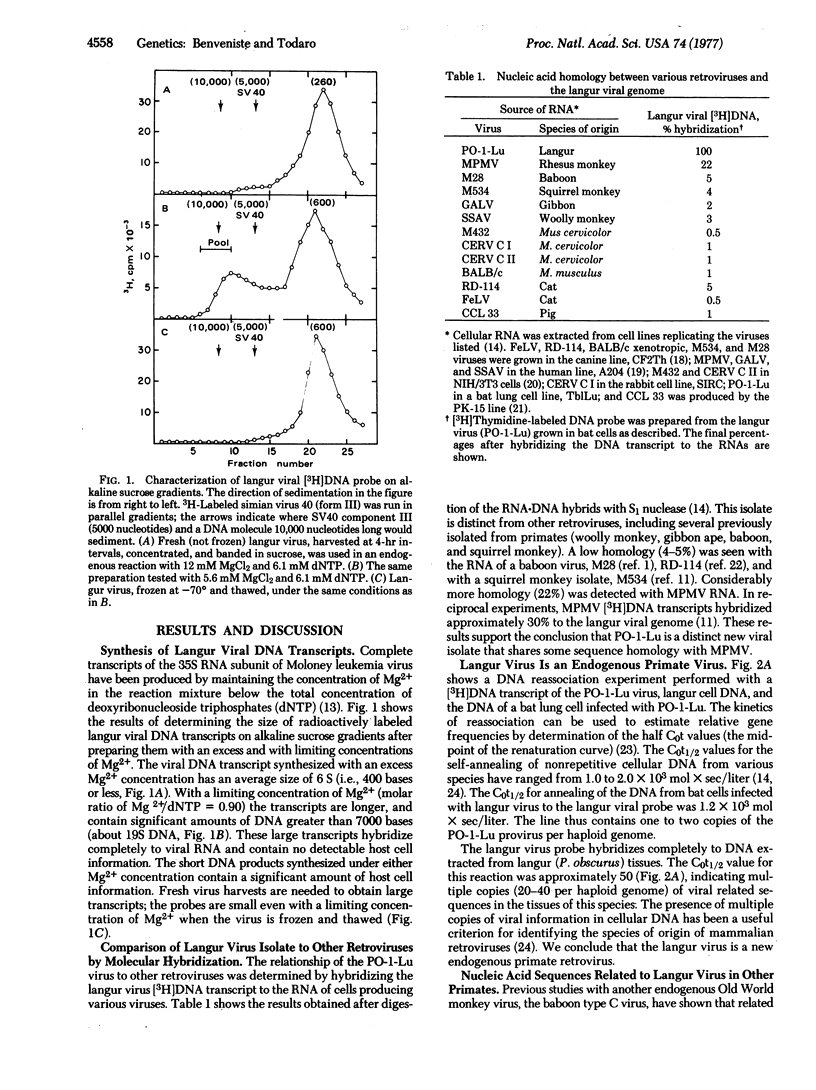
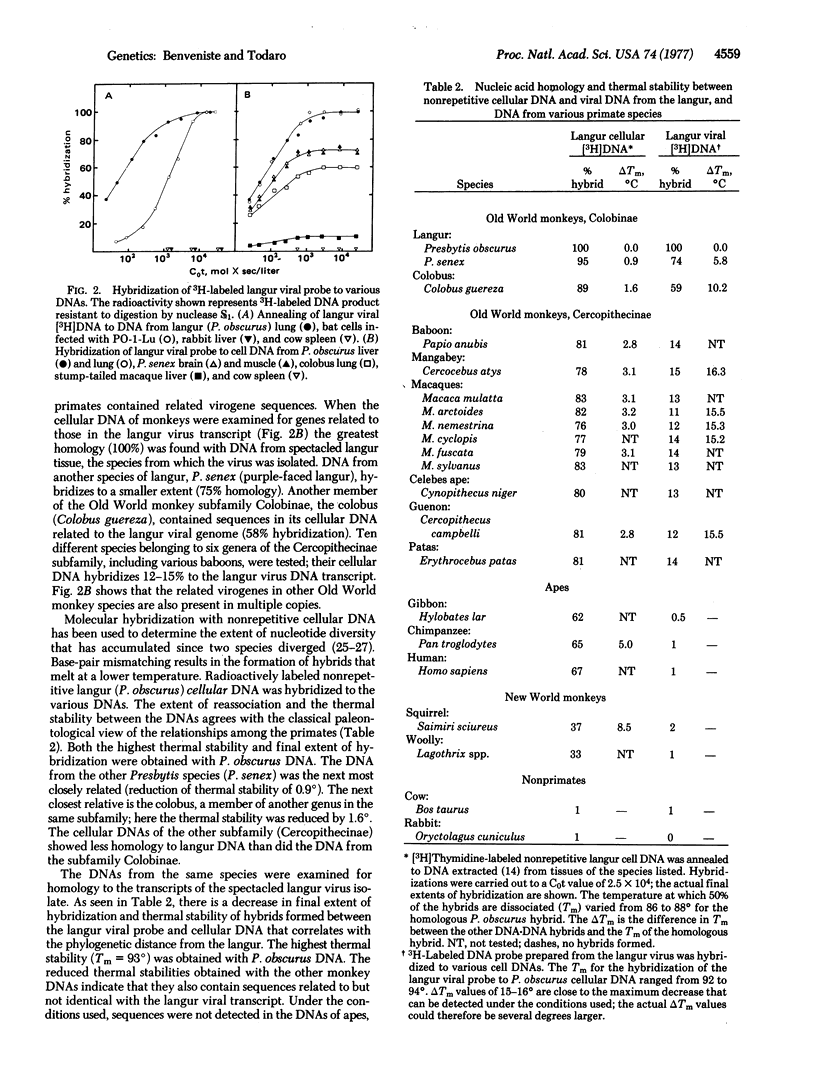
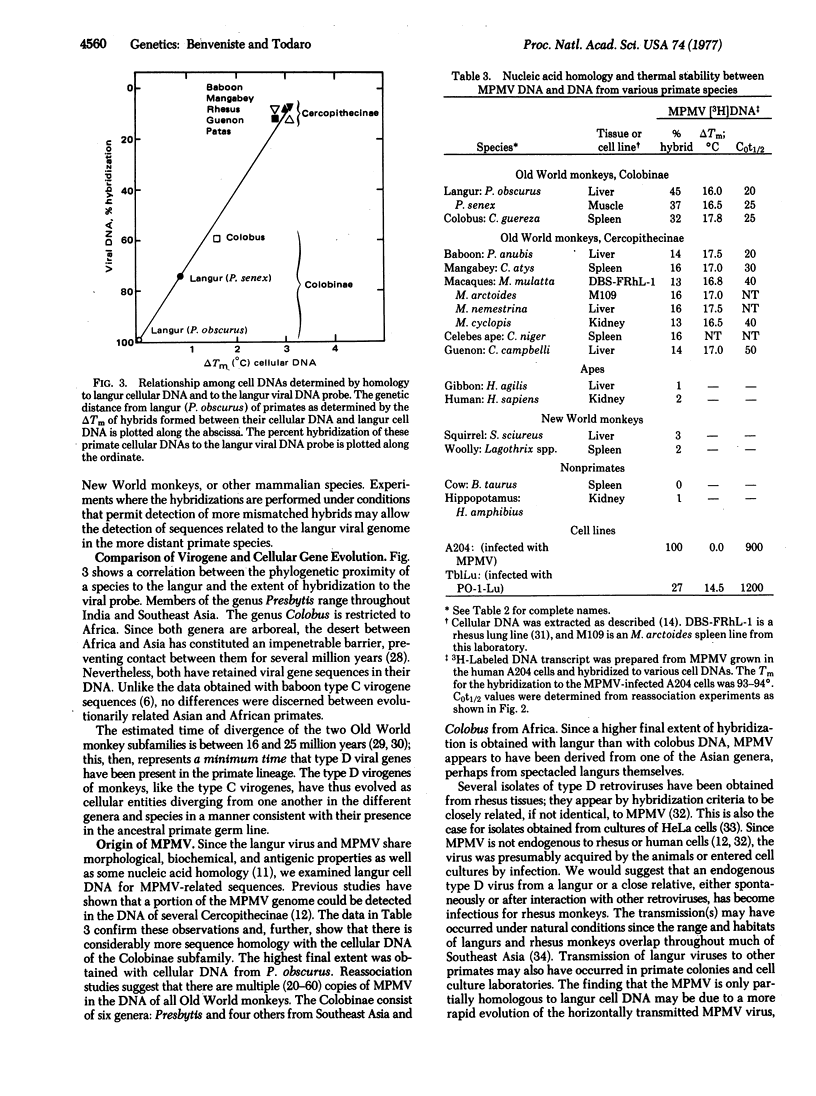
Abstract
Gene sequences related to a retrovirus (oncornavirus type D) isolated from a lung cell culture from spectacled langur (Presbytis obscurus) are found in multiple copies (20-40 per haploid genome) in langur cellular DNA; partially homologous virogene sequences are present in the DNA of related Old World monkey species. Primates thus contain gene sequences for at least two distinct classes of genetically transmitted oncornaviruses, the type C class (isolated from baboons) and the type D class described here. The langur virus is partially related to Mason-Pfizer monkey virus, a type D retrovirus isolated from rhesus monkeys. Nucleic acid hybridization studies suggest that Mason-Pfizer monkey virus, now infectious among primates, was derived from an endogenous virus of langurs or from another member of the primate sub-family Colobinae.
Keywords: type D virus, Mason-Pfizer monkey virus, endogenous baboon type C virus, DNA·DNA hybridization
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Roy-Burman P. Partial characterization of RD114 virus by DNA-RNA hybridization studies. Nat New Biol. 1973 Jul 11;244(132):59–62. doi: 10.1038/newbio244059a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Callahan R., Sherr C. J., Chapman V., Todaro G. J. Two distinct endogenous type C viruses isolated from the asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol. 1977 Mar;21(3):849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974 Dec 6;252(5483):456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: I. Nucleic acid from baboon type C virus as a measure of divergence among primate species. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4513–4518. doi: 10.1073/pnas.71.11.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Homology between type-C viruses of various species as determined by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3316–3320. doi: 10.1073/pnas.70.12.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Multiple divergent copies of endogenous C-type virogenes in mammalian cells. Nature. 1974 Nov 8;252(5479):170–173. doi: 10.1038/252170a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Chopra H. C., Mason M. M. A new virus in a spontaneous mammary tumor of a rhesus monkey. Cancer Res. 1970 Aug;30(8):2081–2086. [PubMed] [Google Scholar]
- Colcher D., Drohan W., Schlom Mason-Pfizer virus RNA genome: relationship to the RNA of morphologically similar isolates and other oncornaviruses. J Virol. 1976 Mar;17(3):705–712. doi: 10.1128/jvi.17.3.705-712.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Colcher D., Schochetman G., Schlom J. Distribution of Mason-Pfizer virus-specific sequences in the DNA of primates. J Virol. 1977 Jul;23(1):36–43. doi: 10.1128/jvi.23.1.36-43.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Peebles P. T., Nomura S., Haapala D. K. Isolation of RD-114-like oncornavirus from a cat cell line. J Virol. 1973 Jun;11(6):978–985. doi: 10.1128/jvi.11.6.978-985.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom H., Bauer H., Ogura H., Wigand R., Fischer A. B. Detection of oncornavirus-like particles in HeLa cells. I. Fine structure and comparative morphological classification. Int J Cancer. 1974 Feb 15;13(2):246–253. doi: 10.1002/ijc.2910130212. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Goldberg R. J., Scolnick E. M., Parks W. P., Yakovleva L. A., Lapin B. A. Isolation of a primate type-C virus from a lymphomatous baboon. Int J Cancer. 1974 Dec 15;14(6):722–730. doi: 10.1002/ijc.2910140605. [DOI] [PubMed] [Google Scholar]
- Kohne D. E. Evolution of higher-organism DNA. Q Rev Biophys. 1970 Aug;3(3):327–375. doi: 10.1017/s0033583500004765. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J., Benveniste R. E., Callahan R., Coon H. G. Isolation from the asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D. M., Todaro G. J. Endogenous type C virus from a cat cell clone with properties distinct from previously described feline type C virus. Virology. 1973 May;53(1):142–151. doi: 10.1016/0042-6822(73)90473-x. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M., Gardner M. B., Rongey R. W., Rasheed S., Sarma P. S., Huebner R. J., Hatanaka M., Oroszlan S., Gilden R. V. C-type virus released from cultured human rhabdomyosarcoma cells. Nat New Biol. 1972 Jan 5;235(53):3–6. doi: 10.1038/newbio235003a0. [DOI] [PubMed] [Google Scholar]
- Neiman P. E. Measurement of RD114 virus nucleotide sequences in feline cellular DNA. Nat New Biol. 1973 Jul 11;244(132):62–64. doi: 10.1038/newbio244062a0. [DOI] [PubMed] [Google Scholar]
- Nelson-Rees W. A., Owens R. B., Arnstein P., Kniazeff A. J. Source, alterations, characteristics and use of a new dog cell line (Cf2Th). In Vitro. 1976 Oct;12(10):665–669. doi: 10.1007/BF02797468. [DOI] [PubMed] [Google Scholar]
- Quintrell N., Varmus H. E., Bishop J. M., Nicholson M. O., McAllister R. M. Homologies among the nucleotide sequences of the genomes of C-type viruses. Virology. 1974 Apr;58(2):568–575. doi: 10.1016/0042-6822(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. W., Goodman N. C., Cho J. R., Ruprecht R. M., Redfield R. R., Spiegelman S. The presence of unique DNA sequences after viral induction of leukemia in mice. (RNA tumor virus-nucleic acid hybridization-insertion of viral DNA). Proc Natl Acad Sci U S A. 1974 May;71(5):1705–1709. doi: 10.1073/pnas.71.5.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Lieber M. M., Sherr C. J. Characterization of a type C virus released from the porcine cell line PK(15). Virology. 1974 Mar;58(1):65–74. doi: 10.1016/0042-6822(74)90141-x. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E. Baboons and their close relatives are unusual among primates in their ability to release nondefective endogenous type C viruses. Virology. 1976 Jul 1;72(1):278–282. doi: 10.1016/0042-6822(76)90331-7. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Tevethia S. S., Melnick J. L. Isolation of an RD-114 related type-C virus from feline sarcoma virus-transformed baboon cells. Intervirology. 1973;1(5):399–404. doi: 10.1159/000148868. [DOI] [PubMed] [Google Scholar]
- Wallace R. E., Vasington P. J., Petricciani J. C., Hopps H. E., Lorenz D. E., Kadanka Z. Development and characterization of cell lines from subhuman primates. In Vitro. 1973 Mar-Apr;8(5):333–341. doi: 10.1007/BF02619057. [DOI] [PubMed] [Google Scholar]
- Wright S. E., Neiman P. E. Base-sequence relationships between avian ribonucleic acid endogenous and sarcoma viruses assayed by competitive ribonucleic acid-deoxyribonucleic acid hybridization. Biochemistry. 1974 Mar 26;13(7):1549–1554. doi: 10.1021/bi00704a035. [DOI] [PubMed] [Google Scholar]



