Abstract
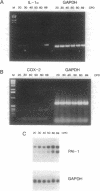
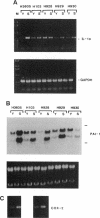
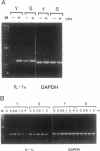
Human umbilical vein endothelial cell (HUVEC) senescence in vitro is characterized by the loss of proliferative potential and an increase in cell size. Because HUVEC senescence in one strain (H101) has been characterized by the increase in the steady-state mRNA level for the signal-peptideless cytokine, interleukin (IL) 1 alpha, we have examined young and senescent populations of five additional HUVEC strains (H3605, H103, H928, H929, and H930) to determine whether the elevated levels of IL-1 alpha mRNA could be observed in all HUVEC strains. Consistent with the data from strain H101, strains H3605 and H930 also exhibited a low steady-state level of the IL-1 alpha mRNA in young populations compared to elevated levels of IL-1 alpha mRNA in the senescent populations. However, three strains (H103, H928, and H929) did not exhibit reduced levels of IL-1 alpha mRNA in the young populations, and interestingly, strain H928, at times, expressed relatively high IL-1 alpha mRNA levels in the young populations. In addition, expression of the steady-state level of plasminogen activator inhibitor 1 and cyclooxygenase 2 was elevated in senescent populations of all HUVEC strains examined, whereas young populations exhibited a low level of expression for these genes regardless of the IL-1 alpha mRNA level. Further, the level of the IL-1 alpha polypeptide was elevated in senescent HUVEC populations relative to young populations that expressed either a high or low level of the IL-1 alpha mRNA. We have also demonstrated that the elevated level of IL-1 alpha mRNA in the senescent population of strain H3605 may be regulated by mRNA stability; however, this mechanism does not apply to all the HUVEC strains examined in this study. Thus, we suggest that while mRNA levels of the IL-1-response genes for plasminogen activator inhibitor 1 and cyclooxygenase 2 are appropriate markers for HUVEC senescence, HUVEC strain-specific post-transcriptional mechanisms may exist to regulate the function of IL-1 alpha as a modifier of HUVEC senescence in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern S. M., Miyata T., Sadler J. E. Regulation of human tissue factor expression by mRNA turnover. J Biol Chem. 1993 Jan 25;268(3):2154–2159. [PubMed] [Google Scholar]
- Bohjanen P. R., Petryniak B., June C. H., Thompson C. B., Lindsten T. AU RNA-binding factors differ in their binding specificities and affinities. J Biol Chem. 1992 Mar 25;267(9):6302–6309. [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly R. P., Fenton M. J., Kaufman J. D., Gerrard T. L. IL-1 expression in human monocytes is transcriptionally and posttranscriptionally regulated by IL-4. J Immunol. 1991 May 15;146(10):3431–3436. [PubMed] [Google Scholar]
- Fenton M. J., Vermeulen M. W., Clark B. D., Webb A. C., Auron P. E. Human pro-IL-1 beta gene expression in monocytic cells is regulated by two distinct pathways. J Immunol. 1988 Apr 1;140(7):2267–2273. [PubMed] [Google Scholar]
- Garfinkel S., Haines D. S., Brown S., Wessendorf J., Gillespie D. H., Maciag T. Interleukin-1 alpha mediates an alternative pathway for the antiproliferative action of poly(I.C) on human endothelial cells. J Biol Chem. 1992 Dec 5;267(34):24375–24378. [PubMed] [Google Scholar]
- Gay C. G., Winkles J. A. The half-lives of platelet-derived growth factor A- and B-chain mRNAs are similar in endothelial cells and unaffected by heparin-binding growth factor-1 or cycloheximide. J Cell Physiol. 1991 Apr;147(1):121–127. doi: 10.1002/jcp.1041470116. [DOI] [PubMed] [Google Scholar]
- Ghezzi P., Dinarello C. A. IL-1 induces IL-1. III. Specific inhibition of IL-1 production by IFN-gamma. J Immunol. 1988 Jun 15;140(12):4238–4244. [PubMed] [Google Scholar]
- Gimenez-Gallego G., Rodkey J., Bennett C., Rios-Candelore M., DiSalvo J., Thomas K. Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies. Science. 1985 Dec 20;230(4732):1385–1388. doi: 10.1126/science.4071057. [DOI] [PubMed] [Google Scholar]
- Gorospe M., Kumar S., Baglioni C. Tumor necrosis factor increases stability of interleukin-1 mRNA by activating protein kinase C. J Biol Chem. 1993 Mar 25;268(9):6214–6220. [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYFLICK L., MOORHEAD P. S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961 Dec;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Haines D. S., Gillespie D. H. RNA abundance measured by a lysate RNase protection assay. Biotechniques. 1992 May;12(5):736–741. [PubMed] [Google Scholar]
- Hasegawa N., Yamamoto M., Imamura T., Mitsui Y., Yamamoto K. Evaluation of long-term cultured endothelial cells as a model system for studying vascular ageing. Mech Ageing Dev. 1988 Dec;46(1-3):111–123. doi: 10.1016/0047-6374(88)90119-4. [DOI] [PubMed] [Google Scholar]
- Herzyk D. J., Allen J. N., Marsh C. B., Wewers M. D. Macrophage and monocyte IL-1 beta regulation differs at multiple sites. Messenger RNA expression, translation, and post-translational processing. J Immunol. 1992 Nov 1;149(9):3052–3058. [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., Carlton D. P., McIntyre T. M., Zimmerman G. A., Prescott S. M. Molecular cloning of human prostaglandin endoperoxide synthase type II and demonstration of expression in response to cytokines. J Biol Chem. 1993 Apr 25;268(12):9049–9054. [PubMed] [Google Scholar]
- Kruys V., Marinx O., Shaw G., Deschamps J., Huez G. Translational blockade imposed by cytokine-derived UA-rich sequences. Science. 1989 Aug 25;245(4920):852–855. doi: 10.1126/science.2672333. [DOI] [PubMed] [Google Scholar]
- Kumar S., Millis A. J., Baglioni C. Expression of interleukin 1-inducible genes and production of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4683–4687. doi: 10.1073/pnas.89.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le P. T., Lazorick S., Whichard L. P., Haynes B. F., Singer K. H. Regulation of cytokine production in the human thymus: epidermal growth factor and transforming growth factor alpha regulate mRNA levels of interleukin 1 alpha (IL-1 alpha), IL-1 beta, and IL-6 in human thymic epithelial cells at a post-transcriptional level. J Exp Med. 1991 Nov 1;174(5):1147–1157. doi: 10.1084/jem.174.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E. M., Mueller S. N. Cultured vascular endothelial cells as a model system for the study of cellular senescence. Int Rev Cytol Suppl. 1979;(10):67–76. doi: 10.1016/s0074-7696(08)60613-0. [DOI] [PubMed] [Google Scholar]
- Maciag T. Angiogenesis. Prog Hemost Thromb. 1984;7:167–182. [PubMed] [Google Scholar]
- Maciag T., Hoover G. A., Stemerman M. B., Weinstein R. Serial propagation of human endothelial cells in vitro. J Cell Biol. 1981 Nov;91(2 Pt 1):420–426. doi: 10.1083/jcb.91.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier J. A., Voulalas P., Roeder D., Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990 Sep 28;249(4976):1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Norioka K., Hara M., Kitani A., Hirose T., Hirose W., Harigai M., Suzuki K., Kawakami M., Tabata H., Kawagoe M. Inhibitory effect of human recombinant interleukin-1 alpha and beta on growth of human vascular endothelial cells. Biochem Biophys Res Commun. 1987 Jun 15;145(2):969–975. doi: 10.1016/0006-291x(87)91060-6. [DOI] [PubMed] [Google Scholar]
- Ratnasabapathy R., Hwang S. P., Williams D. L. The 3'-untranslated region of apolipoprotein II mRNA contains two independent domains that bind distinct cytosolic factors. J Biol Chem. 1990 Aug 15;265(23):14050–14055. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sawada H., Kan M., McKeehan W. L. Opposite effects of monokines (interleukin-1 and tumor necrosis factor) on proliferation and heparin-binding (fibroblast) growth factor binding to human aortic endothelial and smooth muscle cells. In Vitro Cell Dev Biol. 1990 Feb;26(2):213–216. doi: 10.1007/BF02624115. [DOI] [PubMed] [Google Scholar]
- Schleef R. R., Bevilacqua M. P., Sawdey M., Gimbrone M. A., Jr, Loskutoff D. J. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J Biol Chem. 1988 Apr 25;263(12):5797–5803. [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Schuler G. D., Cole M. D. GM-CSF and oncogene mRNA stabilities are independently regulated in trans in a mouse monocytic tumor. Cell. 1988 Dec 23;55(6):1115–1122. doi: 10.1016/0092-8674(88)90256-5. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Whitney R. G. Intraclonal variation in proliferative potential of human diploid fibroblasts: stochastic mechanism for cellular aging. Science. 1980 Jan 4;207(4426):82–84. doi: 10.1126/science.7350644. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Vakalopoulou E., Schaack J., Shenk T. A 32-kilodalton protein binds to AU-rich domains in the 3' untranslated regions of rapidly degraded mRNAs. Mol Cell Biol. 1991 Jun;11(6):3355–3364. doi: 10.1128/mcb.11.6.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]
- Wessendorf J. H., Garfinkel S., Zhan X., Brown S., Maciag T. Identification of a nuclear localization sequence within the structure of the human interleukin-1 alpha precursor. J Biol Chem. 1993 Oct 15;268(29):22100–22104. [PubMed] [Google Scholar]
- Wisdom R., Lee W. The protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitors. Genes Dev. 1991 Feb;5(2):232–243. doi: 10.1101/gad.5.2.232. [DOI] [PubMed] [Google Scholar]
- Zhu X., Komiya H., Chirino A., Faham S., Fox G. M., Arakawa T., Hsu B. T., Rees D. C. Three-dimensional structures of acidic and basic fibroblast growth factors. Science. 1991 Jan 4;251(4989):90–93. doi: 10.1126/science.1702556. [DOI] [PubMed] [Google Scholar]