Abstract
Objective
Attention-deficit/hyperactivity disorder (ADHD) is a common and extensively treated psychiatric disorder in children, which often persists into adulthood. The core diagnostic symptoms include inappropriate levels of hyperactivity, impulsivity, and/or pervasive inattention. Another crucial aspect of the disorder involves aberrations in temporal perception, which have been well documented in behavioral studies and recently have been the focus of neuroimaging studies. These fMRI studies have shown reduced activation in anterior cingulate and prefrontal cortices in ADHD using a time-interval discrimination task, whereby participants distinguish intervals differing by only hundreds of milliseconds.
Method
We utilized magnetoencephalography (MEG) to evaluate the cortical network serving temporal perception during a continuous, long-duration (minutes) time estimation experiment. Briefly, medicated and un-medicated persons with ADHD, and a control group responded each time they estimated 60 s had elapsed for an undisclosed amount of time in two separate MEG sessions. All MEG data were transformed into regional source activity, and subjected to spectral analyses to derive amplitude estimates of gamma-band activity.
Results
Compared to controls, un-medicated patients were less accurate time estimators and had weaker gamma activity in the anterior cingulate, supplementary motor area, and left prefrontal cortices. Following medication, these patients exhibited small but significant increases in gamma across these same neural regions and significant improvements in time estimation accuracy, which correlated with the gamma activity increases.
Conclusions
We found deficient gamma activity in brain areas known to be crucial for timing functions, which may underlie the day-to-day abnormalities in time perception that are common in ADHD.
Keywords: time, temporal perception, prefrontal, stimulant
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is the most common neurobehavioral disorder in children and adolescents in the United States (Center for Disease Control and Prevention [CDC], 2005). For almost 65% of affected youths, at least some of the clinically impairing symptoms (e.g., inattention) persist into adulthood, while another 15% of affected youths exhibit the full-blown disorder as adults (Faraone, Biederman, & Mick, 2006). In most cases, the severity of hyperactivity symptoms decrease as the child approaches adulthood, but the inattention and impulsivity symptoms are maintained and generally become the dominant features of the disorder during adult life (Seidman et al., 2004; Wilens et al., 2009). Several studies have shown that impaired temporal perception is intricately linked to symptoms of impulsivity, and is another problem that persists across the lifespan in patients with ADHD (Rubia, Halari, Christakou, & Taylor, 2009a; Toplak, Dockstader, & Tannock, 2006). Although investigations in this area are relatively rare, deficits have been identified in several distinct domains of timing function, including motor timing, sensory expectation, time discrimination, time estimation, and temporal foresight (Dockstader, Gaetz, Cheyne, & Tannock, 2009; Dockstader et al., 2008; Rubia et al., 2009a; Toplak et al., 2006; Toplak, Jain, & Rosemary, 2008; Valera et al., 2010; Valko et al., 2010). The most well studied aspect is likely fine-grain temporal discrimination, which involves discriminating intervals that differ by s and/or tens-to-hundreds of ms. Numerous studies investigating such fine-grained discrimination in ADHD have demonstrated aberrations in temporal processing (Rubia, Noorloos, Smith, Gunning, & Sergeant, 2003; Rubia, Smith, & Taylor, 2007; Rubia et al., 2009a; Smith, Taylor, Rogers, Newman, & Rubia, 2002; Toplak, Rucklidge, Hetherington, John, & Tannock, 2003; Toplak et al., 2006; Valera et al, 2010). Finally, there is also evidence that among measures of executive function, deficient temporal discrimination is the most robust group discriminator between persons with and without ADHD (Rubia et al., 2007).
The brain circuits that serve temporal perception and timing functions in healthy adults are widespread and vary based on the duration of the interval being estimated (Buhusi & Meck, 2005, 2009; Koch, Oliveri, & Caltagirone, 2009; Morillon, Kell, & Giraud, 2009). The core regions include the cerebellum and basal ganglia, which are critical for precise motor and perceptual timing (Koch et al., 2009; also see Rubia et al., 2009a). The supplementary motor area (SMA) and the anterior cingulate cortices (ACC) apparently serve multiple roles including estimation of both short and long duration intervals, as well as basic motor-perceptual timing (Buhusi & Meck, 2005; Morillon et al., 2009). Lastly, bilateral anterior frontal cortices and prefrontal cortices (PFC) seem to be particularly involved in the estimation of longer time intervals, and may even help to track the duration of intervals parametrically (Buhusi & Meck, 2005, 2009; Koch et al., 2009; Morillon et al., 2009; also discussed in Rubia et al., 2009a). In regards to ADHD, Smith and colleagues demonstrated hypoactivation in the ACC, SMA, dorsolateral PFC, and right anterior frontal cortices during discrimination of intervals of hundreds of ms in medication-naïve ADHD adolescents compared to their healthy peers (Smith, Taylor, Brammer, Halari, & Rubia, 2008). The fMRI activity detected in the ACC and PFC was also positively correlated with task performance across groups (Smith et al., 2008). Using a virtually identical task, this group later showed that activation in left anterior frontal cortex, left orbito-frontal cortex, right PFC, right cerebellum, and the ACC increased following stimulant treatment (relative to placebo) in adolescents with ADHD (Rubia et al., 2009a). Notably, this study largely replicated earlier findings of group differences (Smith et al., 2008), and also demonstrated that several regions including basal ganglia were more active after placebo than stimulant treatment (Rubia et al., 2009a). Other investigators have found abnormalities in the basal ganglia and cerebellum of patients with ADHD, in addition to the cortical areas normally associated with time estimation, using tasks that more purely probe sensorimotor timing (Valera et al., 2010). However, beyond these few studies of time estimation, a large corpus of neuroimaging investigations has repeatedly demonstrated structural brain abnormalities and functional aberrations within these same neural regions of persons with ADHD (Bush, 2010; Paloyelis, Mehta, Kuntsi, & Asherson, 2007; Vaidya & Stollstorff, 2008). Abundant evidence also suggests that the behavioral manifestation of ADHD is better characterized as a brain network abnormality involving multiple neural regions, and not a product of dysfunction in one or more particular brain areas (Konrad & Eickhoff, 2010).
In this study, we implemented an extended duration (i.e., minutes) time estimation experiment, in which participants were required to estimate the passage of 60 s in four consecutive intervals. This task is clearly less structured and more burdensome to sustained attention and working memory faculties compared with the time-interval discrimination and motor timing tasks used in previous studies. However, it also nicely complements these studies as the current behavioral task probes a distinct aspect of the time perception construct. Fundamentally, this implementation involves no visual stimuli and no motor response (with the exception of a rare vocal response), but necessitates that the participant concentrate on time passage for an extended period and report their “estimations.” During task performance, magnetoencephalography (MEG) data were acquired, which are a direct measure of ongoing neurophysiological activity that provides high temporal resolution with good spatial precision.
Our primary focus was on the amplitude of high-frequency (i.e., 30–100 Hz) neural oscillations in the brain regions previously linked to timing functions. Such gamma oscillations are critical to proper network function (e.g., see Fries, 2009; Uhlhaas et al., 2009), and are known to have a central role in attention disorders (Uhlhaas & Singer, 2006). Furthermore, recent reports have shown that the amplitude of gamma activity is reduced in children with ADHD (Barry et al., 2009, 2010) and, unlike age-matched controls, is not correlated with real time cognitive performance (Lenz et al., 2008, 2010); although it is notable that only a few studies have examined gamma activity in ADHD.
Thus, we evaluated population-level neural activity during an extended (i.e., min) time estimation task in medicated and un-medicated adults with ADHD, and their non-ADHD peers. Our main hypotheses were based on the current neuroimaging literature regarding brain areas and mechanisms that are most crucial to temporal perception. We examined the effects of group and medication-status on high-frequency neural activity at the level of brain regions (e.g., ACC), which were well within the spatial accuracy of MEG. We hypothesized that un-medicated adults with ADHD would be relatively poor estimators of time and would have significantly weaker gamma activity in the ACC and PFC. We also predicted that stimulant administration would improve their accuracy in estimating the passage of time and, in concert, would significantly increase high-frequency gamma activity in the same neocortical regions.
Methods and Materials
Subject Selection
We studied 12 adults (4 females) with attention-deficit/hyperactivity disorder (ADHD) inattentive type, and 12 adults (4 females) without ADHD. At enrollment, mean age was 41.83 years in the patient group (range: 30–58 years-old) and 40.08 years in the control group (range: 28–62 years-old). Participants in the control group were individually-matched to participants in the ADHD group in regards to age, sex, ethnicity, and handedness (1 participant in each group was left-handed). All participants in the ADHD group had shown a satisfactory clinical response to a mixture of amphetamine salts, extended release formula (MAS-XR; e.g., Adderall, Extended Release, Shire Pharmaceuticals, Lexington, MA, USA), and had been prescribed the same regularly-monitored dosage for at least 6 months prior to study enrollment. For this study, a satisfactory clinical response was defined as, mutual agreement between patient and clinician that relief of target symptoms, functional impairment, and distress related to ADHD had been consistently well-managed by the maintenance dosage of their prescription medication. Diagnoses were based on a semi-structured comprehensive psychiatric assessment by a board certified psychiatrist (MWW) utilizing DSM-IV diagnostic criteria, the Adult ADHD Symptom Rating Scale (ASRS, v1.1; Kessler et al., 2005), and collateral history. None of the participants were currently diagnosed with anxiety, depressive, or other disorders during the study. About half of the patients had a history of comorbid anxiety, and most of the patients (and several controls) had a history of minor depressive symptoms. Exclusionary criteria included any significant medical illness that indirectly affects CNS function (e.g., HIV-AIDS, Lupus, Cancer, etc.), neurological disorder, history of head trauma, and current substance abuse. Written informed consent was obtained from all participants following the guidelines of the University of Nebraska Medical Center Institutional Review Board, who reviewed and provided ethical approval for all experimental procedures conducted in this study.
Experimental Paradigm
All participants were scheduled for MEG early in the morning (e.g., 07:30–08:00) and, for the group with ADHD, about 24 hr since their last stimulant dosage (i.e., morning of the previous day). During the MEG recording, participants were instructed to keep their eyes closed and continuously estimate the passage of time in 60-s (1 min) increments, until notified to stop by lab personnel. Essentially, participants were asked to speak aloud each time they estimated 60 s had elapsed (i.e., “1 min,” “2 min,” etc.), and were told that any means to ‘mark time’ was allowed, except for strategies that involved motor activity (e.g., no counting aloud or with fingers). The actual duration of the experiment was a fixed 4 min, but this was not explained to participants and therefore, some reached much higher or lower estimates (e.g., 7 min) during the 4-min recording period. Throughout all MEG recording sessions, a member of the study team monitored participants closely for any movements, and recorded the timing of participant’s behavioral responses, using a real time audiovisual feed from inside the MEG room. After completing their first MEG session, participants with ADHD were orally administered their standard daily dosage of MAS-XR, and moved to the patient waiting area. Participants in the control group were simply moved to the same waiting area (no treatment). Approximately 75 min later, all participants returned to the MEG room, and completed a second (identical) session of time estimation. Note that positioning in the MEG chair and preparation took an additional 6–12 min per participant. Accuracy was recorded for each estimation response and converted to a non-compounding error value; for example, an initial estimate of 60 s at an actual time of 93 s would be a 33 s error, and a subsequent estimate of 60 s at an actual time of 152 s would be a 1 s error (i.e., 152 – 93 = 59).† These error values were averaged across trials for each participant per MEG recording session.
MAS-XR medications are commonly used to treat ADHD in adolescents and adults. Upon administration, blood plasma concentration levels rise sharply then begin to asymptote toward a peak of 20–30 ng/mL at 6–8 hr post-administration. This is followed by a gradual decline in plasma concentration level over the next 16–18 hr (Clausen, Read, & Tulloch, 2005; Tulloch, Zhang, McLean, & Wolf, 2002; Weisler, 2005). However, the degree to which the brain’s response curve follows the plasma concentration curve is entirely unknown. We chose 75 min because this is when the slope of the plasma concentration curve begins to flatten out, and because most prior studies of behavior and/or neuroimaging have used between 60 and 90 min from dosage to testing as the delay period (Barry et al., 2009; Chamberlain et al., 2008; Dodds et al., 2009;Peterson et al., 2009; Rubia et al., 2009a, 2009b; Weinbruch et al., 2005; for an exception, see Bush et al., 2009). Thus, 75 min was near the middle of the “standard” window.
Structural Magnetic Resonance Imaging (MRI)
High-resolution neuroanatomic images were acquired using a Philips Achieva 3T X-series scanner (Philips Healthcare, Andover, MA, USA). The T1-weighted sagittal images were obtained with an eight-channel head coil using a 3D fast field echo sequence with the following parameters: field of view, 24 cm; slice thickness, 1 mm with no gap; in-plane resolution, 1.0 × 1.0 mm; sense factor, 1.5. The structural volumes were aligned parallel to the anterior and posterior commissures and used for MEG coregistration.
MEG Data Acquisition & MRI Coregistration
All recordings were conducted in a one-layer magnetically-shielded room (MSR) with active shielding engaged. With an acquisition bandwidth of 0.1 – 330 Hz, neuromagnetic responses were sampled continuously at 1 kHz using an Elekta Neuromag system (Helsinki, Finland) with 306 MEG sensors (204 planar gradiometers and 102 magnetometers). Using MaxFilter (Elekta), each MEG data set was transformed into a standard device-centered head position, individually corrected for head motion during the recording session, and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu & Simola, 2006; Taulu, Simola, & Kajola, 2005).
Prior to MEG measurement, four coils were attached to the subject’s head and the locations of these coils, together with the three fiducial points (i.e., nasion, left and right periauricles) and scalp surface, were determined with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the subject was positioned inside the MSR, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed each coil to be localized in reference to the sensors throughout the recording session. Since coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system (including the scalp surface points), each participant’s MEG data was co-registered with their structural T1-weighted MRI data using a least-squares approach prior to source space analyses. Each participant’s MRI data was aligned parallel to the anterior and posterior commissures and transformed into the Talairach coordinate system (Talairach & Tournoux, 1988). The same transform parameters were then applied to their functional MEG data.
MEG Source Analyses
Following noise reduction and head-motion correction, the magnetic time series was transformed into a 29-node regional source model via inverse spatial filtering. Essentially, a 29-point grid with dual orthogonal orientations per point was constructed, and each orientation was used as an inverse spatial filter for the continuous 204-sensor time series data from each 4 min recording (Hoechstetter et al., 2004; Scherg, Ille, Bornfleth, & Berg, 2002; Scherg et al., 2004). After transformation into source space, the current-amplitude time series for each of the two orthogonal orientations per source was divided into epochs of 2048 ms duration (2048 data points per epoch). Artifact rejection was based on a fixed threshold method supplemented with visual inspection. Artifact-free epochs from each recording were transformed into the frequency domain using discrete Fourier analyses, and these ~115 epochs were averaged to obtain mean spectra for each orientation per brain area. Overall, the mean number of accepted epochs in controls was 113.82 (SD: 7.2) in session 1 and 114.67 (SD: 6.12) in session 2, whereas patients with ADHD had 115.90 (SD: 9.24) accepted epochs in the first session and 112.78 (SD: 8.11) in session 2. For each of the 29 regional sources, the amplitude per band was summed across the two orthogonal orientations to yield the total current-amplitude per frequency-band for the particular brain region. In this study, we focused all statistical analyses on neural activity in 6 of these 29 brain regions.
Based on previous studies and our hypotheses, we examined gamma spectral amplitude changes within three discrete bands (30–56 Hz, 64–82 Hz, and 82–106 Hz) in the ACC, SMA, right and left anterior frontal cortices, and the right and left PFC (i.e., six regions-of -interest (ROIs); see Figure 1) using repeated-measures ANOVAs. The precise bandwidths were selected to divide up the spectra into ranges, while avoiding the frequencies around 60 Hz and 120 Hz to prevent contamination from the electric power mains in the USA. In addition, these three gamma bands proved to be especially interesting in a previous study of ADHD (Wilson, Franzen, Heinrichs-Graham, White, Knott, & Wetzel, 2013). We initially examined the behavioral time estimation data using a 2 × 2 mixed-model ANOVA, with session as a within-subjects factor and group as a between subjects factor. Subsequently, we report the results of an omnibus 6 × 3 × 2 mixed-model ANOVA, with location (6 brain regions) and frequency bin (3 levels) as within-subjects factors, and group (with/without ADHD) as a between-subjects factor. Data from each group were then evaluated for evidence of treatment effects using a separate omnibus 6 × 3 × 2 mixed-model ANOVA, with location (6 brain area), frequency bin (3 levels), and treatment status (2 levels) as within-subject factors. Finally, we performed an exploratory analysis using mixed-model ANOVAs to evaluate the lower-frequency oscillations for evidence of amplitude differences between patients with ADHD and controls. All significant interaction effects detected through the ANOVA’s were followed-up with t-tests (described below), and statistical tests were two-tailed except where noted. All statistical analyses were conducted in SPSS (IBM Software, Armonk, New York, USA). MEG pre-processing and source modeling used the Brain Electrical Source Analysis (BESA version 5.3.2; MEGIS Software GmbH, Grafelfing, Germany) software, and MEG-MRI coregistration and spatial normalization used the BrainVoyager QX (Version 2.2; Brain Innovations, Maastricht, The Netherlands) software.
Figure 1.
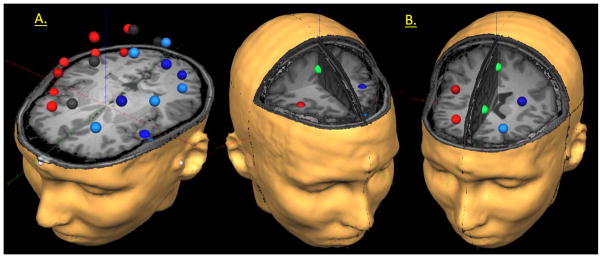
Representative Example of the 29-node Regional Source Model and Current Regions of Interest. (A) For each participant, a 29-node (grid-point) model was fitted to their MRI following coregistration, and this model was used to estimate regional neural activity during the time estimation task. In (A) above, the model can be seen overlaid on the MRI of a participant with ADHD. The different colors are only meant to aid in visually grouping the regional sources corresponding to similar brain areas. Note that the regional sources are spaced equidistant apart, and that each represents activity over an extended cortical area (i.e., > 1cm3). Thus, the time series of each node reflects the average neural activity over that brain region, and not the amount of activation at a precise neuroanatomical coordinate (e.g., a voxel in Talairach space). (B) Regions of interest (ROI) for the current study are shown in reference to two different axial slices to highlight their particular locations. On the left, a more superior axial slice illustrates the center of the right anterior frontal cortices ROI (dark red), the left anterior frontal cortices ROI (dark blue), and the supplementary motor area (SMA; green) ROI. To the right, an inferior axial slice highlights the center of the left (light blue) and right (light red) prefrontal cortices ROI (PFC), and the anterior cingulate cortices ROI (ACC; green). ROIs were chosen a priori based on previous studies of timing function in healthy participants.
Results
Behavioral Data
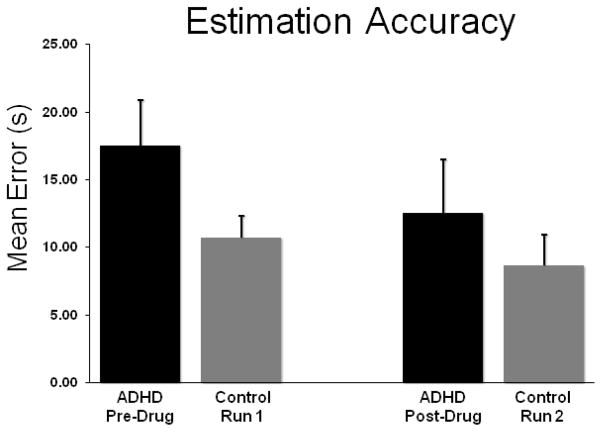
All participants were able to complete the time estimation experiment successfully. Responses were first converted to a non-compounding average error value (see Methods), and these data were subjected to a mixed-model ANOVA with session (within-subjects) and group (between-subjects) as factors.† This mixed-model ANOVA indicated a session-by-group interaction effect F(1, 22) = 3.81 (p = 0.05), and a main effect of session F(1, 22) = 14.41 (p < 0.01). The group main effect was not significant. Follow-up independent-samples t-tests on the session-by-group interaction term indicated that un-medicated patients (session one) were significantly less accurate than controls (t(22) = 1.84, p = 0.04; one-tailed), while medicated patients did not differ from controls (t(22) = 0.65, p = 0.52). Additionally, follow-up paired-samples t-tests revealed a significant improvement in estimation accuracy following medication administration in patients (t(11) = 3.74, p < 0.01), but no significant changes in controls between sessions 1 and 2. Given that participants completed only 3–5 trials per session, the marginal (i.e., p = 0.04; one-tailed) between-group differences were not surprising. Finally, descriptive statistics indicated that the session main effect was caused by significantly better accuracy in session two compared with session one, regardless of group.
MEG Data: Group Comparisons
An omnibus 6 × 3 × 2 mixed-model ANOVA with location (6 regions) and frequency (3 levels) as within-subjects factors, and group (with/without ADHD) as a between-subjects factor was conducted using the session one data from controls and un-medicated patients with ADHD. This analysis indicated that all interaction effects and main effects were significant (all p’s < 0.003). However, the main effect of location was not relevant to our hypotheses and is potentially contaminated by partial volume effects (i.e., differences in the sensitivity of the imaging device to neural activity in different brain regions). Likewise, the main effect of frequency was also confounded because of the general relationship between frequency and power (i.e., 1/f). In the physical world, power decreases with increasing frequency and thus lower frequencies always have greater power. In other words, there is always more power in the delta band (1–4 Hz) compared with the theta (4–7 Hz) and alpha (8–12 Hz) bands. The same principle holds for lower and upper gamma bands. Thus, we did not further examine location or frequency-bin main effects, or the location-by-frequency interaction effect.
Of the other variables, this ANOVA indicated a location-by-frequency-by-group three-way interaction effect F(10, 220) = 2.81 (p < 0.01), a frequency-by-group interaction F(2, 44) = 7.29 (p < 0.01), a location-by-group interaction F(5, 110) = 2.96 (p < 0.01), and a main effect of group F(1, 22) = 8.62 (p < 0.01). Post-hoc (independent-sample) t-tests of the three-way, location-by-frequency-by-group, interaction term indicated that un-medicated patients with ADHD had significantly weaker low-, mid-, and high-gamma band activity relative to controls in most ROIs (see Table 1 and Figures 3–5). One exception was the right PFC, where there were no group differences regardless of gamma range. In regards to the location-by-group interaction term, neural activity was significantly stronger in controls compared with un-medicated patients, regardless of the specific gamma frequency, in the SMA (t(22) = 2.70, p < 0.01), ACC (t(22) = 2.83, p < 0.01), left anterior frontal cortices (t(22) = 2.66, p = 0.01, right anterior frontal cortices t(22) = 2.84, p < 0.01), and the left PFC (t(22) = 3.22, p < 0.01). The frequency-by-group interaction effect indicated that, across all six ROIs, gamma activity in each of the three frequency bins was stronger in controls than in un-medicated patients (low-gamma: t(22) = 2.99, p < 0.01; mid-gamma: t(22) = 2.94, p < 0.01; high-gamma: t(22) = 2.95, p <0.01). Finally, the group main effect signified that activity was generally stronger in controls regardless of neural region or specific gamma-band.
Table 1.
Group Differences in Regional Gamma Activity
| Frequency | Region | t-value | p-value |
|---|---|---|---|
| Low Gamma (30–56 Hz) | ACC | 2.94 | p < 0.01 |
| L Anterior FC | 2.73 | p < 0.01 | |
| R Anterior FC | 3.04 | p < 0.01 | |
| LPFC | 3.28 | p < 0.01 | |
| SMA | 2.80 | p < 0.01 | |
|
| |||
| Mid Gamma (64–82 Hz) | ACC | 2.81 | p < 0.01 |
| L Anterior FC | 2.68 | p = 0.01 | |
| R Anterior FC | 2.78 | p < 0.01 | |
| LPFC | 3.25 | p < 0.01 | |
|
| |||
| High Gamma (82–106 Hz) | ACC | 2.86 | p < 0.01 |
| L Anterior FC | 2.76 | p < 0.01 | |
| R Anterior FC | 2.84 | p < 0.01 | |
| LPFC | 3.33 | p < 0.01 | |
| SMA | 2.87 | p < 0.01 | |
All statistical results reflect control group > un-medicated patient group
ACC = anterior cingulate cortex; L Anterior FC = left anterior frontal cortices; R Anterior FC = right anterior frontal cortices; LPFC = left prefrontal cortex; SMA = supplementary motor area
Figure 3.
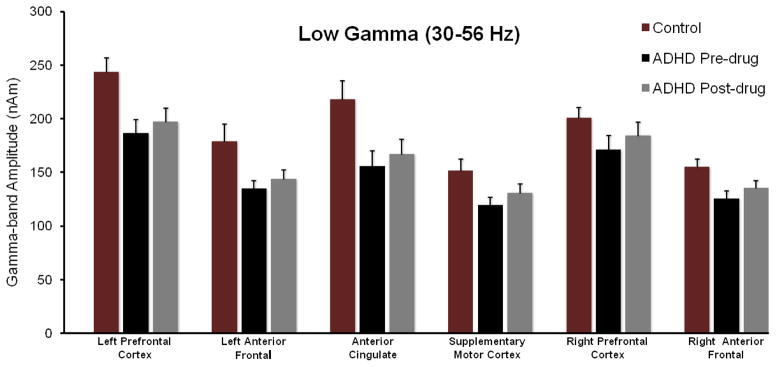
MEG Data Summary for the Low Gamma-band (30–56 Hz). Group averages for the controls in Session 1 (maroon), un-medicated adults with ADHD (black), and medicated adults with ADHD (grey) are shown separately for each ROI along the x-axis. Data for controls in Session 2 did not statistically differ from that of Session 1, and thus is not shown. The y-axis reflects the total current amplitude (nAm) within this frequency-band for the given ROI. Error bars indicate one standard error of the mean (SEM). Figures 4 and 5 follow this identical format in regards to color, x-/y-axes, and error bars. Our key findings indicated that un-medicated participants with ADHD had less 30–56 Hz activity overall (main effect) compared with non-ADHD participants, and this effect was significant for each ROI except the right PFC and SMA. In addition, stimulant administration was associated with a regionally nonspecific increase in 30–56 Hz activity in adults with ADHD.
Figure 5.
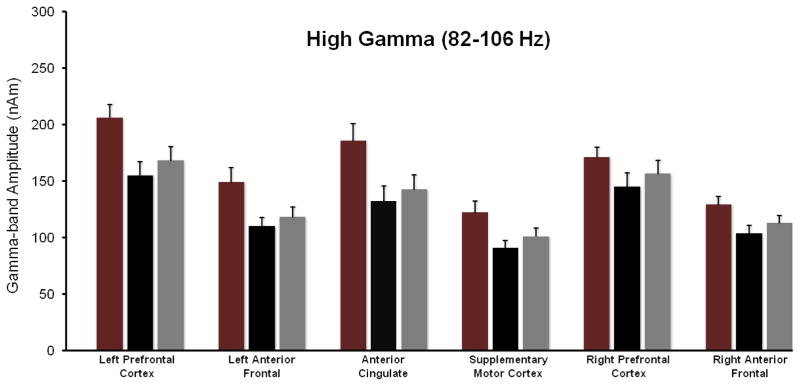
MEG Data Summary for the High Gamma-band (82–106 Hz). Figure follows the format of Figures 3–4. As shown, the un-medicated adults with ADHD had significantly reduced high-gamma activity in the left and right anterior frontal cortices, SMA, ACC, and the left PFC relative to their non-ADHD peers. The administration of MAS-XR medication was associated with an overall increase in high-gamma activity (regionally nonspecific) in the ADHD group. Lastly, the increased 82–106 Hz activity in the SMA (after treatment) was significantly correlated with the improvement in behavioral performance (i.e., estimation accuracy). Error bars indicate one SEM.
To evaluate the regional specificity of these findings, two-sample t-tests (un-medicated patients with ADHD and healthy controls) were conducted on posterior inferior frontal regions (i.e., inferior frontal gyrus, near classic Broca’s area, and its right hemispheric homologue) for each of the three gamma bands. These two-tailed tests revealed no group differences in any band. Essentially, neither left nor right posterior inferior frontal cortices showed gamma-band differences between groups (p-values from 0.25 to 0.90).
MEG Data: MAS-XR Treatment Effects
As with the group comparisons, an omnibus ANOVA was initially computed using location (6 brain area), frequency bin (3 levels), and treatment status (2 levels) as within-subject factors. Groups were evaluated separately to ascertain the effects of MAS-XR and the passage of ~75 min (without intervention). In participants with ADHD, the frequency-by-location F(10, 110) = 14.26 (p < 0.01) and frequency-by-session F(2,22) = 6.31 (p < 0.01) interaction effects were significant, along with the main effects of location F(5, 55) = 22.55 (p < 0.01), frequency F(2, 22) = 335.30 (p < 0.01), and session F(1, 11) = 10.88 (p < 0.01). The other 2- and 3-way interactions were not significant. As with the group comparisons, we did not further evaluate the main effects of frequency or location nor their interaction. Follow-up (paired-sample) t-tests of the frequency-by-session interaction indicated that activity increased in the low-gamma band (t(11) = 3.12, p < 0.01), mid-gamma (t(11) = 3.38, p < 0.01), and high-gamma range (t(11) = 3.4, p < 0.01) following MAS-XR administration (see Figures 3–5). The main effect of session indicated that gamma activity was significantly stronger in session 2 (medicated) compared with session 1 (un-medicated), across brain regions and ranges of gamma activity. In contrast to patients, only the frequency-by-location F(10, 110) = 28.11 (p < 0.01) interaction effect and the main effects of location F(5, 55) = 37.25 (p < 0.01) and frequency F(2, 22) = 277.08 (p < 0.01) were significant in controls; none of which were further explored due to potential confounds (see above). In controls, the main effect of MEG session and its interaction effects did not approach significance.
MEG Exploratory Analyses
Although not directly related to our hypotheses, we examined whether patients with ADHD had abnormal lower-frequency oscillations in an effort to inform future studies. Using four 6 × 2 × 2 ANOVAs (6 brain regions, 2 sessions, 2 groups), we evaluated the amplitude of delta (1–4 Hz), theta (4–7 Hz), alpha (8–14 Hz), and beta activity (14–30 Hz) for differences between patients with ADHD and controls. The group-by-session interaction effect and group main effect were not significant for theta, alpha, or beta activity. In contrast, the 6 × 2 × 2 mixed ANOVA for delta activity indicated a location-by-group interaction effect F(5, 110) = 6.03 (p < 0.01), a main effect of location F(5, 110) = 54.87 (p < 0.01), and a main effect of group F(1, 22) = 21.52 (p < 0.01). The main effect of session was not significant nor was the session-by-group interaction term (p > 0.80). Consistent with our prior comparisons, we did not further evaluate the main effect of location. Follow-up independent-samples t-tests on the location-by-group interaction effect showed that controls had stronger delta activity than patients with ADHD in the SMA (t(22) = 3.18, p < 0.01), ACC (t(22) = 4.36, p < 0.01), left anterior frontal cortices(t(22) = 3.74, p < 0.01), right anterior frontal cortices (t(22) = 3.49, p < 0.01), left PFC (t(22) = 4.64, p < 0.01), and right PFC (t(22) = 3.43, p < 0.01). Group means suggested the most prominent differences were in the left PFC. Finally, the group main effect indicated that delta activity was significantly stronger in controls relative to patients with ADHD, regardless of medication status.
MEG & Behavioral Correlations
To examine the relationship between performance in the time estimation task and our primary MEG measures, we conducted a series of exploratory Pearson-correlation analyses using the gamma activity estimates (per band) corresponding to each ROI per participant. In controls, the results indicated that performance in the time estimation task was robustly correlated with low-, mid-, and high-gamma activity in all ROIs, except the left anterior frontal cortices (see Table 2). There was no relationship between performance and gamma activity in any ROI in the un-medicated patients (all p’s > 0.32), but a few regionally-specific associations did emerge after treatment (see Table 2).
Table 2.
Significant Correlations with Behavioral Performance
| Group | Region | Frequency | r-value | p-value |
|---|---|---|---|---|
| Controls | ACC | Low Gamma | −0.58 | p < 0.05 |
| Mid Gamma | −0.59 | p < 0.05 | ||
| High Gamma | −0.60 | p < 0.05 | ||
|
| ||||
| R Anterior FC | Low Gamma | −0.68 | p < 0.05 | |
| Mid Gamma | −0.67 | p < 0.05 | ||
| High Gamma | −0.67 | p < 0.05 | ||
|
| ||||
| LPFC | Low Gamma | −0.62 | p < 0.05 | |
| Mid Gamma | −0.63 | p < 0.05 | ||
| High Gamma | −0.65 | p < 0.05 | ||
|
| ||||
| RPFC | Low Gamma | −0.69 | p < 0.05 | |
| Mid Gamma | −0.71 | p < 0.05 | ||
| High Gamma | −0.70 | p < 0.05 | ||
|
| ||||
| SMA | Low Gamma | −0.72 | p < 0.05 | |
| Mid Gamma | −0.69 | p < 0.05 | ||
| High Gamma | −0.69 | p < 0.05 | ||
|
| ||||
| Medicated Patients | L Anterior FC | Low Gamma | −0.61 | p < 0.05 |
| SMA | Low Gamma | −0.58 | p < 0.05 | |
ACC = anterior cingulate cortex; L Anterior FC = left anterior frontal cortices; R Anterior FC = right anterior frontal cortices; LPFC = left prefrontal cortex; RPFC = right prefrontal cortex; SMA = supplementary motor area
We also evaluated whether improvement in time estimation accuracy was associated with the increased gamma activity in particular brain regions of patients. These analyses indicated that low (30–56 Hz) and mid range (64–82 Hz) gamma activity increases in the ACC and SMA, following MAS-XR administration, were positively correlated with improvement in estimation accuracy (ACC, low-gamma: r(12) = 0.61 (p < 0.05); ACC, mid-gamma: r(12) = 0.57 (p < 0.05); SMA, low-gamma: r(12) = 0.64 (p < 0.05); SMA, mid-gamma: r(12) = 0.69 (p < 0.05)). Likewise, high-gamma activity increases were also positively correlated with improved time estimation accuracy in the SMA, r(12) = 0.72 (p < 0.01).
Discussion
We evaluated behavioral accuracy and high-frequency brain oscillations in the SMA, ACC, left and right anterior frontal cortices, and the left and right PFC during a continuous, extended-duration, time estimation task in matched groups of adults with and without ADHD. Both groups also underwent a second MEG session (after stimulant administration for ADHD participants), which provided preliminary evidence for how stimulant medications modulate task performance and neural activity. Our most important findings include inferior performance in the time estimation task, and robust reductions across the gamma-frequency spectrum in unmedicated adults with ADHD relative to their non-ADHD peers. These group differences were larger for the higher-frequency bins, with patients exhibiting gamma deficits in all ROIs except the right PFC. This study’s secondary findings establish a preliminary link between stimulant medications, enhanced time estimation accuracy, and increased gamma activity across brain regions serving timing functions. Furthermore, the amplitude of low- and mid-range gamma activity in the ACC and SMA showed positive correlations with improvement in time estimation accuracy (i.e., persons who were very improved estimators also had large increases in gamma activity). Finally, our exploratory analyses suggested that delta activity may also be abnormal in patients with ADHD, and that such reductions may not be responsive to stimulant medications. Below, we discuss the implications of these results for the symptomatology of ADHD, the neural mechanisms of pharmacotherapy, and overall network function in affected patients.
Unlike previous studies of timing function in ADHD (Rubia et al., 2009a; Smith et al., 2008), the current study found significant deviations in behavioral performance in conjunction with clear evidence of aberrant neural activity. These previous studies utilized a time-interval discrimination task, which was substantially less burdening to cognitive faculties than the current task, as it primarily involved discriminating amongst short-duration temporal intervals (i.e., hundreds of ms). Thus, the presence of a moderate behavioral deficit in the un-medicated patients of the current study was not surprising. The results likely reflect the temporal perception problems that these patients experience in their day-to-day life, as well as the overall difficulty of the task. In regards to brain function, we observed significant deficits in gamma oscillatory activity in cortical regions that are known to be central to timing functions, especially those not directly linked to sensorimotor timing (Buhusi & Meck, 2005, 2009; Koch et al., 2009; Morillon et al., 2009; Rubia et al., 2009a; Smith et al., 2008; Valera et al., 2010). Specifically, we found that oscillatory gamma activity was broadly (30–106 Hz) reduced bilaterally in the anterior frontal cortices, ACC, SMA, and the left PFC of un-medicated adults with ADHD. Although there have been a large number of neurophysiological investigations of ADHD, almost all of these have focused exclusively on low-frequency neural activity in children with ADHD (for review, see Barry, Clarke, & Johnstone, 2003), and our exploratory analyses of delta activity add to this literature. However, several recent studies have quantified and more fully evaluated higher-frequency brain responses (i.e., beta-band and above), and these reports have shown reduced gamma activity in children with ADHD (Barry et al., 2009, 2010). There is also evidence that the amplitude of gamma responses, unlike age-matched controls, is not correlated with real time cognitive performance in ADHD children (Lenz et al., 2008, 2010), which again is fully consistent with the current results. These recent studies have not reported regional specificity in regards to gamma abnormalities, but due to the electrical conductivity properties of the skull, such EEG voltage measurements are sometimes limited in this regard.
Gamma-band (30–120 Hz) activity is known to occur across the neocortex and is crucially dependent upon the integrity of local interneuronal networks, which function as GABA-gated pacemakers for cortical oscillatory activity (Bartos, Vida, & Jonas, 2007; Fries, Nikolic, & Singer, 2007; Singer, 1999). An organizing hypothesis anchored by extensive neurophysiological data postulates that gamma oscillations are crucial in coordinating information processing (e.g., see Fries, 2009; Uhlhaas et al., 2009). Essentially, gamma-frequency neural activity likely mediates interactions between distinct brain regions by linking the stimulus and/or intrinsic processing, which is accomplished by an individual brain area, into the overall processing of the distributed neural system (Fries, 2009; Singer, 1999). Thus, the broad deficits we observed in oscillatory gamma responses may suggest that individual brain regions are unable to generate sufficient high-frequency activity, and thereby are unable to effectively interact with other nodes of the brain networks that serve timing functions. Importantly, there is converging evidence for network-level deficits in right fronto-parietal attention systems and the default-mode network in persons with ADHD (Konrad and Eickhoff, 2010), which could reflect the same mechanisms that we observed in the neural circuits that serve timing functions. Such a basic-level aberration, in the generation or maintenance of gamma oscillatory activity, may explain the absolute breadth of diverse pathophysiological findings in the existent ADHD neuroimaging literature (Paloyelis et al., 2007; Vaidya & Stollstorff, 2008), as it could be expressed across a wide variety of behavioral tasks and be indirectly detected using a multitude of distinct neuroimaging measurements.
Although not our primary focus, the current study found that stimulant administration modulated gamma activity in adults with ADHD. Previous EEG studies of children with ADHD had shown that stimulant medications decrease neural activity in the theta-band (4–7.5 Hz; Clarke, Barry, Bond, McCarthy, & Selikowitz, 2002; Clarke et al., 2003; Clarke, Barry, McCarthy, Selikowitz, & Johnstone, 2007), and one MEG study reported the opposite effect of an increase in frontal theta following stimulant treatment in children and adolescents with ADHD (Wienbruch, Paul, Bauer, & Kivelitz, 2005). Despite the central role gamma activity plays in disorders of attention (Uhlhaas & Singer, 2006), no previous pharmacological EEG/MEG study has evaluated gamma-frequency responses, or any neural activity faster than 24 Hz. Our preliminary results suggest that stimulants (i.e., MAS-XR) directly or indirectly increase gamma activity in a regionally non-specific way. In short, we observed a small but significant increase across all regions of interest following MAS-XR administration, which was correlated with the improved estimation accuracy in both the SMA and ACC. The effect of stimulant treatment was also relatively large for the low- and high-gamma bands compared with the mid-range (64–82 Hz) in our study. However, following treatment, the amplitude of gamma activity (across all bands) in patients remained well-below that observed in controls. Of course, medicated patients continue to experience minor ADHD symptoms, as the medication is only able to significantly decrease the severity of symptoms and cannot completely suppress all signs of ADHD symptomatology. Moreover, it is unlikely that gamma activity is the only aspect of neurophysiology that reflects ADHD symptoms, and a full normalization of gamma activity would not necessarily be connected to a complete absence of breakthrough symptoms. Finally, it is important to recognize that these findings should be considered preliminary until replicated with a placebo-controlled study. To date, only one neuroimaging study (Rubia et al., 2009a, 2009b) has utilized a healthy control group, a placebo-treatment group, and a drug-treatment group, and this study utilized the same 13 children at two different time points to form both the placebo and treatment groups (i.e., an AB/BA cross-over design).
Although this cross-over study and other fMRI studies cannot directly address the topic of reduced gamma activity, they have provided significant insight into the brain areas and mechanisms modulated by stimulant medications in patients (mostly children) with ADHD. Some of their more pertinent findings have included, enhanced suppression of neural activity in the ventral anterior cingulate node of the default-mode network following stimulant ingestion in adolescents with ADHD (Peterson et al., 2009). Using an attention-demanding interference task, increased activation in the ACC, PFC, and parietal areas has been found following stimulant administration in adults with ADHD (Bush et al., 2008). As discussed in the introduction, Rubia et al. (2009a) showed activation was increased in the left anterior frontal cortex, right PFC, right cerebellum, and the ACC following stimulant (relative to placebo) administration in adolescents with ADHD, who were completing a short-interval time discrimination task. In the same cohort, Rubia et al. (2009b) also found that the ACC and several other brain regions were modulated by stimulant medications when these patients completed a rewarded continuous performance task.
In addition to the regionally-specific findings, some of these fMRI studies have examined functional connectivity and found that those with ADHD exhibited reduced functional connectivity in fronto-parietal networks, and other activated brain areas, in the un-medicated state. Following stimulant administration, these same patients showed enhanced functional interactivity in fronto-parietal networks, and between ACC and PFC regions (Peterson et al., 2009; Rubia et al., 2009). The current data showing oscillatory gamma increases in these same areas of the PFC and ACC clearly complement these studies. It is consistent with both the regionally-specific findings and the functional hypo-connectivity observed in ADHD patients who are in the un-medicated state. Such wide convergence is especially important in this area, as neuroimaging studies to date have used a wide range of participants (i.e., children, adolescents, children and adolescents, or adults) and, with the exception of Rubia et al. (2009a–b), have not included placebo-treatment control subjects and healthy subjects in the same study.
Before closing, it is important to acknowledge the limitations of this work including the modest sample sizes, the focus on inattentive subtype, the relatively old age of our ADHD patient group, the inherent complexity of a long-duration time estimation task, the potential that practice effects partially confounded the treatment effects, and the lack of a placebo-control group. As stated above, the use of placebo-control groups has been rare in pharmacological brain imaging studies to date, but such practice would clearly strengthen the conclusions of work in this arena, including the current study. In regards to subtypes, we focused on the inattentive subtype here as it is slightly more common in our clinic, which is located in an above average socio-economic area. That said, it is unclear how well the ADHD subtypes actually map onto adult patients; in our experience, inattentive symptoms are almost always the primary complaint amongst adult patients. In regards to generalizing our findings, it should be noted that our patients with ADHD were older than what is common in studies of ADHD. Past studies have enrolled subjects who were closer to 25 years old on average, where our subjects were almost 42 years old on average. Finally, with respect to our task, it is possible that practice effects partially contributed to the significant medication effect that we observed in the accuracy of patients with ADHD. Although controls showed a much smaller improvement from session-one to session-two, thus practice effects were probably a small contributor to the observed improvement. It is important to acknowledge that estimating such long intervals of time inherently involves both working memory and sustained attention, and these functions likely contribute to the deficits observed here. It is possible that patients had difficulty sustaining attention, and disengaged and reengaged multiple times during specific trials. This would have likely affected their estimation accuracy. Differentiating activation and performance differences linked to such cognitive faculties, from those that are more purely attributable to abnormal timing function, would require several parallel experiments and should be a focus of future work, which could also extend the current observations to younger persons with ADHD.
Figure 2.
Time Estimation Accuracy. The actual time corresponding to the estimation response per 60 s interval was manually recorded and converted a non-compounding error value, which was then averaged across trials for each participant per MEG recording session. This average error rate (in s) is shown on the y-axis with each group separated along the x-axis. Un-medicated adults with ADHD were significantly less accurate than their adult counterparts without ADHD. There was a significant improvement in estimation accuracy following stimulant administration in the ADHD group, and a non-significant improvement from Session 1 to Session 2 in controls. The medicated patients did not statistically differ from controls in Session 2. Error bars indicate one standard error of the mean.
Figure 4.
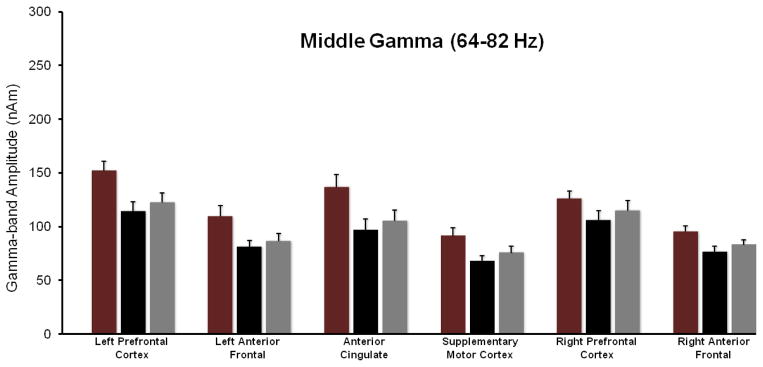
MEG Data Summary for the Mid-Gamma range (64–82 Hz). This figure follows the format of Figure 3 (and 5). In MEG Session 1, healthy adults without ADHD exhibited greater 64–82 Hz activity relative to the un-medicated ADHD group in the SMA, right and left anterior frontal cortices, ACC, and the left PFC. Following stimulant treatment, mid-gamma activity increased across all ROIs (main effect), and in the SMA and ACC regions this increase was significantly correlated with the improvement in estimation accuracy seen in the ADHD group.
Acknowledgments
The Center for Magnetoencephalography at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. TWW has been supported by a grant from the National Institutes of Health. This work was also supported by a grant from the Nebraska Banker’s Association to TWW.
Footnotes
It is important to recognize that the use of non-compounding, compared with compounding error is a major difference that would significantly affect the results. In the compounding case, if a participant estimates 60 s at an actual time of 70s than they would need to estimate the subsequent 60 s at an actual time of 120 s (i.e., 10 s short) to achieve an error free value (0 s) for the 2nd trial. In the non-compounding case, the same participant who was 10 s over on the first trial would need to estimate the second 60-s period to conclude at an actual time of 130 s (i.e., 60 s after they responded on the first trial, irrespective of actual time). In the current study, controls often misestimated the first trial, but not the subsequent trials which resulted in a relatively low average error. However, if the error rate had been tied to actual time (i.e., compounded across trials) their mistake on trial 1 would have been additive to that of all remaining trials. In contrast to controls, the participants with ADHD seemed to perform progressively worse as the session evolved, thus their scores would have been relatively less affected by compounding the error.
Disclosure/Conflicts of Interest
Dr. Wilson has no biomedical financial interests or potential conflicts of interest to report. Dr. White reported consulting fees from Philips Healthcare, serves on the advisory board for Bayer HealthCare Pharmaceuticals Inc., and has received grant funding from Bracco Diagnostics Inc. Dr. Wetzel has received speaker honoraria from Shire. Ms. Knott and Heinrichs-Graham have reported no biomedical financial interests or conflicts of interest.
References
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Bruggemann JM. Acute atomoxetine effects on the EEG of children with attention-deficit/hyperactivity disorder. Neuropharmacology. 2009;57:702–707. doi: 10.1016/j.neuropharm.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Dupuy FE. Resting-state EEG gamma activity in children with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology. 2010;121:1871–1877. doi: 10.1016/j.clinph.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in Attention-Deficit/Hyperactivity Disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology. 2003;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews Neuroscience. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relative time sharing: New findings and an extension of the resource allocation model of temporal processing. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2009;364:1875–1885. doi: 10.1098/rstb.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Bush G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J. Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Archives of General Psychiatry. 2008;65:102–114. doi: 10.1001/archgenpsychiatry.2007.16. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Mental health in the United States: Prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder – United States, 2003. Morbidity and Mortality Weekly Report. 2005;54:842–847. [PubMed] [Google Scholar]
- Chamberlain SR, Hampshire A, Müller U, Rubia K, Del Campo N, Craig K, Regenthal R, Suckling J, Roiser JP, Grant JE, Bullmore ET, Robbins TW, Sahakian BJ. Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biological Psychiatry. 2009;65:550–555. doi: 10.1016/j.biopsych.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Bond D, McCarthy R, Selikowitz M. Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2002;164:277–284. doi: 10.1007/s00213-002-1205-0. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Brown CR, Croft RJ. Effects of stimulant medications on the EEG of children with Attention- Deficit/Hyperactivity Disorder Predominantly Inattentive type. International Journal of Psychophysiology. 2003;47:129–137. doi: 10.1016/s0167-8760(02)00119-8. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M, Johnstone SJ. Effects of stimulant medications on the EEG of girls with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology. 2007;118:2700–2708. doi: 10.1016/j.clinph.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Clausen SB, Read SC, Tulloch SJ. Single- and multiple-dose pharmacokinetics of an oral mixed amphetamine salts extended-release formulation in adults. CNS Spectrums. 2005;10(12 Suppl 20m):6–15. [PubMed] [Google Scholar]
- Dockstader C, Gaetz W, Cheyne D, Tannock R. Abnormal neural reactivity to unpredictable sensory events in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;66:376–383. doi: 10.1016/j.biopsych.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Dockstader C, Gaetz W, Cheyne D, Wang F, Castellanos FX, Tannock R. MEG event-related desynchronization and synchronization deficits during somatosensory processing in individuals with ADHD. Behavioral and Brain Functions. 2008;4:8. doi: 10.1186/1744-9081-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds CM, Müller U, Clark L, van Loon A, Cools R, Robbins TW. Methylphenidate has differential effects on blood oxygenation level-dependent signal related to cognitive subprocesses of reversal learning. Journal of Neuroscience. 2008;28:5976–5982. doi: 10.1523/JNEUROSCI.1153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: A meta-analysis of follow-up studies. Psychological Medicine. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annual Review of Neuroscience. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W. The gamma cycle. Trends in Neurosciences. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, Howes MJ, Jin R, Secnik K, Spencer T, Ustun TB, Walters EE. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychological Medicine. 2005;35:245–256. doi: 10.1017/s0033291704002892. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2009;364:1907–1918. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Eickhoff SB. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping. 2010;31:904–916. doi: 10.1002/hbm.21058. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz D, Krauel K, Flechtner HH, Schadow J, Hinrichs H, Herrmann CS. Altered evoked gamma-band responses reveal impaired early visual processing in ADHD children. Neuropsychologia. 2010;48:1985–1993. doi: 10.1016/j.neuropsychologia.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Lenz D, Krauel K, Schadow J, Baving L, Duzel E, Herrmann CS. Enhanced gamma-band activity in ADHD patients lacks correlation with memory performance found in healthy children. Brain Research. 2008;1235:117–132. doi: 10.1016/j.brainres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Morillon B, Kell CA, Giraud AL. Three stages and four neural systems in time estimation. Journal of Neuroscience. 2009;29:14803–14811. doi: 10.1523/JNEUROSCI.3222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: A systematic literature review. Expert Review of Neurotherapeutics. 2007;7:1337–1356. doi: 10.1586/14737175.7.10.1337. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during stroop task performance in youths with ADHD. American Journal of Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperactive behavior: The effect of methylphenidate on motor timing. Journal of Abnormal Child Psychology. 2003;31:301–313. doi: 10.1023/a:1023233630774. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychology. 2007;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E. Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention-deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 2009a;364:1919–1931. doi: 10.1098/rstb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009b;57:640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ille N, Bornfleth H, Berg P. Advanced tools for digital EEG review: virtual source montages, whole-head mapping, correlation, and phase analysis. Jouranl of Clinical Neurophysiology. 2002;19:91–112. doi: 10.1097/00004691-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Scherg M, Bast T, Hoechstetter K, Ille N, Weckesser D, Bornfleth H, Berg P. Brain source montages improve the non-invasive diagnosis in epilepsy. International Congress Series. 2004;1270:15–19. [Google Scholar]
- Seidman LJ, Doyle A, Fried R, Valera E, Crum K, Matthews L. Neuropsychological function in adults with attention-deficit/hyperactivity disorder. Psychiatric Clinics of North America. 2004;27:261–282. doi: 10.1016/j.psc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Rogers JW, Newman S, Rubia K. Evidence for a pure time perception deficit in children with ADHD. Journal of Child Psychology and Psychiatry. 2002;43:529–542. doi: 10.1111/1469-7610.00043. [DOI] [PubMed] [Google Scholar]
- Smith A, Taylor E, Brammer M, Halari R, Rubia K. Reduced activation in right lateral prefrontal cortex and anterior cingulate gyrus in medication-naïve adolescents with attention deficit hyperactivity disorder during time discrimination. Journal of Child Psychology and Psychiatry. 2008;49:977–985. doi: 10.1111/j.1469-7610.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Physics in Medicine and Biology. 2006;51:1759–1768. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS) IEEE Transactions on Signal Processing. 2005;53:3359–3372. [Google Scholar]
- Toplak ME, Rucklidge JJ, Hetherington R, John SCF, Tannock R. Time perception deficits in attention-deficit/hyperactivity disorder and comorbid reading difficulties in child and adolescent samples. Journal of Child Psychology and Psychiatry. 2003;44:888–903. doi: 10.1111/1469-7610.00173. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: findings to date and new methods. Journal of Neuroscience Methods. 2006;151:15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Jain U, Rosemary T. Executive and motivational processes in adolescents with attention-deficit/hyperactivity disorder (ADHD) Behavioral and Brain Functions. 2008;1:8. doi: 10.1186/1744-9081-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch SJ, Zhang Y, McLean A, Wolf KN. SLI381 (Adderall XR), a two-component extended-release formulation of mixed amphetamine salts: bioavailability of three test formulations and comparisons of fasted, fed, and sprinkled administration. Pharmacotherapy. 2002;22:1405–1415. doi: 10.1592/phco.22.16.1405.33687. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolić D, Singer W. Neural synchrony in cortical networks: history, concept and current status. Frontiers in Integrative Neuroscience. 2009;3:17. doi: 10.3389/neuro.07.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Stollstorff M. Cognitive neuroscience of attention deficit hyperactivity disorder: Current status and working hypotheses. Developmental Disabilities Research Reviews. 2008;14:261–267. doi: 10.1002/ddrr.40. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Spencer RM, Zeffiro TA, Makris N, Spencer TJ, Faraone SV, Biederman J, Seidman LJ. Neural substrates of impaired sensorimotor timing in adult attention-Deficit/Hyperactivity disorder. Biological Psychiatry. 2010;68:359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko L, Schneider G, Doehnert M, Muller U, Brandeis D, Steinhausen HC, Drechsler R. Time processing in children and adults with ADHD. Journal of Neural Transmission. 2010;117:1213–1228. doi: 10.1007/s00702-010-0473-9. [DOI] [PubMed] [Google Scholar]
- Weisler RH. Safety, efficacy and extended duration of action of mixed amphetamine salts extended-release capsules for the treatment of ADHD. Expert Opinion on Pharmacotherapy. 2005;6:1003–1018. doi: 10.1517/14656566.6.6.1003. Review. [DOI] [PubMed] [Google Scholar]
- Wienbruch C, Paul I, Bauer S, Kivelitz H. The influence of methylphenidate on the power spectrum of ADHD children - an MEG study. BMC Psychiatry. 2005;5:29. doi: 10.1186/1471-244X-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Faraone SV, Martelon M, Westerberg D, Spencer TJ. Presenting ADHD symptoms, subtypes, and comorbid disorders in clinically referred adults with ADHD. Journal of Clinical Psychiatry. 2009;70:1557–1562. doi: 10.4088/JCP.08m04785pur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Franzen JD, Heinrichs-Graham E, White ML, Knott NL, Wetzel MW. Broadband neurophysiological abnormalities in the medial prefrontal region of the default-mode network in adults with ADHD. Human Brain Mapping. 2013;34:566–574. doi: 10.1002/hbm.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]