure performed. In the IV acetaminophen group, 58% of patients underwent hip replacement surgery and 42% of patients underwent knee replacement; in the group not receiving IV acetaminophen, 43% and 57% of patients underwent hip and knee replacement, respectively (Table 1). However, the difference in type of procedure performed between the groups did not reach statistical significance.
Table 1. Demographic and clinical characteristics of study subjects.
| Characteristic | IV APAP (n = 88) | Not given IV APAP (n = 88) | P |
| Mean age, years (±SD) | 62.3 (12.8) | 61.6 (11.9) | .696 |
| Female, n (%) | 33 (37.5) | 33 (37.5) | 1 |
| Mean weight, kg (±SD) | 89.4 (24.5) | 86 (21.1) | .319 |
| Race, n (%) | |||
| Caucasian | 67 (76.1) | 73 (82.9) | .246 |
| African American | 20 (22.7) | 13 (14.8) | – |
| Other | 1 (1.1) | 2 (2.3) | – |
| Type of procedure, n (%) | |||
| Hip replacement | 51 (58) | 38 (43.2) | .07 |
| Knee replacement | 37 (42) | 50 (56.8) | – |
| Opioid tolerance, n (%)a | 3 (3.4) | 1 (1.1) | .62 |
Note: IV APAP = intravenous acetaminophen.
Opioid tolerance defined as using ≥60 mg of oral morphine equivalents per day before undergoing hip or knee replacement.
Outcomes
Results from the primary and secondary endpoints are listed in Table 2. For the primary study endpoint, there was not a statistically significant difference mean 24-hour postoperative opioid consumption in oral morphine equivalents for patients who received intraoperative IV acetaminophen (149.3 ± 98.7 mg) and patients who did not receive IV acetaminophen (147.2 ± 122.6 mg) during the study period. A post hoc analysis comparing mean opioid consumption in hip and knee replacements revealed that patients who underwent hip replacement (124.6 ± 113.6 mg) utilized less oral morphine equivalents compared to patients who underwent knee replacement (172.4 ± 103.5 mg) during the 24 hours following surgery (P = .004). There was also no statistically significant difference in mean length of PACU stay between the IV acetaminophen group (163.1 ± 74.6 minutes) and the group not administered IV acetaminophen (169.4 ± 78.2 minutes).
Table 2. Primary and secondary outcome measures.
| Factor or measure | IV APAP (n = 88) | Not given IV APAP (n = 88) | P |
| 24-hour postoperative opioid consumption, mg | |||
| Mean opioid consumption (±SD)a | 149.3 (98.7) | 147.2 (122.6) | .904 |
| Median opioid consumption (range)a | 132.1 (0-446.5) | 118.4 (0-741) | – |
| PACU time, min | |||
| Mean PACU time (±SD) | 163.1 (74.6) | 169.4 (78.2) | .588 |
| Median PACU time (range; min) | 149 (45-450) | 162 (60-494) | – |
| Safety endpoints, n (%) | |||
| Patients with RASS score ≤ -4 | 0 (0) | 0 (0) | – |
| Patients requiring naloxone bolus | 0 (0) | 0 (0) | – |
Note: IV APAP = intravenous acetaminophen; PACU = postanesthesia care unit; RASS = Richmond Agitation and Sedation Scale.
In oral morphine equivalents.
Adjunctive analgesic utilization data for the 2 groups are listed in Table 3. The one difference between the groups as a whole reaching statistical significance was use of pregabalin or gabapentin (53.4% in the group not receiving IV acetaminophen compared to 29.5% of subjects in the IV acetaminophen group). A post hoc breakdown of the data by type of procedure within each of the different adjunctive analgesics revealed that hip replacement patients who did not receive IV acetaminophen were significantly more likely to receive bupivacaine than their counterparts who received intraoperative IV acetaminophen.
Table 3. Adjunctive analgesic utilization during 24-hour post-operative period, n/N (%).
| Adjunctive analgesic | IV APAPa (n = 88) | Not given IV APAP (n = 88) | P |
| Oral acetaminophen | 57 (64.8) | 58 (65.9) | 1 |
| Hip | 25/51 (49) | 24/38 (63.2) | .203 |
| Knee | 32/37 (86.5) | 34/50 (68) | .075 |
| Celecoxib | 34 (38.6) | 44 (50) | .172 |
| Hip | 11/51 (21.6) | 12/38 (31.6) | .333 |
| Knee | 23/37 (62.2) | 32/50 (64) | 1 |
| Bupivacaine | 20 (22.7) | 31 (35.2) | .096 |
| Hip | 17/51 (33.3) | 25/38 (65.8) | .003 |
| Knee | 3/37 (8.1) | 6/50 (12) | .727 |
| Ropivacaine | 32 (36.4) | 42 (47.7) | .169 |
| Hip | 0/51 (0) | 2/38 (5.3) | .18 |
| Knee | 32/37 (86.5) | 40/50 (80) | .569 |
| Gabapentin or pregabalin | 26 (29.5) | 47 (53.4) | .002 |
| Hip | 8/51 (15.7) | 12/38 (31.6) | .122 |
| Knee | 18/37 (48.6) | 35/50 (70) | .049 |
| NSAIDs (oral or IV) | 3 (3.4) | 4 (4.5) | 1 |
| Hip | 3/51 (5.9) | 2/38 (5.3) | 1 |
| Knee | 0/37 (0) | 2/50 (4) | .501 |
| Tramadol | 2 (2.2) | 2 (2.2) | 1 |
| Hip | 1/51 (2) | 0/38 (0) | 1 |
| Knee | 1/37 (2.7) | 2/50 (4) | 1 |
Note: APAP = acetaminophen; IV = intravenous; NSAIDs = nonsteroidal anti-inflammatory drugs (nonselective agents only).
Another post hoc of adjunctive analgesic utilization was conducted based on the type of procedure performed, regardless of whether or not patients received intraoperative IV acetaminophen (see Table 4). Patients who underwent knee replacement were significantly more likely to use oral acetaminophen (75.9% vs 55.1%), celecoxib (63.2% vs 25.8%), ropivacaine (82.8% vs 2.2%), and gabapentin or pregabalin (62.1% vs 22.5%) during the 24-hour postoperative period than those who underwent hip replacement. Hip replacement patients were significantly more likely to use bupivacaine during the 24-hour postoperative period (47.2% vs 10.3%) than knee replacement patients.
Table 4. Adjunctive analgesic utilization by type of procedure during 24-hour postoperative period, n (%).
| Adjunctive analgesic | Hip replacement (n = 89) | Knee replacement (n = 87) | P |
| Oral acetaminophen | 49 (55.1) | 66 (75.9) | .004 |
| Celecoxib | 23 (25.8) | 55 (63.2) | <.001 |
| Bupivacaine | 42 (47.2) | 9 (10.3) | <.001 |
| Ropivacaine | 2 (2.2) | 72 (82.8) | <.001 |
| Gabapentin or pregabalin | 20 (22.5) | 54 (62.1) | <.001 |
| NSAIDs (oral or IV) | 5 (5.6) | 2 (2.3) | .444 |
| Tramadol | 1 (1.1) | 3 (3.4) | .365 |
Note: IV = intravenous; NSAIDs = nonsteroidal anti-inflammatory drugs (nonselective agents only).
RASS scores and incidence of naloxone bolus administration were assessed to determine the frequency of oversedation and need for acute opioid reversal in both subject groups. No subjects in each group experienced a RASS score less than or equal to -4 or required naloxone bolus administration for acute opioid reversal during the 24-hour postoperative period (Table 2).
Discussion
The current ASA guidelines on perioperative pain management recommend utilizing nonopioid analgesics to improve pain management and potentially decrease opioid use.11 In this retrospective study, we attempted to determine the impact of a single, 1 g, intraoperative IV acetaminophen dose on postoperative opioid consumption in adults who underwent hip or knee replacement at DUH. The reasons for completing this study include increased use of this agent at DUH, differences in study design for the procedurerelated pain studies cited in the prescribing information, and the high cost per dose of the drug. Although IV acetaminophen reaches higher peak serum16 and cerebrospinal fluid concentrations17 than its oral counterpart, there is a significant cost difference between the 2 agents. The average wholesale price (AWP) for 1 vial of IV acetaminophen (containing a single 1 g dose) is $14.52, compared to an AWP of $9.96 for 100 oral tablets of 500 mg acetaminophen.24
Because this medication is frequently utilized at DUH, this study sought to ascertain the impact of IV acetaminophen when utilized as a single, intraoperative dose.
In this study, there was not a significant difference in average opioid consumption between subjects who received intraoperative IV acetaminophen and subjects who did not receive IV acetaminophen during the study period. Furthermore, the difference in average length of PACU stay between the 2 patient groups was not statistically significant, although additional factors beyond pain control (such as bed availability within the hospital or treating health care provider) may have also impacted length of stay. There were also no patients in either group who experienced a RASS score of less than or equal to -4 or lower or required naloxone bolus administration for acute opioid reversal.
A divergence noted between the 2 patient groups was the utilization of adjunctive analgesics during the 24-hour postoperative period (see Table 3). A higher percentage of subjects in the group that did not receive IV acetaminophen were administered celecoxib, bupivacaine, ropivacaine, and a GABA agent (pregabalin or gabapentin) compared to the intraoperative IV acetaminophen group. However, only the difference in subjects receiving a GABA agent reached statistical significance between the groups. This finding was particularly interesting because it suggested a decrease in utilization of nonopioid adjunctive analgesics in the IV acetaminophen group as opposed to an opioid-sparing effect. It is possible the differences observed in adjunctive analgesic utilization could be a reflection of improved postoperative pain control in the intraoperative IV acetaminophen group. Additionally, it could be reflective of a maximum threshold health care providers at DUH have for administering opioids to patients presenting with acute postoperative pain.
There were several limitations to this study. This was a retrospective, single-center, observational cohort, so results may not be generalizable to other institutions, procedures, or patient populations. Due to the nature of the study design, accuracy of documentation during the perioperative period was heavily relied upon during data collection. Since the impact of a single intra-operative IV acetaminophen dose on 24-hour postoperative opioid consumption was examined, there may have been a flattening of the potential opioid-sparing effect observed with IV acetaminophen due to the pharmacokinetics of this agent. Although this opens up the possibility of underestimating the true impact of the agent, a previous MUE revealed that a majority of hip and knee replacement patients received a single intraoperative dose. For this reason, the study design was executed to most closely align with actual practice at DUH during that time.
Opioid consumption is used in this study as a surrogate marker of pain control in both subject groups, but pain scores were not documented during the 24-hour study period. Pain scores were not documented, because there were not consistent time points or intervals during the study period at which subjects had pain intensity or relief assessed. This limited the utility of making comparisons between the 2 groups for this endpoint.
Another potential limitation to the study was the different adjunctive analgesia regimens received by patients in the perioperative period (see Table 3). To eliminate potential confounders during the intraoperative period, patients were excluded if they received intravenous ketamine or NSAIDs intraoperatively. However, because this was a retrospective study, there were no controls placed on the adjunctive analgesic regimens that subjects received prior to induction of anesthesia or during the 24-hour postoperative period. Due to this, subjects may have received different preoperative and postoperative adjunctive analgesic regimens based on health care provider preferences.
Although most of the demographics potentially impacting opioid consumption (eg, age, weight, level of opioid tolerance) were fairly well matched, there were numerically different percentages of hip and knee replacement procedures within each of the groups; this difference was not significant. A post hoc analysis comparing mean opioid consumption in hip and knee replacements revealed that patients who underwent hip replacement (124.6 ± 113.6 mg) utilized less oral morphine equivalents compared to patients who underwent knee replacement (172.4 ± 103.5 mg) during the 24 hours following surgery (P = .004).
A post hoc analysis of adjunctive analgesic utilization by procedure (see Table 4) revealed that knee replacement patients were significantly more likely to use oral acetaminophen, celecoxib, ropivacaine, and a GABA agent than hip replacement patients, whereas hip replacement patients were more likely to utilize bupivacaine. As previously mentioned, there were more hip replacement patients in the intra-operative IV acetaminophen group and knee replacement patients in the group that did not receive IV acetaminophen.
Finally, we set out to report the incidence of oversedation (RASS score ≤ -4) and need for acute opioid reversal (naloxone bolus administration). When assessing oversedation, zero subjects experienced a RASS score less than -2, likely due to close monitoring from nursing staff. Although several subjects utilized a naloxone infusion for opioid-related pruritus, no subjects necessitated a bolus administration of naloxone for acute opioid reversal. Events requiring naloxone bolus administration for reversal, such as severe respiratory depression, are infrequently observed in the general population. For this reason, a much larger study would need to be conducted to adequately assess opioid-related oversedation and respiratory depression.
Other administration times of IV acetaminophen in relation to surgery have been investigated beyond what was evaluated in the 2 pivotal, postoperative pain management studies. Jokela and colleagues25 evaluated the administration of IV acetaminophen 1 g every 6 hours for 24 hours at the induction of anesthesia in women undergoing abdominal laparoscopic hysterectomy. Subjects in the IV acetaminophen group required less oxycodone than placebo during the 24-hour postoperative study period (P = .031). Moon and colleagues26 investigated the effect of a single 2 g IV acetaminophen dose given 30 minutes prior to induction of anesthesia in women undergoing laparoscopic surgery. This study noted a 30% decrease in postoperative hydromorphone consumption in the group of patients given IV acetaminophen preoperatively during the 24 hours following surgery (P = .013). Khalili and colleagues27 examined the impact of IV acetaminophen 15 mg/kg given as a single dose 30 minutes prior to surgery initiation or immediately preceding skin closure compared to placebo in patients who underwent lower extremity surgery. Both groups receiving IV acetaminophen experienced decreased pain scores compared with placebo at 6 hours after surgery (P < .001). Differences in pain scores between the groups at 12, 18, and 24 hours were not significant. Patients receiving IV acetaminophen 30 minutes prior to surgery initiation consumed the lowest amount of meperidine for rescue analgesia during the 24-hour postoperative period (P < .01).
A systematic review and meta-analysis published in 2013 evaluated the effect of the timing of IV acetaminophen on postoperative pain and opioid consumption.28 When patients received IV acetaminophen prophylactically, a concomitant reduction in pain was observed (odds ratio [OR], 0.66; 95% CI, 0.47-0.93), but not a reduction in postoperative opioid consumption (OR, 0.89; 95% CI, 0.64-1.22). Further investigation into the timing of IV acetaminophen administration may be warranted so the maximum benefits of the medication can be realized.
Since the completion of this study, policies surrounding the use of IV acetaminophen at DUH have changed. When DUH went live with its new computerized physician order entry system in June 2013, the automatic stop time for an IV acetaminophen order changed to 48 hours. In December 2013, it was changed back to 24 hours for all approved orders. The drug was also subsequently removed from all DUH order sets. If a prescriber wishes to use IV acetaminophen in a patient taking oral medication, he or she must seek authorization from the on-call pharmacy administrator. Currently, there have not been any policy modifications that would directly impact the utilization of IV acetaminophen by prescribers during the intraoperative setting for patients undergoing hip or knee replacement.
Conclusions
In this retrospective cohort study, there was no statistically significant difference observed in postoperative opioid consumption between randomly selected subjects who received intraoperative IV acetaminophen and randomly selected subjects who were not given IV acetaminophen. However, these results may have been influenced by confounding factors such as type of procedure performed and adjunctive analgesia regimen received. There was a negligible difference between the 2 groups in average length of time spent in the PACU. No subjects in either study group required naloxone boluses for acute opioid reversal or met prespecified oversedation criteria. Further investigation is warranted to ascertain the timing of IV acetaminophen administration in relation to procedure completion that will provide the greatest benefit to patients in a costeffective manner.
Acknowledgments
Financial support/disclosures: The authors would like acknowledge John Pura, MPH, for statistical support throughout the study, and the Duke University Hospital Department of Pharmacy, which provided funding for the study. Dr. Raiff and Dr. Vaughan are clinical pharmacists in the Center for Medication Policy at Duke University Hospital.
Dr. McGee is the Director of the Center for Medication Policy at Duke University Hospital. The authors have no conflicts of interest to disclose.
References
- 1.Apfelbaum JL, Chen C, Mehta SS, et al. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–540. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ.Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–1625. [DOI] [PubMed] [Google Scholar]
- 3.Wells N, Pasero C, McCaffery M.Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol. I Rockville, MD: Agency for Healthcare Research and Quality; 2008:469–497. [PubMed] [Google Scholar]
- 4.The Joint Commission. Facts about pain management. 2012. http://www.jointcommission.org/assets/1/18/pain_management.pdf Accessed May1, 2013.
- 5.US Department of Health and Human Services, Centers for Medicare and Medicaid. HCAHPS: Patients’ Perspectives of Care Survey. http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/HospitalHCAHPS.html Accessed April30, 2013.
- 6.American Hospital Association (AHA). Hospital value-based purchasing program: The final rule. 2011. http://www.americangovernance.com/education/webinars/policy/pdf/final_rule_vbp_regulatory_advisory.pdf Accessed May1, 2013.
- 7.Veterans Health Administration. VHA/DoD Clinical Practice Guideline for the Management of Postoperative Pain. Washington, DC: Author; 2002. [Google Scholar]
- 8.Wheeler M, Oderda GM, Ashburn MA, et al. Adverse events associated with postoperative opioid analgesia: A systematic review. J Pain. 2002;3:159–180. [DOI] [PubMed] [Google Scholar]
- 9.Oderda GM, Said S, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: Impact on costs and length of stay. Ann Pharmacother. 2007;41:400–407. [DOI] [PubMed] [Google Scholar]
- 10.Roberts GW, Bekker TB, Carlsen HH, et al. Postoperative nausea and vomiting are strongly influenced by postoperative opioid use in a dose-dependent manner. Anesth Analg. 2005;101:1343–1348. [DOI] [PubMed] [Google Scholar]
- 11.Oderda G.Challenges in the management of acute post-surgical pain. Pharmacotherapy. 2012;32:6S–11S. [DOI] [PubMed] [Google Scholar]
- 12.ASA Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. [DOI] [PubMed] [Google Scholar]
- 13.Pergolizzi JV, Raffa RB, Tallarida R, et al. Continuous multimechanistic postoperative analgesia: A rationale for transitioning from intravenous acetaminophen and opioids to oral formulations. Pain Pract. 2012;12:159–173. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi K, Baratta JL, Heitz JW, et al. Acute pain management in the postanesthesia care unit. Anesthesiol Clin. 2012;30:1–15. [DOI] [PubMed] [Google Scholar]
- 15.Jahr JS, Filocamo P, Singh S.Intravenous acetaminophen: A review of pharmacoeconomic science for perioperative use. Am J Ther. 2013;20:189–199. [DOI] [PubMed] [Google Scholar]
- 16. Ofirmev [package insert]. San Diego, CA: Cadence Pharmaceuticals; October2013. [Google Scholar]
- 17.Singla NK, Parulan C, Samson R, et al. Plasma and cerebrospinal fluid pharmacokinetic parameters after single-dose administration of intravenous, oral, or rectal acetaminophen. Pain Pract. 2012;12:523–532. [DOI] [PubMed] [Google Scholar]
- 18.Sinatra RS, Jahr JS, Reynolds LW, et al. Efficacy and safety of single and repeated administration of 1 gram intra-venous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822–831. [DOI] [PubMed] [Google Scholar]
- 19.Wininger SJ, Miller H, Minkowitz HS, et al. A randomized, double-blind, placebo-controlled, multicenter, repeat-dose study of two intravenous acetaminophen dosing regimens for the treatment of pain after abdominal laparoscopic surgery. Clin Ther. 2010;32:2348–2369. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Adult Cancer Pain Guidelines Version 1.2013. Opioid principles, prescribing, titration, maintenance, and safety. http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf Accessed February5, 2013.
- 21.Murray A, Hagen NA.Hydromorphone. J Pain Symptom Manage. 2005;29:S57–66. [DOI] [PubMed] [Google Scholar]
- 22.Sessler CN, Gosnell M, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care patients. Am J Respir Crit Care Med. 2002;166:1338–1344. [DOI] [PubMed] [Google Scholar]
- 23.Rathmell JP, Pino CA, Taylor R, et al. Intrathecal morphine for postoperative analgesia: A randomized, controlled, dose-ranging study after hip and knee arthroplasty. Anesth Analg. 2003;97:1452–1457. [DOI] [PubMed] [Google Scholar]
- 24.Acetaminophen. Lexi-Drugs Online [Internet]. Hudson, OH: Lexi-Comp, Inc. 1978-2013 [cited May 1, 2013]. Available via institutional subscription at: http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6264. [Google Scholar]
- 25.Jokela R, Ahonen J, Seitsonen E, et al. The influence of ondansetron on the analgesic effect of acetaminophen after laparoscopic hysterectomy. Clin Pharmacol Ther. 2010;87:672–678. [DOI] [PubMed] [Google Scholar]
- 26.Moon YE, Lee YK, Lee J, et al. The effects of preoperative intravenous acetaminophen in patients undergoing abdominal hysterectomy. Arch Gynecol Obstet. 2011;284:1455–460. [DOI] [PubMed] [Google Scholar]
- 27.Khalili G, Janghorbani M, Saryazdi H, et al. Effect of pre-emptive and preventive acetaminophen on postoperative pain score: A randomized, double-blind trial of patients undergoing lower extremity surgery [published online ahead of print April 6, 2013]. J Clin Anesth. [DOI] [PubMed] [Google Scholar]
- 28.Apfel CC, Turan A, Souza K, et al. Intravenous acetaminophen reduces postoperative nausea and vomiting: A systematic review and meta-analysis. Pain. 2013;154:677–689. [DOI] [PubMed] [Google Scholar]


