Abstract
Objectives
The extent and relevance of altered bone metabolism for statural growth in children with chronic kidney disease is controversial. We analyzed the impact of renal dysfunction and recombinant growth hormone therapy on a panel of serum markers of bone metabolism in a large pediatric chronic kidney disease cohort.
Methods
Bone alkaline phosphatase (BAP), tartrate-resistant acid phosphatase 5b (TRAP5b), sclerostin and C-terminal FGF-23 (cFGF23) normalized for age and sex were analyzed in 556 children aged 6–18 years with an estimated glomerular filtration rate (eGFR) of 10–60 ml/min/1.73m2. 41 children receiving recombinant growth hormone therapy were compared to an untreated matched control group.
Results
Standardized levels of BAP, TRAP5b and cFGF-23 were increased whereas sclerostin was reduced. BAP was correlated positively and cFGF-23 inversely with eGFR. Intact serum parathormone was an independent positive predictor of BAP and TRAP5b and negatively associated with sclerostin. BAP and TRAP5B were negatively affected by increased C-reactive protein levels. In children receiving recombinant growth hormone, BAP was higher and TRAP5b lower than in untreated controls. Sclerostin levels were in the normal range and higher than in untreated controls. Serum sclerostin and cFGF-23 independently predicted height standard deviation score, and BAP and TRAP5b the prospective change in height standard deviation score.
Conclusion
Markers of bone metabolism indicate a high-bone turnover state in children with chronic kidney disease. Growth hormone induces an osteoanabolic pattern and normalizes osteocyte activity. The osteocyte markers cFGF23 and sclerostin are associated with standardized height, and the markers of bone turnover predict height velocity.
Background
Mineral and bone disorder (MBD) is a common complication of chronic kidney disease (CKD) in children. CKD-MBD encompasses complex abnormalities of bone turnover and mineralization, serum minerals and regulatory hormones, [1–3], may impact on linear growth and, by promoting vascular calcification, is considered a major cause of early cardiovascular morbidity in CKD. Treatment with recombinant growth hormone (rhGH) constitutes an approved and effective treatment for growth retardation in CKD. The effect of rhGH on bone metabolism is of particular interest since CKD-MBD and growth retardation are often connected.
However, monitoring of CKD-MBD is challenging. Bone biopsy is considered the gold standard procedure but, due to its invasiveness, currently recommended only in exceptional clinical situations [2]. Whereas plain X rays lack sensitivity, bone density measurements are more informative but still involve repetitive exposure to radiation. Hence, serum parathormone (PTH), calcium and phosphorus constitute the mainstay of CKD-MBD monitoring in routine clinical practice.
In 2006 KDIGO first recommended a detailed evaluation of emerging new biomarkers that may reflect bone cell activity in patients with CKD [1]. For children, the particular need to investigate potential associations to linear growth was emphasized. Here, we investigated in a large pediatric CKD population a panel of serum proteins potentially reflecting the activity of different bone cell types. Bone alkaline phosphatase (BAP), an osteoblast enzyme, is a sensitive and specific marker of bone formation and remodeling. The KDIGO 2009 guidelines suggest measuring either intact serum parathormone (iPTH) or BAP, since markedly altered levels are informative of bone turnover. In children BAP levels peak during infancy and puberty, mirroring the physiological activation of bone formation during these periods of rapid longitudinal growth. Tartrate-resistant acid phosphatase 5b (TRAP5b) degrades bone matrix proteins and is considered a specific marker of late osteoclast differentiation [4]. The peptide sclerostin is a novel key regulator of bone turnover [5]. It is released mainly by osteocytes as a paracrine negative feedback signal regulating bone formation by inhibiting the differentiation of osteochondral precursor cells to osteoblasts [6]. Finally, fibroblast growth factor 23 (FGF-23) is a key endocrine player in the regulation of bone mineral metabolism. Synthesized and released by osteocytes and osteoblasts [7] and targeting the renal tubule, FGF-23 plays an important role in maintaining mineral and vitamin D homeostasis.
Whereas the mechanisms and pathways of bone metabolism and the biological functions of the biomarker proteins are well established, their precise association to CKD-related abnormalities of bone metabolism and their role in height and growth of children with CKD is still controversial. Age- and gender-related differences and usually small available sample sizes add a further level of complexity to the interpretation of circulating bone markers in pediatric studies. Recently, pediatric reference values have been established for BAP, TRAP5b, sclerostin and c-terminal FGF-23 (cFGF-23), allowing age- and gender independent assessment of bone turnover in chronically diseased children [8].
The 4C Study consortium is following the largest cohort of children with CKD assembled to date [9]. We utilized the 4C cohort to characterize the patterns of these novel serum bone markers in pediatric CKD and to describe their associations with endpoints of statural growth, i.e. height standard deviation score (SDS) and its change over time, in children with and without concomitant recombinant growth hormone (GH) treatment.
Methods
Ethics statement
This study has been conducted within the framework of the 4C study. The 4C study was approved by the Institutional Review Boards (IRB) and Ethics Committees (EC) of all participating study sites. Informed written consent for participation in the 4C study and analysis of data and biosamples was obtained from parents/guardians on behalf of minors/children who also gave their consent to participate in the study. The study has been conducted according to the ethical principles of the Helsinki Declaration of 1964. The following Ethics committees (EC) and Institutional Review Boards (IRB) approved the 4C Study: Austria: EC of the University of Vienna; EC of the University of Innsbruck. Czech Republic: EC of the University of Prague. France: Comité de protection des personnes “Est IV”, Strasbourg. Germany: EC Charité—Universitätsmedizin Berlin; EC of the Medical Faculty of the University of Cologne; Ethikkommission der sächsischen Länderkammer; EC of Friedrich-Alexander-University Erlangen; EC of the University of Duisburg-Essen; EC of Albert-Ludwig University Freiburg; Ethikkommission der Ärztekammer Hamburg; EC and IRB of Hannover Medical School; EC and IRB of the University of Heidelberg; EC of the Medical Faculty of the University of Jena; EC of the Medical Faculty of the University of Leipzig; EC of Philipps University Marburg; Ethikkommission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms Universität Münster; EC of the Medical Faculty of the University of Rostock. Italy: Comitato Etico Indipendente dell’Azienda Ospedaliero-Universitaria di Bologna, Policlinico S.Orsola-Malpighi; Comitato di Etica dell’IRCCS Istituto Giannina Gaslini di Genova; Comitato Etico dell’IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena di Milano; Ethical Committee for clinical practice of the General Hospital-University of Padova; Comitato Etico per la Sperimentazione Clinica dell’IRCCS Ospedale Pediatrico Bambino Gesu’ di Roma. Lithuania: EC and IRB of Vilnius University. Poland: EC at Children’s Memorial Health Institute, Warsaw. Portugal: EC of the University of Porto. Serbia: EC of the University of Belgrade. Switzerland: EC of the Canton Bern; EC of the Canton Zurich. Turkey: EC of the University of Cukurova, Adana; Non-interventional Clinical Researches Ethics Boards of Hacettepe University, Ankara; EC of the University of Cerrahpasa, Istanbul; EC and IRB of the University of Ege, Izmir. United Kingdom: Great Ormond Street Hospital and UCL Institute of Child Health research EC.
Study population
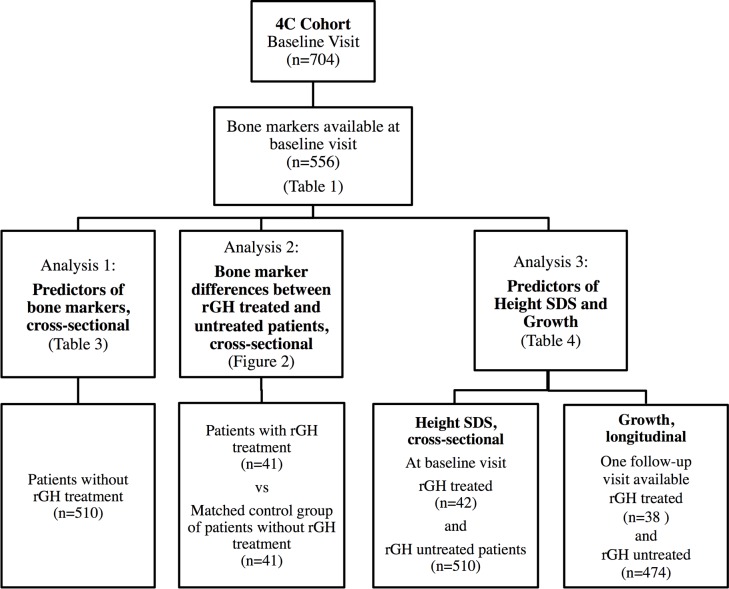
All patients were participants of the 4C Study (The Cardiovascular Comorbidity in Children with Chronic Kidney Disease Study, registered at ClinicalTrials.gov August 7, 2009, Identifier NCT01046448). The study is following children with CKD (eGFR 10–60 ml/min/1.73m2) aged 6–18 years. Clinical and biochemical examinations are performed every 6 months. Enrolment started in September 2009. All patients enrolled by December 2011 with available blood samples for bone marker analysis were included into this substudy of bone marker analysis. Bone markers were analyzed cross-sectionally. For the analysis of predictors of longitudinal growth, a follow-up clinical visit 6 (or alternatively 12) months after time of blood sampling was mandatory. Separate analyses were performed in order to 1) cross-sectionally analyze CKD-related predictors of bone markers, 2) compare bone markers of patients with rhGH treatment to a matched control group without rhGH treatment, and 3) analyze the influence of CKD-related factors and rhGH treatment on height and longitudinal growth. For patient selection and criteria for each analysis see Fig. 1.
Fig 1. Patient inclusion criteria and subgroups for cross-sectional analysis of bone markers, influence of rhGH treatment and predictors of height and growth.
The bone marker panel was measured in the baseline visit blood sample, concomitantly with a centralized biochemical analysis including serum creatinine, cystatin C, urea, C-reactive protein (CRP), bicarbonate, iPTH, calcium, phosphate and 25-hydroxy-vitamin D (25OHD). Estimated GFR (eGFR, ml/min/1.73m2) was calculated from creatinine, cystatine c, urea and height [10]. CKD stage was defined according to KDOQI [11]. Hypocalcemia was defined as a serum calcium level below 2.0 mmol/l; hyperphosphatemia was defined as serum phosphorus >1.87 mmol/L for patients aged 6 to 12 years and >1.45 mmol/L for those aged 12 years and older. A detailed patient and medication history was recorded. Physical activity was categorized into four levels depending on the hours of sports and other strenuous physical activity performed per week (0: none, 1: 1–2 hours/week, 2: 3–4 hours/week, 3: >4 hours/week). Patients were classified as rhGH treated or untreated when treatment modality was consistent over at least 3 months before bone marker analysis.
Renal diagnoses were categorized as CAKUT (congenital anomaly of the kidney and urinary tract), glomerulopathies (congenital/infantile/syndromal nephrotic syndrome, minimal-change nephropathy, focal segmental glomerulosclerosis, membranous nephropathy, mesangioproliferative glomerulonephritis, membranoproliferative glomerulonephritis, rapidly progressive glomerulonephritis, post-infectious glomerulonephritis), nephropathies due to systemic inflammatory disorders (Wegener’s granulomatosis, systemic lupus erythematosus Henoch-Schonlein purpura nephritis and IgA nephropathy), hemolytic uremic syndrome (HUS), metabolic disorders (cystinosis, oxalosis, nephrocalcinosis), other disorders (interstitial nephropathy, renovascular disease, post-ischemic chronic renal failure, other), or unknown.
Laboratory analyses
ELISA kits were used for quantitative determination of cFGF-23 (Immutopics, San Clemente, CA, USA), sclerostin (Tecomedical, Sissach, Switzerland), BAP (Quidel, San Diego, CA, USA) and TRAP5b (Quidel). All assays were performed essentially as described by the manufacturers and all samples were assayed in duplicate. The inter-/intra-assay coefficients of variation for cFGF-23, sclerostin, BAP and TRAP5b were 5.1%/5.0%; 9.3%/5.9%; 5.5%/5.0%; and 4.2%/2.8%, respectively. If duplicate results differed by more than 5% (sclerostin: 7%), measurements were repeated on a second aliquot.
Biostatistical approaches
The bone markers were normalized and standard deviation scores (SDS) were calculated for age and sex as proposed by Fischer et al., whose results on reference values were based on values obtained from 424 healthy children aged 0.1 and 21 years [8].
The influence of renal function and CKD stage on subject characteristics was tested by ANOVA for CKD stage, correlation analysis for eGFR and Kruskal-Wallis and chi square tests for categorical variables. The association of sex, age, BMI SDS, physical activity, metabolic status (serum calcium, bicarbonate, phosphorus, 25OHD) and renal function (eGFR, albuminuria) with standardized bone marker concentrations was evaluated by stepwise multivariate regression analysis in patients without rhGH treatment. For the assessment of the association of bone markers with longitudinal growth, the prospective change in height SDS was calculated over 12 months whenever available or annualized from the 6-month change if a complete 12-month follow-up was not available. The predictive value of serum bone markers on height and growth were tested by stepwise multivariate regression analysis correcting for age, sex, metabolic parameters and renal function in patients with and without rhGH treatment using rhGH treatment as confounding variable.
In all multivariate analyses, variables with a p value <0.15 were kept in the model during the selection procedure. Variables were tested for normality and log-transformed in case of violation of normality assumption.
To compare bone marker levels of patients with and without rhGH treatment with the best possible exclusion of confounding factors, the group of patients with rhGH treatment were compared to an untreated control group matched individually for age, sex, eGFR, CRP, and iPTH. Of the original cohort, the control group was selected out of 186 patients who were not treated with rhGH and were living in the same countries as children with rhGH treatment. Matching of rhGH treated and untreated patients was performed using SAS 9.2. Matches were available for 41 out of 42 rhGH treated patients. Post hoc testing for significant differences of matching variables and additional confounding factors between groups was performed.
Paired t-tests were applied to test for differences of bone marker levels between the matched groups. Statistical analyses were performed using SAS 9.2 and R. A p-value ≤ 0.05 was considered statistically significant.
Results
Subject characteristics
A cohort of 556 children and adolescents (365 males) participating in the 4C Study was studied. The patients were enrolled at 55 centers in Turkey (50%), Germany (15%), France (9%), the UK (6%), Italy (5%), Poland (4%), Austria (2.5%), Serbia (2.5%), Lithuania (2%), Switzerland (2%), Portugal (1%), and the Czech Republic (1%). Subject characteristics are given in Table 1. Underlying diseases comprised CAKUT in 74%, glomerulopathies in 6%, metabolic diseases in 3.5%, HUS in 2%, systemic vasculitis in 1%, others in 9.5% and unknown in 4% of the patients, respectively. The prevalence and severity of albuminuria (p<0.0003), hyperphosphatemia (p<.0001), hyperparathyroidism (p<0.0001), and metabolic acidosis (p<0.0009) increased with decreasing eGFR, whereas serum albumin, 25OHD, and CRP were not statistically significantly associated with renal function. Children with advanced CKD stages were shorter in stature (p<0.0001), but growth rates as expressed by the prospective change in height SDS did not differ significantly between CKD stages (p = 0.32).
Table 1. Subject characteristics.
| All | CKD Stage | |||
|---|---|---|---|---|
| 3 | 4 | 5 | ||
| N | 556 | 219 | 296 | 41 |
| Age (years) | 12.2 ± 3.3 | 12.3 ± 3.2 | 12.2 ± 3.4 | 12.1 ± 3.1 |
| % male | 66 | 67 | 66 | 59 |
| CKD duration (years) | 6.0 ± 4.6 | 6.1 ± 4.7 | 6.3 ± 4.5 | 4.3 ± 4.1 |
| BMI SDS | 0.08 ± 1.28 | 0.02 ± 1.39 | 0.12 ± 1.24 | 0.15 ± 0.97 |
| Height SDS | -1.38 ± 1.39 | -1.05 ± 1.31 | -1.58 ± 1.39 | -1.64 ± 1.47 |
| ∆ height SDS (per year) | -0.04 ± 0.38 | -0.02 ± 0.38 | -0.03 ± 0.37 | -0.13 ± 0.49 |
| Midparental height (cm) | 170.7 ± 9.4 | 171.3 ± 10.3 | 170.6 ± 9.2 | 168.7 ± 9.97 |
| Physical activity level | ||||
| 0 | 133 (23.9%) | 43 (19.6%) | 71 (24%) | 19 (46.3%) |
| 1 | 111 (20.0%) | 39 (17.8%) | 65 (22%) | 7 (17.1%) |
| 2 | 79 (14.2%) | 37 (16.8%) | 40 (13.5%) | 2 (4.9%) |
| 3 | 231 (41.6%) | 99 (45.2%) | 119 (40.2%) | 13 (31.7%) |
| Serum bicarbonate (mM) | 21.2 ± 3.8 | 21.6 ± 3.5 | 21.0 ± 3.8 | 20.5 ± 4.4 |
| Serum calcium (mM) | 2.21 ± 0.23 | 2.20 ± 0.21 | 2.22 ± 0.23 | 2.22 ± 0.27 |
| Hypocalcemia | 74 (13.3%) | 31 (14.6%) | 38 (12.8%) | 5 (12.2%) |
| Serum phosphate (mM) | 1.54 ± 0.37 | 1.47 ± 0.36 | 1.63 ± 0.35 | 1.67 ± 0.64 |
| Hyperphosphatemia | 183 (32.9%) | 54 (24.7%) | 102 (18.4%) | 27 (65.9%) |
| Serum iPTH (pmol/l) | 12.3 (7.1, 20.2) | 9.2 (5.9, 15.2) | 14.6 (8.7, 23.9) | 17.8 (9.4, 36.3) |
| Serum 25OHD (μg/l) | 11.0 (6.6, 18.1) | 12.4 7.34, 17.6) | 10.7 (6.4, 18.3) | 9.2 (5.5, 20.0) |
| CRP (mg/l) | 0.56 (0.22, 2.06) | 0.55 (0.21, 2.01) | 0.55 (0.3, 2.06) | 0.98 (0.22, 3.16) |
| Albuminuria (mg/g creatinine) | 331 (86, 1343) | 192 (56, 749) | 443 (112, 1669) | 982 (359, 2330) |
| Medications | ||||
| rhGH | 42 (7.6%) | 11 (5.0%) | 26 (8.8%) | 5 (12.2%) |
| Vitamin D supplement | 74 (13.3%) | 21 (9.6%) | 42 (14.2%) | 11 (26.8%) |
| Active vitamin D analogue | 281 (50.5%) | 89 (40.8%) | 165 (55.9%) | 25 (61.0%) |
| Phosphate binders | 254 (45.7%) | 82 (37.6%) | 142 (48.1%) | 29 (70.7%) |
Data are given as mean ± SD, median (interquartile range) or n (%) as appropriate.
At the time of bone marker assessment, 42 children (7.4%) were receiving rhGH treatment, excluding 4 patients who either started or stopped rhGH therapy within 3 months prior to analysis. 38 patients were consistently treated with rhGH during the follow-up period of 6–12 months. rhGH treated patients originated from 7 of the 12 participating countries, had a longer preceding duration of CKD (8.1±4.3 vs 5.9±4.5 years, p<0.003), were taller (-0.89±0.87 vs. -1.4±1.4 SDS, p<0.0002) and showed marginally better current growth rates (0.07±0.38 vs. -0.04±0.38, p = 0.08) compared to all non-rhGH treated patients.
Effectors of serum bone marker concentrations
Compared to healthy controls, standardized BAP, TRAP5b and cFGF-23 were significantly increased and Sclerostin decreased in the CKD population (Fig. 2 and Table 2). In univariate analysis BAP SDS was positively correlated to TRAP5b SDS (r = 0.58,p<0.0001) and inversely to cFGF-23 SDS (r = -0.2,p<0.0001). Sclerostin showed no correlation with TRAP5b, BAP or cFGF-23 (data not shown).
Fig 2. Distribution of serum bone marker concentrations in 510 pediatric CKD patients. Data are expressed as standard deviation scores (SDS).
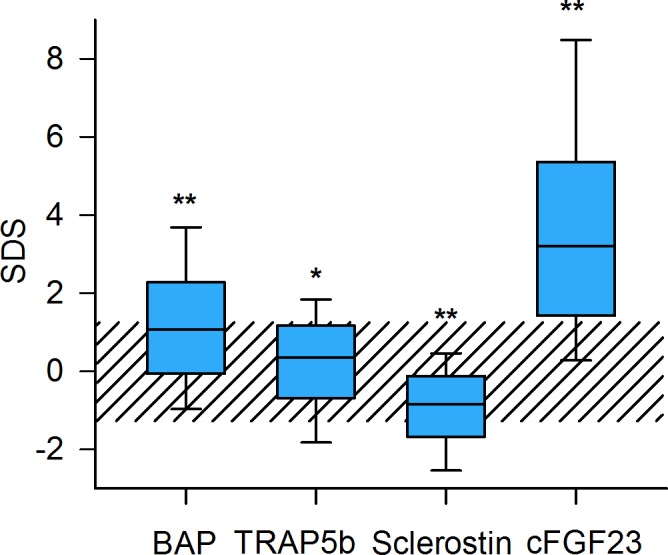
The shaded area depicts the normal range (5th to 95th percentile of biomarker concentrations in healthy children)[8]. Asterisks indicate significant deviation from distribution in the reference population (*: p<0.05, **: p<0.0001 compared to healthy controls).
Table 2. Distribution of serum bone markers by CKD stage.
| All | p | CKD Stage | |||
|---|---|---|---|---|---|
| 3 | 4 | 5 | |||
| BAP (U/L) | 147 ± 84 | 146 ± 74 | 151 ± 92 | 127 ± 73 | |
| BAP SDS | 1.25 ± 2.06 | <0.0001 | 1.27 ± 1.75 | 1.28 ± 2.27 | 0.91 ± 2.14 |
| TRAP5b (U/L) | 13.1 ± 6.6 | 13.3 ± 6.1 | 13.0 ± 7.0 | 12.8 ± 6.6 | |
| TRAP5b SDS | 0.13 ± 1.56 | 0.05 | 0.18 ± 1.35 | 0.10 ± 1.69 | 0.16 ± 1.7 |
| Sclerostin (μg/L) | 0.31 ± 0.12 | 0.30 ± 0.12 | 0.31 ± 0.12 | 0.33 ± 0.16 | |
| Sclerostin SDS | -0.99 ± 1.17 | <0.0001 | -1.05 ± 1.16 | -0.94 ± 1.12 | -1.0 ± 1.6 |
| cCFGF-23 (kRU/L) | 183 (112, 321) | 128 (89, 221) A | 226 (136, 355) B | 654 (321, 1224) B | |
| cCFGF-23 SDS | 3.21 (1.43, 5.46) | <0.0001 | 1.76 (0.67, 3.81) A | 3.85 (1.95, 6.12) B | 7.85 (4.94, 14.3) C |
Data are given as mean ± SD or median (interquartile range) P values indicate difference of age- and sex-adjusted SDS from reference population. Different superscript letters indicate significant differences (p<0.05) between CKD stages (according to ANOVA using Student-Newman-Keuls grouping).
A, B & C: Student-Newman-Keuls Grouping—Means with the same letter are not significantly different.
In stepwise multivariate analysis, BAP SDS decreased and cFGF-23 SDS increased with declining eGFR, while TRAP5b SDS and Sclerostin showed no statistically significant association to renal function (Table 3). Albuminuria was associated with higher TRAP SDS and glomerulopathies as renal diagnosis were associated with lower BAP SDS. While inflammatory renal disease did not show any association to bone markers, BAP and TRAP5b appeared to reflect the impact of the current inflammatory status on bone metabolism as indicated by their independent associations with CRP levels. BAP and TRAP5b SDS were closely associated with iPTH (Table 3). Sclerostin SDS was predicted inversely by iPTH and positively by 25OHD levels. Patients with 25OHD deficiency (25OHD <16 μg/L) had significantly lower sclerostin and sclerostin SDS levels than patients higher 25OHD levels (sclerostin 0.30 vs 0.36 μg/l, p<0.0001 and -1.07 vs -0.61 SDS, p<.0001).
Table 3. Predictors of standardized bone marker concentrations; results of stepwise multiple linear regression analyses.
| BAP SDS | TRAP SDS | Sclerostin SDS | cFGF-23 SDS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ß ± SE | Part.R2 | p | ß ±SE | Part.R2 | p | ß ±SE | Part.R2 | p | ß ±SE | Part.R2 | p | |
| Intercept | -2 ±0.75 | 0.008 | -5.07 ±0.98 | <0.001 | -0.84 ± 0.45 | 0.06 | -1.23 ± 1.98 | 0.53 | ||||
| Sex (m = 1, f = 2) | -0.35 ±0.18 | 0.01 | 0.02 | 0.32 ±0.14 | 0.005 | 0.005 | -0.33 ±0.11 | 0.026 | 0.0004 | |||
| Age (y) | 0.06 ±0.03 | 0.007 | 0.05 | 0.15 ±0.02 | 0.089 | <0.0001 | 0.30 ±0.07 | 0.028 | <0.0001 | |||
| Phys Activity (0–3) | -0.17 ±0.07 | 0.01 | 0.02 | -0.11 ±0.05 | 0.006 | 0.05 | ||||||
| eGFR (ml/min/1.73m2) | 0.03 ±0.01 | 0.014 | 0.004 | 0.02 ±0.008 | 0.005 | 0.08 | -0.21 ±0.02 | 0.13 | <0.0001 | |||
| Plasma iPTH (pM, log) | 0.77 ±0.11 | 0.077 | <0.0001 | 0.44 ±0.09 | 0.038 | <0.0001 | -0.26 ±0.06 | 0.045 | <0.0001 | |||
| Serum Pi (mM) | 1.77 ±0.68 | 0.015 | 0.004 | |||||||||
| Serum calcium (mmol/l) | 0.55 ±0.33 | 0.005 | 0.1 | |||||||||
| Serum 25OHD (µg/l) | 0.23 ±0.12 | 0.07 | 0.05 | 0.16 ±0.09 | 0.006 | 0.09 | 0.22 ± 0.07 | 0.017 | 0.003 | |||
| Serum bicarbonate (mM) | 0.03 ±0.01 | 0.006 | 0.08 | 0.22 ±0.06 | 0.025 | 0.0002 | ||||||
| Serum CRP (mg/L, log) | -0.22 ±0.06 | 0.037 | <0.0001 | -0.20 ±0.04 | 0.054 | <0.0001 | ||||||
| Albuminuria (g/g creatinine) | 0.107 ±0.04 | 0.006 | 0.008 | |||||||||
| Glomerulopathy | -0.65 ±0.37 | 0.076 | 0.04 | -0.34 ± 0.23 | 0.004 | 0.13 | ||||||
| Model R2 | 0.173 | 0.22 | 0.106 | 0.2 | ||||||||
Serum cFGF-23 concentrations were strongly and independently affected by eGFR, serum phosphorus and bicarbonate levels. In contrast to the other bone markers, there was no association with iPTH or CRP.
Serum bone markers and statural growth
Standardized cFGF-23 and sclerostin levels were independently positively associated with height SDS (Table 4). These relationships added independently to significant positive associations of age, eGFR and negative effects of metabolic acidosis on height SDS.
Table 4. Predictors of baseline and prospective change in standardized height in total cohort; results of stepwise multiple linear regression analyses.
| Height SDS | Change in height SDS per year | |||
|---|---|---|---|---|
| ß ± SE | p | ß ± SE | p | |
| Age (y) | 0.06 ± 0.02 | 0.0004 | 0.009 ± 0.001 | 0.09 |
| Sex (male) | -0.52 ± 0.14 | 0.0002 | ||
| Height SDS | - | -0.03 ± 0.013 | 0.01 | |
| Midparental height (cm) | 0.06 ± 0.008 | <.0001 | ||
| BMI SDS | -0.09 ± 0.04 | 0.037 | ||
| Physical activity level (0–4) | 0.05 ± 0.01 | 0.001 | ||
| rhGH therapy | 0.15 ± 0.07 | 0.04 | ||
| BAP SDS | 0.03 ± 0.01 | 0.002 | ||
| TRAP SDS | 0.03 ± 0.01 | 0.04 | ||
| Sclerostin SDS | 0.13 ± 0.05 | 0.005 | ||
| cFGF-23 SDS | 0.04 ± 0.01 | 0.001 | ||
| eGFR (ml/min/1.73m2) | 0.03 ± 0.007 | <.0001 | 0.002 ±0.002 | 0.21 |
| Serum bicarbonate (mM) | 0.04 ± 0.015 | 0.01 | ||
| Serum Pi (mM) | 0.14 ± 0.05 | 0.005 | ||
| Serum 25OHD (μg/l, log) | 0.006 ± 0.004 | 0.2 | 0.05 ± 0.02 | 0.04 |
| Serum CRP (mg/L, log) | -0.07 ± 0.04 | 0.07 | ||
| Model R2 | 0.24 | 0.10 | ||
The prospective change in height SDS during one year of observation was positively associated with higher BAP and TRAP5b SDS, lower baseline height SDS and rhGH therapy.
Interestingly, physical activity showed a positive association to prospective growth.
A case-control study was performed to obtain an unbiased analysis of the impact of rhGH on the bone marker pattern. For 41 rhGH treated patients an equal number of untreated control subjects matched by age, sex, country of residence, CKD duration, eGFR and serum phosphorus, iPTH and CRP was identified (see Table 5).
Table 5. Subject Characteristics for groups matched for GH treatment.
| GH untreated Controls | GH Treated Cases | |
|---|---|---|
| n | 41 | 41 |
| Age (years) | 11.9 ± 3.76 | 12.44 ± 3.24 |
| Sex: male | 35% | 35% |
| CKD duration (years) | 7.79 ± 3.73 | 8.16 ±4.37 |
| Height SDS | -0.78 ± 0.91 | -0.89 ± 0.88 |
| ∆ Height SDS | 0.0 ± 0.39 | 0.06 ± 0.39 |
| BMI SDS | 0.1 ± 1.17 | 0.01 ± 1.27 |
| eGFR (ml/min/1.73m2) | 24.9 ±8.9 | 24.9 ± 8.95 |
| Albuminuria (mg/g) | 601 ± 899 | 1534 ± 5426 |
| CRP (mg/dl) | 0.57 ± 0.7 | 0.49 ± 0.55 |
| Bicarbonate (mmol/l) | 22.4 ± 3.0 | 21.7 ± 13.0 |
| iPTH (pmol/l) | 11.6 ± 6.4 | 12.0 ± 5.8 |
| Calcium (mmol/l) | 2.3 ± 0.2 | 2.29 ± 0.26 |
| Phosphate (mmol/l) | 1.60 ± 0.39 | 1.58 ± 0.27 |
| 25OH Vitamin D (μg/l) | 16.9 ± 9.5 | 21.7 ± 13.0 |
| 1,25 OH Vitamin D (ng/l) | 89.6 ± 73.0 | 88.6 ± 78.4 |
| Diagnosis | ||
| Glomerulopathies | 5 (12%) | 2 (5%) |
| CAKUT | 30 (73%) | 29 (71%) |
| Hemolytic Uremic Syndrome | 2 (5%) | 2 (5%) |
| Inflammatory | 0 (0%) | 1 (2%) |
| Metabolic | 0 (0%) | 1 (2%) |
| Others | 4 (10%) | 6 (15%) |
| Physical Activity Level | ||
| 0 | 14 (34%) | 7 (17%) |
| 1 | 9 (22%) | 13 (32%) |
| 2 | 7 (17%) | 14 (34%) |
| 3 | 11 (27%) | 7 (17%) |
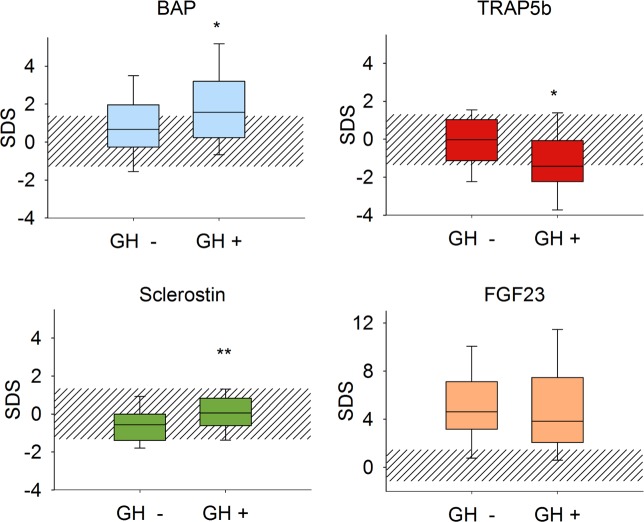
BAP SDS was significantly more increased in rhGH treated patients than in the untreated children (p<0.05) (Fig. 3, Table 6). Conversely, TRAP5b SDS was significantly lower in rhGH treated patients than in untreated controls (p<0.05). Sclerostin SDS in rhGH treated patients was not different from healthy controls but significantly higher than in the matched rhGH untreated patients (p<0.005). cFGF-23 did not differ by rhGH treatment status.
Fig 3. Distribution of serum bone marker concentrations in children with and without rhGH treatment (n = 41 per group).
Data are expressed as standard deviation scores (SDS). The shaded area depicts the normal range (5th to 95th percentile of biomarker concentrations in healthy children)8. Asterisks indicate significant deviation from distribution in the reference population (*: p<0.05, **: p<0.01).
Table 6. Bone markers in groups matched for GH treatment.
| GH untreated group | GH treated group | p | |
|---|---|---|---|
| BAP | 144 ± 84 | 182 ± 87 | 0.03 |
| BAP SDS | 0.97 ± 1.93 | 1.93 ± 1.96 | 0.02 |
| TRAP5b | 11.9 ± 8.08 | 8.83 ± 4.82 | 0.03 |
| TRAP5b SDS | -0.36 ± 1.96 | -1.25 ± 1.75 | 0.05 |
| Sclerostin | 0.35 ± 0.16 | 0.46 ± 0.19 | 0.01 |
| Sclerostin SDS | -0.63 ± 1.09 | 0.04 ± 0.96 | 0.005 |
| cFGF23 | 407 ± 457 | 380 ± 417 | n.s. |
| cFGF23 SDS | 5.36 ± 4.07 | 4.72 ± 4.17 | n.s. |
Discussion
This is the first comprehensive study of novel circulating markers of bone activity performed in a large, representative cohort of children with moderate to severe CKD. Within the pediatric age range, bone formation rate and bone turnover are essentially based on the developmental state of the skeleton. Relating our results to recently established age-specific reference values [8] allowed us to account for the physiological variability of bone markers during childhood. We demonstrated abnormal distributions of the osteoblast marker BAP, the osteoclast marker TRAP5b and the osteocyte markers cFGF-23 and sclerostin, compatible with a major alteration of bone turnover in this population. Furthermore, we observed significant changes of the bone marker profile in children undergoing rhGH treatment.
BAP and TRAP
BAP levels are reflective of osteoanabolism and those of TRAP5b of bone resorption[12,13]. Both markers are correlated with serum intact PTH, but are superior to PTH in predicting bone histology and prospective changes in bone mass[13,14]. Previous studies of BAP and TRAP5B in children with CKD yielded heterogeneous results. In a small single-center study, BAP did not differ between children with CKD and healthy controls [15], possibly due to inclusion of a wide age range encompassing the physiological concentration peaks during infancy and puberty. For TRAP5b, elevated levels were found in children with CKD stage 5 but not in stage 3 and 4 [16]. Normalizing BAP and TRAP5B plasma levels for age and sex in this large pediatric cohort with CKD stage 3 to 5, we observed a clear increase of BAP and a slight but significant increase of TRAP5B levels. This finding is compatible with a preferential high bone turnover in this patient group. A small association of BAP SDS with eGFR emerged in multivariate but not univariate analysis, indicating a higher bone turnover rate in earlier CKD stages. However, the association was weak, suggesting a predominant role of factors other than renal function. When controlling for age, sex, eGFR and underlying renal disease the main predictors of increased BAP and TRAP5b levels were low CRP and high iPTH levels. The negative association of BAP and TRAP5b with CRP may indicate a link between inflammation and reduced bone turnover or adynamic bone disease independent of the underlying renal disease, whereas the independent positive association with iPTH supports a potential role of these bone markers for the assessment of high bone turnover.
Sclerostin
In healthy children, sclerostin levels are higher in boys, decline during puberty and are positively correlated with the cortical porosity index [17]. In this pediatric CKD cohort, serum sclerostin levels were also higher in boys but did not change with age.
In adult CKD patients sclerostin levels increased progressively with advanced CKD [18]. However, in mice with progressive CKD sclerostin levels were found increased in early disease but decreased in advanced renal failure [19]. In keeping with that study we found overall reduced sclerostin levels in this cohort of patients with moderate to severe CKD. There is also experimental and clinical evidence of sclerostin suppression by PTH [19–21]. Accordingly, low sclerostin levels were predictive of high bone turnover in adults with CKD [22]. Our results confirm an inverse relationship of sclerostin and iPTH, adding to the notion that the osteoanabolic effects of iPTH may be partially related to the inhibition of sclerostin release.
Also, the bulk of sclerostin in healthy bone is released by osteocytes deep within the bone matrix whereas osteocytes near zones of bone remodeling produce little sclerostin [23]. Consistent with the observed high BAP and TRAP5b levels suggesting a high bone turnover state, the low plasma sclerostin concentrations in this cohort might indicate a relative preponderance of zones of bone remodeling over compartments with mature osteocytes and constant sclerostin production.
Little is known to date about the interaction of sclerostin with vitamin D metabolism. Increased 1,25-dihydroxy-vitamin D hydroxylase activity has recently been demonstrated in sclerostin knockout mice [24]. The independent positive association of 25OHD and sclerostin levels observed here might reflect the impact of vitamin D status on mineralized bone mass. Since the majority of children in this cohort were vitamin D insufficient or deficient, the decreased sclerostin levels might in part be explained by impaired vitamin D action.
FGF-23
FGF-23 functions as a physiological endocrine regulator of serum phosphate and 1,25-dihydroxy vitamin D levels [25]. Here, we confirm for children the tight and independent regulation of circulating FGF-23 by eGFR and serum phosphate levels.
Plasma FGF-23 levels are age-dependent and increase early in the course of CKD prior to any abnormalities of serum phosphorus, calcium or PTH [8,26]. They correlate well with FGF-23 synthesis in the bone, as demonstrated in a study of 32 pediatric and young adult CKD patients [27]. FGF-23 is mainly expressed by osteocytes of the trabecular periphery. In dialyzed children circulating FGF-23 levels were found related to the process of osteoid maturation and mineralization time [28]. FGF-23 is highly expressed at sites of new bone formation [29] and appears to regulate osteoblast matrix mineralization in an age-dependent fashion [30,31]. In a study of predialysis children FGF-23, but not PTH or serum phosphorus, independently predicted bone formation [32]. All this evidence suggests a direct role of FGF-23 in bone formation and mineralization. While our study cannot provide insights into the bone morphology of children with CKD, it adds to the current state of knowledge the important observation that FGF-23 levels are associated with relative height in growing children. We also noted that patients with metabolic acidosis tend to exhibit lower FGF-23 at a given eGFR and serum phosphorus level. This association might indicate a novel mechanistic link between metabolic acdosis and mineral-bone disorder in CKD.
rhGH promotes an osteoanabolic state in CKD
Growth hormone (GH) and its mediator IGF-1 drive longitudinal growth as part of their general anabolic action on tissues. CKD causes partial resistance to endogenous GH action, which can be overcome by administration of recombinant (r)GH at pharmacological doses. The 40 children receiving rhGH therapy in our cohort allowed to assess any effects of rhGH on bone metabolism. When compared to a matched control group of non-treated children, children receiving rhGH displayed significantly higher BAP and lower TRAP levels, consistent with an osteoanabolic action of rhGH.
This interpretation is supported by a randomized trial in dialyzed children investigating the effect of rhGH treatment on bone formation, where children receiving rhGH exhibited a higher bone formation rate than untreated children irrespective of bone histology at baseline [33]. Evidence for increased bone formation rate and bone mass from exposure to IGF1 also comes from conditions such as acromegaly and rhGH treated children receiving long-term corticosteroid therapy [34].
Sclerostin is a potent inhibitor of Wnt signaling, which plays an important role during bone formation. The finding that serum sclerostin levels were significantly higher with a distribution matching that of healthy children, suggests that rhGH might play a role in the regulation of bone formation via this pathway. We were unable to confirm the findings of a small study in GH deficient children showing an increase of cFGF-23 during rhGH supplementation therapy [35]; small changes in cFGF-23 might be overshadowed by the overall increase of cFGF-23 in CKD. Taken together, our findings support a major osteoanabolic effect of rhGH treatment in children with CKD.
Bone markers predict height velocity
We confirmed several established causative factors as independently associated with small stature in CKD, such as low eGFR, metabolic acidosis, inflammation and vitamin D deficiency. Prospective catch-up growth was associated with rhGH treatment, a high physical activity level and higher serum phosphorus, the latter possibly reflecting good nutritional intake. When adjusting for these factors, Sclerostin and cFGF-23 SDS were independently associated with height SDS, and BAP and TRAP SDS were predictive of the prospective change in height. Similarly to this observation, a study in GH deficient patients showed an increase of BAP after treatment with rhGH, which was positively correlated with to improvement of height SDS and gain of bone density[36]. It is readily conceivable that Sclerostin and cFGF-23, representing cell activity in the mineralized bone compartment, be associated with relative body height, the final outcome of bone acquisition during statural growth, whereas BAP and TRAP as markers of bone remodeling rather show an association with short-term changes in growth velocity. Notably iPTH, the conventional biomarker of bone turnover, was not associated with either height or growth rate, confirming previous findings in dialyzed children [37].
In conclusion, in a large cohort of children with predialysis CKD we found important associations of serum bone biomarkers to patient characteristics, traditional markers of bone metabolism and linear growth. Our study benefited from a large sample size, the controlled observational study design and the application of age-specific reference values. However, a limitation results from the lack of a direct assessment of bone status by biopsy or skeletal imaging studies such as DEXA, pqCT or MRI. Also, it should be emphasized that the observed associations cumulatively explained only 10–20% of the overall variation of bone marker concentrations and 10–24% of the variance of growth indicators. Hence, while our study is informative regarding important biological regulators of bone metabolism and growth, other major sources of variation, including the impact of genetic disposition, remain to be explored. We hope that our findings will prompt further evaluation of these biomarkers including bone imaging techniques and bone histomorphometry to further validate their usefulness in the assessment and therapeutic monitoring of bone metabolism in children with CKD.
Acknowledgments
The authors would like to acknowledge all children and their families for their participation in the 4C Study.
The following principal investigators are contributing to the 4C Study: Austria: G. Cortina, Children’s Hospital, Innsbruck; K. Arbeiter, University Children’s Hospital, Vienna. Czech Republic: J. Dusek, University Hospital Motol, Prague. France: J. Harambat, Hôpital des Enfants, Bordeaux; B. Ranchin, Hôpital Femme Mère Enfant et Université de Lyon; M. Fischbach, Hôpital de Hautepierre, Strasbourg. Germany: U. Querfeld, R. Zeller, Charité Children’s Hospital, Berlin; J. Dötsch, University Children’s Hospital, Cologne; M. Galiano, University Children’s Hospital, Erlangen; R. Büscher, University Children’s Hospital, Essen; C. Gimpel, Center for Pediatrics and Adolescent Medicine, Freiburg; M. Kemper, UKE University Children’s Hospital, Hamburg; A. Melk, D. Kracht, Hannover Medical School, Hannover; F. Schaefer, A. Doyon, E. Wuehl, Center for Pediatrics and Adolescent Medicine, Heidelberg; M. Pohl, Center for Pediatrics and Adolescent Medicine, Jena; S. Wygoda, City Hospital St. Georg, Leipzig; N. Jeck, KfH Kidney Center for Children, Marburg; B. Kranz, University Children’s Hospital, Münster; M. Wigger, Children’s Hospital, Rostock. Italy: G. Montini, S. Orsola-Malpighi Hospital, Bologna; A. Trivelli, Istituto Giannina Gaslini, Genova; S. Testa, Fondazione Ospedale Maggiore Policlinico, Milano; E. Vidal, Pediatric Nephrology, Dialysis & Transplant Unit, Padova; C. Matteucci, S. Picca, Ospedale Bambino Gesù, Rome. Lithuania: A. Jankauskiene, University Children’s Hospital, Vilnius. Poland: A. Zurowska, Pediatric and Adolescent Nephrology, Gdansk; D. Drodz, University Children’s Hospital, Krakow; M. Tkaczyk, Polish Mothers Memorial Hospital Research Institute, Lodz; T. Urasinski, Clinic of Pediatrics, Szczecin; M. Litwin, Anna Niemirska, Children’s Memorial Health Institute, Warsaw; M. Szczepanska, Zabrze. Portugal: A. Texeira, Hospital Sao Joao, Porto; Serbia: A. Peco-Antic, University Children’s Hospital, Belgrade. Switzerland: G. Simonetti, Inselspital, Bern; G. Laube, University Children’s Hospital, Zurich. Turkey: A. Anarat, A.K. Bayazit, Cukurova University, Adana; F. Yalcinkaya, University Faculty of Medicine, Ankara; E. Basin, Baskent University Faculty of Medicine, Ankara; N. Cakar, Diskapi Children’s Hospital, Ankara; O. Soylemezoglu, Gazi University Hospital, Ankara; A. Duzova, Y. Bilginer, Hacettepe Medical Faculty, Ankara; H. Erdogan, Dortcelik Children’s Hospital, Bursa; O. Donmez, Uludag University, Bursa; A. Balat, University of Gaziantep; A. Kiyak, Bakirkoy Children’s Hospital, Istanbul; S. Caliskan, N. Canpolat, Istanbul University Cerrahpasa Faculty of Medicine, Istanbul; C. Candan, Goztepe Educational and Research Hospital, Istanbul; M. Civilibal, Haseki Educational and Research Hospital, Istanbul; S. Emre, Istanbul Medical Faculty, Istanbul, H. Alpay, Marmara University Medical Faculty, Istanbul; G. Ozcelik, Sisli Educational and Research Hospital, Istanbul; S. Mir, B. Sözeri, Ege University Medical Faculty; Izmir; O. Yavascan, Tepecik Training and Research Hospital, Izmir; Y. Tabel, Inonu University, Malatya; P. Ertan, Celal Bayar University, Manisa; E. Yilmaz, Children’s Hospital, Sanliurfa. United Kingdom: R. Shroff, Great Ormond Street Hospital, London.
Lead investigators of the 4C Study: F. Schaefer (franz.schaefer@med.uni-heidelberg.de), U. Querfeld (uwe.querfeld@charite.de), A.Melk (melk.anette@mh-hannover.de).
Funding Statement
This study has been made possible by grants of the KfH Foundation for Preventive Medicine, the European Renal Association—European Dialysis and Transplant Association (www.era-edta.org), the German Federal Ministry of Education and Research (reference number: 01EO0802) and Pfizer Deutschland GmbH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moe S, Drueke T, Cunningham J, Goodman W, Martin K, et al. (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Vol. 69 pp. 1945–1953. 10.1038/sj.ki.5000414 [DOI] [PubMed] [Google Scholar]
- 2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group (2009) KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl: S1–S130. 10.1038/ki.2009.188 [DOI] [PubMed] [Google Scholar]
- 3. Wesseling-Perry K, Salusky IB (2013) Chronic kidney disease: mineral and bone disorder in children. YSNEP 33: 169–179. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=23465503&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersson G, Ek-Rylander B, Hollberg K, Ljusberg-Sjölander J, Lång P, et al. (2003) TRACP as an osteopontin phosphatase. J Bone Miner Res 18: 1912–1915. 10.1359/jbmr.2003.18.10.1912 [DOI] [PubMed] [Google Scholar]
- 5. Drüeke TB, Lafage-Proust MH (2011) Sclerostin: Just One More Player in Renal Bone Disease? Clin J Am Soc Nephrol 6: 700–703. 10.2215/CJN.01370211 [DOI] [PubMed] [Google Scholar]
- 6. Galli C, Passeri G, Macaluso GM (2010) Osteocytes and WNT: the mechanical control of bone formation. J Dent Res 89: 331–343. 10.1177/0022034510363963 [DOI] [PubMed] [Google Scholar]
- 7. Lu Y, Feng JQ (2011) FGF23 in Skeletal Modeling and Remodeling. Curr Osteoporos Rep 9: 103–108. 10.1007/s11914-011-0053-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fischer DC, Mischek A, Wolf S, Rahn A, Salweski B, et al. (2012) Paediatric reference values for the C-terminal fragment of fibroblast-growth factor-23, sclerostin, bone-specific alkaline phosphatase and isoform 5b of tartrate-resistant acid phosphatase. Annals of Clinical Biochemistry 49: 546–553. 10.1258/acb.2012.011274 [DOI] [PubMed] [Google Scholar]
- 9. Querfeld U, Anarat A, Bayazit AK, Bakkaloglu AS, Bilginer Y, et al. (2010) The Cardiovascular Comorbidity in Children with Chronic Kidney Disease (4C) study: objectives, design, and methodology. Clin J Am Soc Nephrol 5: 1642–1648. 10.2215/CJN.08791209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, et al. (2009) New Equations to Estimate GFR in Children with CKD. Journal of the American Society of Nephrology 20: 629–637. 10.1681/ASN.2008030287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, et al. (2003) National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics 111: 1416–1421. [DOI] [PubMed] [Google Scholar]
- 12. Ureña P, Hruby M, Ferreira A, Ang KS, de Vernejoul MC (1996) Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol 7: 506–512. [DOI] [PubMed] [Google Scholar]
- 13. Shidara K, Inaba M, Okuno S, Yamada S, Kumeda Y, et al. (2008) Serum Levels of TRAP5b, a New Bone Resorption Marker Unaffected by Renal Dysfunction, as a Useful Marker of Cortical Bone Loss in Hemodialysis Patients. Calcif Tissue Int 82: 278–287. Available: http://www.cof.org.cn/pdf/2008/6/Serum%20Levels%20of%20TRAP5b.pdf. 10.1007/s00223-008-9127-4 [DOI] [PubMed] [Google Scholar]
- 14. Ureña P, de Vernejoul MC (1999) Circulating biochemical markers of bone remodeling in uremic patients. Kidney Int 55: 2141–2156. 10.1046/j.1523-1755.1999.00461.x [DOI] [PubMed] [Google Scholar]
- 15. Behnke B, Kemper M, Kruse HP, Müller-Wiefel DE (1998) Bone alkaline phosphatase in children with chronic renal failure. Nephrol Dial Transplant 13: 662–667. [DOI] [PubMed] [Google Scholar]
- 16. Siomou E, Challa A, Printza N, Giapros V, Petropoulou F, et al. (2011) Serum osteoprotegerin, RANKL and fibroblast growth factor-23 in children with chronic kidney disease. Pediatr Nephrol 26: 1105–1114. 10.1007/s00467-011-1870-5 [DOI] [PubMed] [Google Scholar]
- 17. Kirmani S, Amin S, McCready LK, Atkinson EJ, Melton LJ, et al. (2011) Sclerostin levels during growth in children. Osteoporos Int 23: 1123–1130. 10.1007/s00198-011-1669-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pelletier S, Dubourg L, Carlier MC, Hadj-Aissa A, Fouque D (2013) The Relation between Renal Function and Serum Sclerostin in Adult Patients with CKD. Clin J Am Soc Nephrol 8: 819–823. 10.2215/CJN.07670712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sabbagh Y, Graciolli FG, O'Brien S, Tang W, Reis dos LM, et al. (2012) Repression of osteocyte Wnt/β-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 27: 1757–1772. 10.1002/jbmr.1630 [DOI] [PubMed] [Google Scholar]
- 20. Kramer I, Keller H, Leupin O, Kneissel M (2010) Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol Metab 21: 237–244. 10.1016/j.tem.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 21. Drake MT, Srinivasan B, Mödder UI, Peterson JM, McCready LK, et al. (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95: 5056–5062. 10.1210/jc.2010-0720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere M-C, et al. (2011) Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 6: 877–882. 10.2215/CJN.06550810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poole KES, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, et al. (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19: 1842–1844. 10.1096/fj.05-4221fje [DOI] [PubMed] [Google Scholar]
- 24. Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, et al. (2013) Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. Proc Natl Acad Sci USA 110: 6199–6204. 10.1073/pnas.1221255110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, et al. (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568. 10.1172/JCI19081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, et al. (2010) The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab 95: 1741–1748. 10.1210/jc.2009-1576 [DOI] [PubMed] [Google Scholar]
- 27. Pereira RCR, Juppner HH, Azucena-Serrano CEC, Yadin OO, Salusky IBI, et al. (2009) Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone 45: 8–8. 10.1016/j.bone.2009.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wesseling-Perry K, Pereira RC, Wang H, Elashoff RM, Sahney S, et al. (2009) Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. Journal of Clinical Endocrinology & Metabolism 94: 511–517. 10.1210/jc.2008-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, et al. (2003) FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest 112: 683–692. 10.1172/JCI18399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshiko Y, Wang H, Minamizaki T, Ijuin C, Yamamoto R, et al. (2007) Mineralized tissue cells are a principal source of FGF23. Bone 40: 1565–1573. 10.1016/j.bone.2007.01.017 [DOI] [PubMed] [Google Scholar]
- 31. Wesseling-Perry K (2010) FGF-23 in bone biology. Pediatr Nephrol 25: 603–608. 10.1007/s00467-009-1384-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wesseling-Perry K, Pereira RC, Tseng C-H, Elashoff R, Zaritsky JJ, et al. (2012) Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clin J Am Soc Nephrol 7: 146–152. 10.2215/CJN.05940611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bacchetta J, Wesseling-Perry K, Kuizon B, Pereira RC, Gales B, et al. (2013) The Skeletal Consequences of Growth Hormone Therapy in Dialyzed Children: A Randomized Trial. Clinical Journal of the American Society of Nephrology 8: 824–832. 10.2215/CJN.00330112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bacchetta J, Harambat J, Cochat P, Salusky IB, Wesseling-Perry K (2012) The consequences of chronic kidney disease on bone metabolism and growth in children. Nephrology Dialysis Transplantation 27: 3063–3071. 10.1093/ndt/gfs299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gardner J, Ashraf A, You Z, McCormick K (2011) Changes in plasma FGF23 in growth hormone deficient children during rhGH therapy. J Pediatr Endocrinol Metab 24: 645–650. [DOI] [PubMed] [Google Scholar]
- 36. Tobiume HH, Kanzaki SS, Hida SS, Ono TT, Moriwake TT, et al. (1997) Serum bone alkaline phosphatase isoenzyme levels in normal children and children with growth hormone (GH) deficiency: a potential marker for bone formation and response to GH therapy. Journal of Clinical Endocrinology & Metabolism 82: 2056–2061. 10.1111/cen.12617 [DOI] [PubMed] [Google Scholar]
- 37. Borzych D, Rees L, Soo Ha Il, Chua A, Valles PG, et al. (2010) The bone and mineral disorder of childrenundergoing chronic peritoneal dialysis. Kidney Int 78: 1295–1304. 10.1038/ki.2010.316 [DOI] [PubMed] [Google Scholar]