Abstract
Background
Delirium is common, morbid, and costly, yet remains often unrecognized in most clinical settings. The Confusion Assessment Method (CAM) is the most widely used diagnostic algorithm, and operationalizing its features would represent a substantial advance for clinical care.
Objective
To derive the 3D-CAM, a new 3-minute diagnostic assessment for CAM-defined delirium, and to validate it against a clinical reference standard.
Design
Diagnostic test study
Setting
4 general medicine units in an academic medical center
Participants
201 inpatients aged ≥ 75 years old
Measurements
We identified 20 items that best operationalized the 4 CAM diagnostic features to create the 3D-CAM. For prospective validation, 3D-CAM assessments were administered by trained research assistants. Independently, clinicians performed an extensive assessment that included patient interviews, family interviews, and review of the medical record. These data were considered by an expert panel to determine the presence or absence of delirium and dementia (reference standard). We compared the 3D-CAM delirium determination to the reference standard in all patients and in subgroups with and without dementia.
Results
The 201 participants in the prospective validation study had mean age (SD) of 84 (5.5) years, and 27% had dementia. The expert panel identified delirium in 21%. Median administration time for 3D-CAM was 3 minutes (inter-quartile range: 2–5 minutes). The sensitivity [95% CI] of 3D-CAM was 95% [84%, 99%] and the specificity was 94% [90%, 97%]. The 3D-CAM performed well in patients both with dementia (sensitivity=96% [82%, 100%], specificity=86% [67%, 96%]) and without dementia (sensitivity=93% [66%, 100%], specificity=96% [91%,99%]).
Limitations
Limited to single center, cross-sectional, and medicine patients only
Conclusion
The 3D-CAM operationalizes the CAM algorithm using a 3-minute structured assessment with high sensitivity and specificity relative to a reference standard and could be an important tool for improving recognition of delirium.
Keywords: delirium, aged, diagnostic tests, inpatient, sensitivity and specificity
Introduction
Delirium is common, morbid, and costly in hospitalized elders (1–3). Despite increasing awareness of its importance, most delirium, particularly hypoactive delirium and delirium superimposed on dementia, still goes unrecognized (1–3). Prompt recognition of delirium is the first key step in its appropriate management, which involves careful review for reversible contributors, preventing complications (including ensuring patient safety), and instituting cognitive and physical rehabilitation (1). Evidence suggests that such an approach can shorten the duration of delirium and improve its associated adverse outcomes (1, 3).
The Confusion Assessment Method (CAM), developed in 1990 (4), has been widely adopted, and a recent comparison of diagnostic methods suggests the CAM is the best performing bedside delirium assessment tool (5). While the CAM is widely used to define delirium in the literature (6), it can be challenging to operationalize in the clinical setting, requiring cognitive assessment and substantial interviewer training. Moreover, there is still a great deal of variability in how the CAM is applied, which can lead to differential performance in detecting delirium (5).
A brief, structured mental status assessment that operationalizes the CAM algorithm would be extremely helpful to accelerate widespread ascertainment of delirium in high risk patients (4, 5). Therefore, our overall goal was to develop and validate the 3D-CAM, the 3-Minute Diagnostic Assessment for Delirium using the CAM algorithm. Our current aims were: 1) to create the 3D-CAM using model selection methods to finalize items and to determine thresholds for the presence or absence for each of the 4 CAM diagnostic features, and 2) to prospectively validate the 3D-CAM by comparing it to a reference standard that included an extensive clinical evaluation in a new population of older general medicine patients with a high burden of baseline cognitive impairment and comorbidity.
METHODS
Derivation of the 3D-CAM (for details, see eAppendix 1)
We started with a dataset of 4598 structured delirium assessments from a previously completed multi-site trial of the Delirium Abatement Program (DAP) conducted in 8 post-acute facilities (7). In previously published 3D-CAM derivation work, we mapped over 120 items from this assessment to the four CAM diagnostic features (8), and used item response theory (IRT) (9) to identify the 36 most informative items for the identification of each of these features (10). For more details, see eAppendix 1.
For the current 3D-CAM derivation work, we further reduced this set of 36 items using logistic regression and assembled the most useful subset of items from each of the 4 CAM diagnostic features to create the 3D-CAM. We used regression coefficients to determine weights of each item and thresholds for determining the presence or absence of each of the features: 1) acute change and fluctuating course, 2) inattention, 3) disorganized thinking, and 4) altered level of consciousness. For each feature, the best performing approach weighted each cognitive testing item, patient symptom question, and interviewer observation equally. Moreover, the CAM feature was rated as present if any one of the items (cognitive test result, reported symptom, observation item) was rated as positive. Once each feature is rated, the presence of delirium is determined by the CAM diagnostic algorithm, which requires the presence of features 1 and 2, and either 3 or 4 (see Figure 1 for 3D-CAM items and scoring algorithm).
Figure 1. Overview of 3D-CAM Assessment.
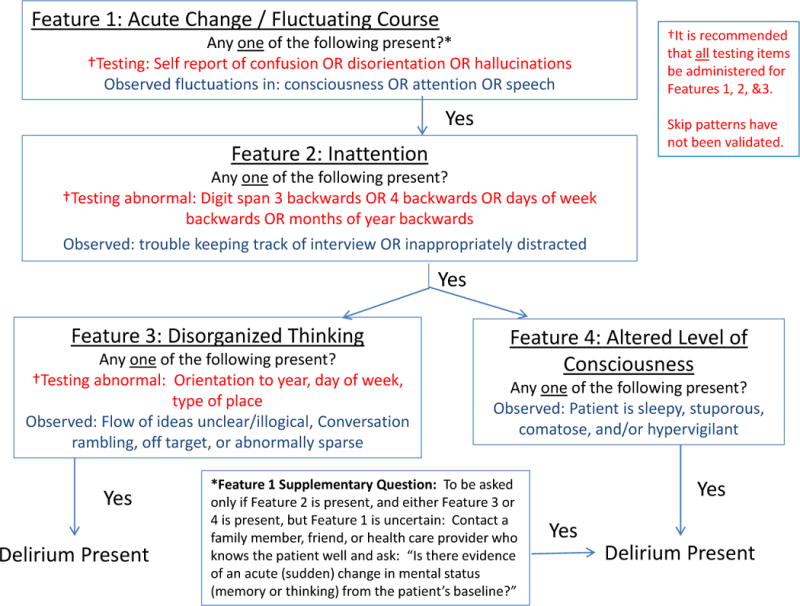
This figure depicts the CAM diagnostic algorithm, with the 3D-CAM items and scoring summarized under each CAM diagnostic feature.
Once we selected the items and defined the scoring algorithm for the 3D-CAM, we made a preliminary assessment of the 3D-CAM’s diagnostic accuracy. Using the DAP dataset of 4598 assessments, we used only the 3D-CAM items and algorithm described above to score the CAM algorithm, and compared the presence or absence of delirium generated from this approach with the results from the full 160-item structured delirium assessment (11). In this initial derivation work, the 3D-CAM achieved 92% sensitivity, 95% confidence interval (C.I.) [90%, 94%] and 93% specificity, 95% C.I. [92%, 93%] relative to the full assessment, which met our goal and allowed us to proceed with the prospective validation.
Prospective Validation Study
Study Population
We enrolled participants from a large urban teaching hospital in Boston, Massachusetts. Inclusion criteria were: 1) age ≥ 75 years old, 2) admitted to general medicine or geriatric medicine services, 3) able to communicate effectively in English, 4) without terminal conditions, 5) expected hospital stay of ≥ 2 days, and 6) not a previous study participant. Experienced clinicians (clinical psychologists and advanced practice nurses) screened for eligible patients. After obtaining approval from the attending physician, each eligible patient was approached for informed consent. If the patient lacked capacity to provide consent, the designated surrogate decision-maker was contacted. The study protocol and informed consent procedures were approved by the Institutional Review Board.
Reference Standard Delirium Assessment
The operational reference standard delirium diagnosis was based on an extensive face-to-face patient interview (45 minutes), medical record review, and input from the patient’s nurse and available family members. This assessment included: 1) reason for hospital admission and hospital course, 2) presence of cognitive concern, both prior to and during the hospitalization, 3) family, social, and functional history, 4) Montreal Cognitive Assessment (MoCA), a 30-item assessment that takes approximately 20 minutes to administer (12), 5) Geriatric Depression Scale (GDS) to evaluate for presence of depressive symptoms (13), 6) medical record review, including quantification of comorbidities using the Charlson index (13), diagnosis of dementia or mild cognitive impairment (MCI) prior to hospitalization, determination of functional status using the basic and Instrumental Activities of Daily Living scales (15, 16), and a list of psychoactive medications administered. If the assessment indicated potential cognitive impairment, (MoCA score≤23) (12), the clinical assessor conducted a proxy interview to assist with determining the patient’s baseline mental status relevant to a possible diagnosis of dementia versus a history of lifelong, developmentally based cognitive limitations. The proxy interview included: 1) ascertainment of a cognitive concern, prior to and during the hospitalization; 2) ascertainment as to whether specific cognitive deficits evident on testing existed prior to hospitalization; 3) confirmation of functional status obtained from the medical record, and 4) a proxy-based screening questionnaire for dementia (the AD-8) (17).
The final delirium diagnoses were adjudicated by a study panel including the clinical assessor (psychologist or advanced practice nurse), the study PI (Marcantonio), a geriatrician, and a board-certified neuropsychologist (O’Connor), using DSM-IV criteria (18) For patients not meeting delirium criteria, the panel adjudicated the presence or absence of subsyndromal delirium (19), defined by the presence of acute change/fluctuating course, plus inattention or disorganized thinking or an altered level of consciousness. The panel was blinded to the results of subsequent 3D-CAM testing (see below). A geropsychiatrist (Metzger) subsequently re-adjudicated a 10% subsample (10 randomly selected participants with delirium and 10 without delirium) blinded to the original results to verify the panel adjudication process. In addition to determining delirium status, the panel adjudicated the presence or absence of cognitive impairment at baseline, including dementia or mild cognitive impairment (MCI) using the National Institute on Aging – Alzheimer’s Association criteria (20, 21) (for details of data used for adjudication of dementia and MCI, see eAppendix 2).
3D-CAM Assessments
Following the reference standard assessment, the 3D-CAM was administered by research assistants (RA’s) who were blinded to the reference standard results. A total of 8 RA’s participated in the validation study, and each evaluated between 4–49 participants, based on participant and RA availability. Before the start of the study, each RA underwent a 1–2 hour training session on the use of the 3D-CAM, including practice administering the instrument to each other and to actual patients. To assess inter-rater reliability, based on a random number sequence 50% of the participants were selected to undergo a second 3D-CAM assessment, blinded both to the reference standard and the first 3D-CAM. All 8 RA’s participated in the reliability study, representing 18 distinct pairs of raters, each evaluating between 1–19 participants, again based on participant and RA availability. To assure temporal proximity, all assessments (the reference standard and the one or two 3D-CAM assessments) were completed within a 2-hour period during the hours of 11 AM and 2 PM (Figure 2).
Figure 2. Prospective Validation Study Flow Diagram.
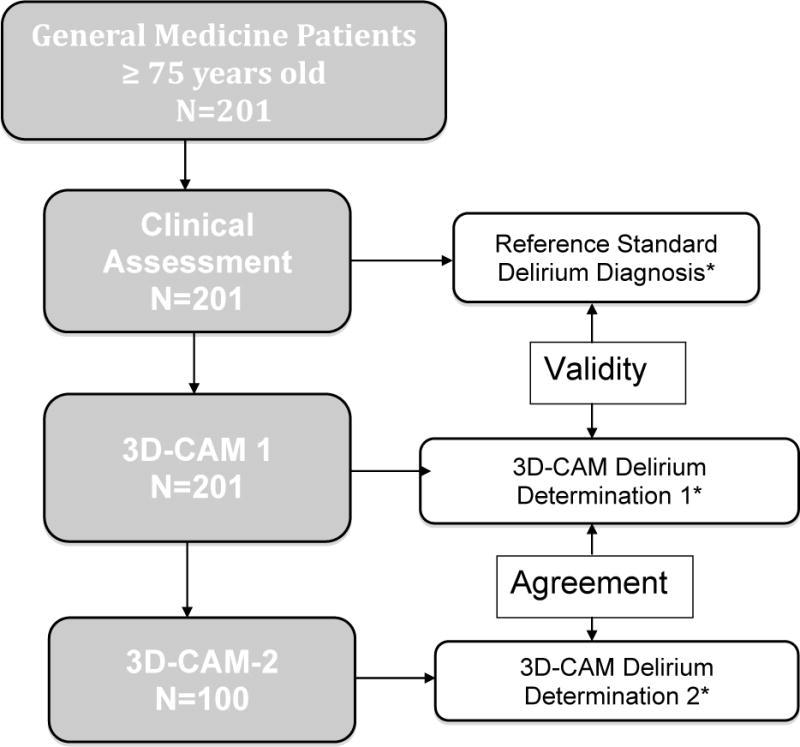
*Presence or absence of delirium as determined by first 3D-CAM assessment was compared to the reference standard delirium diagnosis to assess validity, and to the second 3D-CAM assessment to assess inter-rater agreement
All assessments were completed within 2 hours of each other, and results were strictly blinded from the other assessors
Statistical Analyses
We calculated sensitivity, specificity, and 95% confidence intervals for the 3D-CAM delirium determination compared to the reference standard. We performed subset analyses to determine diagnostic test characteristics of the 3D-CAM stratified by the patient’s baseline cognitive status (normal/MCI vs. dementia). We assessed inter-rater agreement of the two independent 3D-CAM assessments using simple descriptive statistics. We used SAS software, Version 9.3 (SAS Institute, Inc., Cary, NC) for all data analyses.
Role of Funding Agency
The National Institute on Aging had no role in the design, conduct, and analysis, nor in the decision to submit this manuscript for publication.
RESULTS
Prospective Validation Study
Patient Characteristics
A total of 201 patients met inclusion criteria and provided informed consent. The mean age [standard deviation (SD)] was 84 (5.4) years, 62% were women, and 88% self-reported to be white (Table 1). Approximately half had some college education, and 5% had English as a second language but were sufficiently fluent to be enrolled. The mean Charlson comorbidity score (SD) was 3.0 (2.3), indicating a moderate to high level of medical comorbidity (14). Fifty-five percent had dependencies in one or more basic Activities of Daily Living (15), and 81% had dependencies in one or more Instrumental Activities of Daily Living (16). Based on the expert panel operational reference standard assessment, 27 percent of the population was found to have baseline dementia.
Table 1.
Characteristics of the Prospective Validation Study Population (N=201)
| Characteristic | |
|---|---|
| Age, mean (SD) | 84 (5.4) |
| Sex, N (%) female | 125 (62) |
| Race–White, N (%) | 177 (88) |
| Education*, N (%) | |
| Less than High School | 20 (10) |
| High School Graduate | 75 (38) |
| College or More | 100 (49) |
| English second language N (%) | 10 (5) |
| Severe sensory Impairment–Vision, N (%) | 5 (2) |
| Severe sensory Impairment–Hearing, N (%) | 18 (9) |
| Charlson Comorbidity Index (14), mean (SD) | 3.0 (2.3) |
| Activities of Daily Living Dependence (15), N (%) | 110 (55) |
| Instrumental Activities of Daily Living Dependence (16), N (%) | 163 (81) |
| Baseline Cognition—Mild Cognitive Impairment, N (%) | 50 (25) |
| Baseline Cognition–Dementia, N (%) | 55 (27) |
| Delirium by Reference Standard, N (%) | 42 (21) |
| Delirium Psychomotor Features, N (%) | |
| Hypoactive or Normal | 37 (19) |
| Hyperactive or Mixed | 5 (2) |
Education status was missing in 6 (3%) participants
Overall Study Flow, Reference standard and 3D-CAM assessments
Figure 2 depicts the study flow of assessments, which included the reference standard clinical evaluation for delirium, the 3D-CAM assessment, and a second 3D-CAM in a random one half of participants. All of the face-to-face components of the assessments were completed within the desired 2 hour time window. The reference standard assessment for delirium took approximately 1.5 hours to complete including patient interviews, medical record reviews, and proxy interviews. Based on these assessments, 42 participants (21% of the sample) were diagnosed with delirium. Of these, 37 (88% of delirium cases) had either hypoactive or normal psychomotor features with only 5 showing hyperactive or mixed features. In the 20 patients (10 with delirium, 10 without delirium) whose records were re-reviewed by the blinded geropsychiatrist, there was 100% concordance with the expert panel diagnosis. In comparison to the reference standard assessment, the 3D-CAM was completed in a median of 3 minutes (interquartile range 2–5 minutes, overall range 1–15 minutes). Forty-nine participants (24%) were determined to have delirium based on the 3D-CAM.
Diagnostic Test Characteristics of the 3D-CAM
Compared to the reference standard delirium diagnosis, the sensitivity of the 3D-CAM was 95% with 95% confidence intervals (C.I.) of [84%, 99%], while the specificity of 3D-CAM was 94%, with 95% C.I. [90%, 97%] (Table 2). These result in a likelihood ratio positive of 16.8, 95% C.I. [8.9, 31.8], and a likelihood ratio negative of 0.05, 95% C.I. [0.01, 0.20]. Notably, of the 9 “false positives” identified by 3D-CAM, 6 were adjudicated to have subsyndromal delirium based on the reference standard assessment (19). In post-hoc analyses, we examined the effect of regrouping subsyndromal cases with the “positives” based on the reference standard. This resulted in the sensitivity of the 3D-CAM increasing to 46/48 (96%) and specificity increasing to 150/153 (98%).
Table 2.
Diagnostic Test Characteristics of the 3D-CAM Compared to the Reference Standard Delirium Assessment
Diagnostic Test Characteristics Stratified by baseline cognition
We examined the diagnostic test characteristics of 3D-CAM stratified by participants’ baseline cognitive function (Table 3). In the group with either normal baseline cognition or MCI, the sensitivity was 93%, with specificity of 96%. In the dementia subgroup, the sensitivity was excellent at 96%, with slightly lower specificity of 86% (for 95% CI’s and likelihood ratios stratified by baseline cognition, see Table 3).
Table 3.
Diagnostic Test Characteristics of the 3D-CAM Stratified by Baseline Cognitive Function
| 3D-CAM Test Characteristics in Patients with Normal Baseline Cognition or MCI (N=145)
| |||
|---|---|---|---|
| Delirium Diagnosis (− or +) | Reference Standard (+) |
Reference Standard (−) |
3D-CAM Totals |
| 3D-CAM (+) | 13 | 5 | 18 |
| 3D-CAM (−) | 1 | 126 | 127 |
| Reference Standard Totals | 14 | 131 | 145 |
| Test Characteristic† | Sensitivity = 13/14 | Specificity = 126/131 | |
| %, [95% C.I.] | 93% [66%,100%] | 96% [91%,99%] | |
| 3D-CAM Test Characteristics in Patients with Dementia (N=56)
| |||
|---|---|---|---|
| Delirium Diagnosis (− or +) | Reference Standard (+) |
Reference Standard (−) |
3D-CAM Totals |
| 3D-CAM (+) | 27 | 4 | 31 |
| 3D-CAM (−) | 1 | 24 | 25 |
| Reference Standard Totals | 28 | 28 | 56 |
| Test Characteristic† | Sensitivity = 27/28 | Specificity = 24/28 | |
| %, [95% C.I.] | 96% [82%,100%] | 86% [67%,96%] | |
The sensitivity and specificity result in a likelihood ratio positive of 24.3 [10.2, 58.2], and a likelihood ratio negative of 0.07 [0.01, 0.49] for patients with normal baseline cognition or MCI, and a likelihood ratio positive of 6.8 [2.7, 16.8], and a likelihood ratio negative of 0.04 [0.01, 0.29] for patients with dementia.
Inter-rater Agreement
Finally, we examined agreement across raters in the subset of 100 participants who underwent a second 3D-CAM assessment blinded to the first. This demonstrated that the 3D-CAM had excellent inter-rater agreement of 95% (see eTable3 for details).
DISCUSSION
Delirium is an important clinical syndrome to detect. Currently there is no brief instrument well-suited for widespread use across clinical settings. We therefore sought to develop and evaluate the 3D-CAM, a short, structured diagnostic assessment for delirium that can be administered by healthcare delivery staff with minimal additional training. The 3D-CAM demonstrated strong performance characteristics in our study. It was completed in a median of 3 minutes and had excellent sensitivity and specificity relative to a reference standard delirium diagnosis, even in patients with dementia. The slightly lower specificity in the dementia subgroup is attributable to a higher likelihood of false positives inherent in the challenging process of distinguishing symptoms and signs of delirium from dementia (22). The 3D-CAM also had excellent inter-rater agreement. With its brevity and ease of use, 3D-CAM may provide a useful tool for improving widespread detection of delirium in clinical settings.
Hospitalized patients are rarely formally assessed for delirium (1, 3). Studies performed over the past 20 years suggest that the clinical recognition rate has not significantly changed, and remains in the 12–35% range (23–27). Moreover, delirium cases identified tend to be agitated patients who are disruptive to patient care, while hypoactive patients remain unrecognized. Studies have shown that hypoactive patients with delirium have either similar or somewhat worse outcomes compared with agitated patients with delirium (28, 29). The 3D-CAM demonstrated excellent sensitivity in our sample, even though 88% of delirium cases had either hypoactive or normal psychomotor features.
To improve delirium recognition in hypoactive patients, it is important to incorporate results from direct questioning of patients and mental status testing into the delirium assessment and not just rely on interviewer observations. In the initial validation studies, the CAM developers performed a structured mental status assessment consisting of the Mini-Mental State Examination (MMSE) (30) plus recall of a story or digit span before operationalizing the CAM algorithm (4). In a subsequent publication, nurses were interviewed about the CAM diagnostic features on a daily basis using observations from routine clinical care without formal mental status assessment (31). When nurse CAM ratings were compared to researcher CAM ratings following a structured mental status assessment, the sensitivity of the nurse CAM ratings was only 20% per interview, and 33% over the course of the entire hospitalization (31). Thus, in the absence of mental status testing, the CAM algorithm alone did not substantially improve the rate of delirium detection. The 3D-CAM demonstrates that excellent sensitivity for delirium can be achieved with brief mental status testing focused on attention and orientation.
The CAM-ICU is an example of a structured assessment that incorporates a specific set of questions to operationalize each CAM diagnostic feature (32). For instance, attention is assessed using 2 items: the vigilance “A” task, and the picture recognition task from the Attention Screening Examination (33). Because the CAM-ICU was designed to assess delirium in intubated ICU patients, all questions are answerable using non-verbal responses, such as a nod of the head (yes or no). With its brevity and ease of use, the CAM-ICU has greatly enhanced the assessment of delirium in the ICU (34). Yet, recent studies suggest that the CAM-ICU may not be optimized to non-ICU populations, where it demonstrates lower sensitivity relative to a verbal delirium assessment (35–37). For instance, in a recent validation study of 406 people evaluated in an Emergency Department (mean age 73.5 years, dementia prevalence of 5.9%) the CAM-ICU demonstrated a sensitivity of 68–72% (37). The recently developed and validated B-CAM provides an alternative that incorporates verbal testing using items very similar to the CAM-ICU (38). Notably, both the CAM-ICU and the B-CAM put major emphasis on the Richmond Agitation and Sedation Scale (RASS) (39), which detects altered levels of consciousness. Yet, the recently published DSM-5 delirium definition de-emphasizes the importance of altered level of consciousness in delirium and focuses instead on assessment of attention and orientation (40), both key components of the 3D-CAM. Moreover, altered level of consciousness is much less prevalent in delirium outside of the ICU; in our sample, it was present in only 8 of 42 delirium cases (19%).
While physicians and nurses will need to be trained to use the 3D-CAM optimally, its brevity and algorithmic structure should simplify the process. There are clear instructions mapping specific questions to each CAM feature. The 3D-CAM instrument and training manual (available free of charge at www.hospitalelderlifeprogram.org) provide clear explanations of how to code the presence or absence of each feature based on patient responses. These characteristics of the 3D-CAM reduce the amount of judgment required by the assessor and facilitate reproducibility across assessors, consistent with the extremely high inter-rater agreement from this study. The structured nature of 3D-CAM also makes it very amenable to administration via an electronic platform, such as mobile technology. Notably, since only one “positive” item is required to trigger the presence of each feature, the assessment could potentially be shortened further by incorporating skip patterns, an approach that we did not test in our validation study. Finally, the 3D-CAM is entirely compatible with the short form version of the recently published CAM-S delirium severity measure (41). We are developing an algorithm to score the CAM-S using 3D-CAM items, to be presented in future work.
Our approach has several important strengths. First, the 3D-CAM was created using items rigorously selected using IRT to be maximally informative for determining the presence or absence of the 4 CAM diagnostic features. These items were further reduced using model selection techniques in a dataset of 4598 delirium assessments. Our prospective validation study enrolled a sample of over 200 patients with a mean age of over 80 years, substantial comorbidity, and a high burden of baseline cognitive impairment, representing a purposeful “challenge population” for 3D-CAM validation. The 3D-CAM is equally applicable in younger populations with a lower prevalence of dementia, and will likely perform even better since delirium assessment in these patients is more straightforward. Importantly, our study employed a clinical reference standard and a design in which all delirium assessments were administered close in time while the results of each test were kept strictly blinded from the other assessors. Finally, we assessed and confirmed excellent inter-rater agreement for the 3D-CAM ratings and also independently validated a subset of reference standard delirium diagnoses.
Our approach does have several limitations. First, our prospective validation study enrolled only general medicine patients and was conducted at a single site. Thus, our findings should be confirmed in other settings in which delirium is also common and morbid, such as non-ICU surgical wards, palliative care, post-acute care, and other types of hospitals. Second, our prospective validation study assessed patients only on a single hospital day. Additional studies should examine the performance and acceptability of the 3D-CAM when repeated on a daily basis for delirium screening. Third, all of our evaluations were performed during the day shift, and performance during evening and night shifts should also be evaluated. Fourth, the 95% confidence intervals for some test characteristics in our stratified analyses are wide, and should be interpreted accordingly. Finally, we used a paper and pencil, static 3D-CAM assessment, and future studies should examine use of a dynamic assessment employing automated skip patterns on an electronic platform, which might further enhance efficiency.
In conclusion, in our study of general medicine patients with advanced age and a high prevalence of underlying cognitive impairment, the 3D-CAM proved to be a brief, highly reproducible, and valid method for diagnosing delirium using the CAM algorithm. Given these characteristics, the 3D-CAM could be an important component of future efforts to improve systematic case-finding of delirium in high-risk older adults. Further research will focus on developing the optimal strategies for translating the 3D-CAM into routine care, and determining whether improved detection of delirium can result in improved outcomes for vulnerable hospitalized elders.
Supplementary Material
Acknowledgments
The authors wish to thank the contribution of the patients and families at Beth Israel Deaconess Medical Center who participated in this study. Moreover, we would like to thank the clinical assessors Tracee Francis, Leo Waterston, Meghan Collier, and Laura Branford-White as well as the research assistants Kerry Palihnich, Jacqueline Gallagher, Aleksandra Kuczmarska, Ariel Hodara, Benjamin Helfand, and Mary Michaels for all their hard work and dedication to the study.
Funding: This work was supported by the National Institute of Aging grant number R01AG030618 and K24AG035075 to Dr. Marcantonio. Dr. Inouye’s time was supported in part by grants P01AG031720 and K07AG041835 from the National Institute on Aging. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair (Hebrew SeniorLife/Harvard Medical School). The funding agencies had no role in the preparation of this manuscript and the authors retained full autonomy in the preparation of this manuscript.
Footnotes
Authors’ contributions:
Marcantonio: Conception and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, obtained funding, supervision.
Ngo: Conception and design, statistical analysis, interpretation of the data, critical revision of the manuscript.
O’Connor: Conception and design, acquisition of data, critical revision of the manuscript, supervision.
Jones: Conception and design, critical revision of the manuscript.
Crane: Conception and design, critical revision of the manuscript.
Metzger: Conception and design, acquisition of data, critical revision of the manuscript.
Inouye: Conception and design, interpretation of data, critical revision of the manuscript, supervision.
Supplemental material for reviewers only: 3D-CAM Instrument
Drs. Marcantonio and Ngo had full access to the data and can attest to its integrity and validity. All authors gave final approval of the version to be published.
Declaration of conflicts: None declared—the authors retained full autonomy in carrying out this work.
References
- 1.Marcantonio ER. In the clinic Delirium. Ann Intern Med. 2011;154(11):ITC6. doi: 10.7326/0003-4819-154-11-201106070-01006. [DOI] [PubMed] [Google Scholar]
- 2.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–22. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 5.Wong CL, Holroyd-Leduc J, Simel DL, Straus SE. Does this patient have delirium?: value of bedside instruments. JAMA. 2010;304(7):779–86. doi: 10.1001/jama.2010.1182. [DOI] [PubMed] [Google Scholar]
- 6.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823–30. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcantonio ER, Bergmann MA, Kiely DK, Orav EJ, Jones RN. Randomized trial of a delirium abatement program for postacute skilled nursing facilities. J Am Geriatr Soc. 2010;58(6):1019–26. doi: 10.1111/j.1532-5415.2010.02871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang LW, Inouye SK, Jones RN, Fong TG, Rudolph JL, O’Connor MG, et al. Identifying indicators of important diagnostic features of delirium. J Am Geriatr Soc. 2012;60(6):1044–50. doi: 10.1111/j.1532-5415.2012.03996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambleton RK, Swaminathan H, Rogers HJ. Fundamentals of item response theory. Newbury Park, Calif: Sage Publications; 1991. [Google Scholar]
- 10.Yang FM, Jones RN, Inouye SK, Tommet D, Crane PK, Rudolph JL, et al. Selecting optimal screening items for delirium: an application of item response theory. BMC Med Res Methodol. 2013;13:8. doi: 10.1186/1471-2288-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon SE, Bergmann MA, Jones RN, Murphy KM, Orav EJ, Marcantonio ER. Reliability of a structured assessment for nonclinicians to detect delirium among new admissions to postacute care. J Am Med Dir Assoc. 2006;7(7):412–5. doi: 10.1016/j.jamda.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 13.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24(4):709–11. [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MaKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–9. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 16.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86. [PubMed] [Google Scholar]
- 17.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–64. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Psychiatric Disorders, Version 4. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 19.Meagher D, Adamis D, Trzepacz P, Leonard M. Features of subsyndromal and persistent delirium. Br J Psychiatry. 2012;200(1):37–44. doi: 10.1192/bjp.bp.111.095273. [DOI] [PubMed] [Google Scholar]
- 20.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723–32. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 23.Gustafson Y, Brannstrom B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760–5. doi: 10.1111/j.1532-5415.1991.tb02697.x. [DOI] [PubMed] [Google Scholar]
- 24.Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248–53. doi: 10.1067/mem.2002.122057. [DOI] [PubMed] [Google Scholar]
- 25.Kales HC, Kamholz BA, Visnic SG, Blow FC. Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol. 2003;16(1):32–8. doi: 10.1177/0891988702250535. [DOI] [PubMed] [Google Scholar]
- 26.Lemiengre J, Nelis T, Joosten E, Braes T, Foreman M, Gastmans C, et al. Detection of delirium by bedside nurses using the confusion assessment method. J Am Geriatr Soc. 2006;54(4):685–9. doi: 10.1111/j.1532-5415.2006.00667.x. [DOI] [PubMed] [Google Scholar]
- 27.Milisen K, Foreman MD, Wouters B, Driesen R, Godderis J, Abraham IL, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs. 2002;28(11):23–9. doi: 10.3928/0098-9134-20021101-07. [DOI] [PubMed] [Google Scholar]
- 28.Kiely DK, Jones RN, Bergmann MA, Marcantonio ER. Association between psychomotor activity delirium subtypes and mortality among newly admitted post-acute facility patients. J Gerontol A Biol Sci Med Sci. 2007;62(2):174–9. doi: 10.1093/gerona/62.2.174. [DOI] [PubMed] [Google Scholar]
- 29.Yang FM, Marcantonio ER, Inouye SK, Kiely DK, Rudolph JL, Fearing MA, et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics. 2009;50(3):248–54. doi: 10.1176/appi.psy.50.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM., Jr Nurses’ recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467–73. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 32.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 33.Hart RP, Levenson JL, Sessler CN, Best AM, Schwartz SM, Rutherford LE. Validation of a cognitive test for delirium in medical ICU patients. Psychosomatics. 1996;37(6):533–46. doi: 10.1016/S0033-3182(96)71517-7. [DOI] [PubMed] [Google Scholar]
- 34.Brummel NE, Vasilevskis EE, Han JH, Boehm L, Pun BT, Ely EW. Implementing delirium screening in the ICU: secrets to success. Crit Care Med. 2013;41(9):2196–208. doi: 10.1097/CCM.0b013e31829a6f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNicoll L, Pisani MA, Ely EW, Gifford D, Inouye SK. Detection of delirium in the intensive care unit: comparison of confusion assessment method for the intensive care unit with confusion assessment method ratings. J Am Geriatr Soc. 2005;53(3):495–500. doi: 10.1111/j.1532-5415.2005.53171.x. [DOI] [PubMed] [Google Scholar]
- 36.Neufeld KJ, Hayat MJ, Coughlin JM, Huberman AL, Leistikow NA, Krumm SK, et al. Evaluation of two intensive care delirium screening tools for non-critically ill hospitalized patients. Psychosomatics. 2011;52(2):133–40. doi: 10.1016/j.psym.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Han JH, Wilson A, Graves AJ, Shintani A, Schnelle JF, Dittus RS, et al. Validation of the Confusion Assessment Method for the Intensive Care Unit in Older Emergency Department Patients. Acad Emerg Med. 2014;21(2):180–7. doi: 10.1111/acem.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han JH, Wilson A, Vasilevskis EE, Shintani A, Schnelle JF, Dittus RS, et al. Diagnosing delirium in older emergency department patients: validity and reliability of the delirium triage screen and the brief confusion assessment method. Ann Emerg Med. 2013;62(5):457–65. doi: 10.1016/j.annemergmed.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. 5. Washington, D.C.: American Psychiatric Association; 2013. [Google Scholar]
- 41.Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, et al. The CAM-S: Development and Validation of a New Scoring System for Delirium Severity in 2 Cohorts. Ann Intern Med. 2014;160(8):526–33. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


