Abstract
There is a critical need for effective new pharmacotherapies for pain. The paucity of new drugs successfully reaching the clinic calls for a reassessment of current analgesic drug discovery approaches. Many points early in the discovery process present significant hurdles, making it critical to exploit advances in pain neurobiology to increase the probability of success. In this review, we highlight approaches that are being pursued vigorously by the pain community for drug discovery, including innovative preclinical pain models, insights from genetics, mechanistic phenotyping of pain patients, development of biomarkers, and emerging insights into chronic pain as a disorder of both the periphery and the brain. Collaborative efforts between pharmaceutical, academic, and public entities to advance research in these areas promise to de-risk potential targets, stimulate investment, and speed evaluation and development of better pain therapies.
INTRODUCTION
“Pain is a more terrible lord of mankind than even death itself”
(Albert Schweitzer, 1931).
Pain is the most common reason people seek medical care (1). The Institutes of Medicine and the American Pain Society estimate that pain affects more than 100 million adults in the United States and costs about $635 billion each year in medical treatment and lost productivity (2, 3). These numbers will only increase as the world’s population ages. Current pharmacotherapy is dominated by well-established drug classes such as narcotic analgesics and nonsteroidal anti-inflammatory agents. These and other classes of drugs such as cyclooxygenase-2–selective inhibitors, anticonvulsants, and antidepressants are in widespread use for pain treatment. However, not all patients achieve meaningful pain relief, leaving a significant unmet medical need for new, safe, and effective treatments for both acute and chronic pain. The global market for current pain therapeutics is substantial, estimated at $50 billion in 2009, making pain an attractive therapeutic area to the pharmaceutical industry. This review considers issues that may limit, and approaches that may enhance, the probability of success in the discovery and development of new medicines for pain.
PRECLINICAL CHALLENGES IN IDENTIFICATION OF PAIN TARGETS
Target discovery for pain therapeutics
Pain is defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Tissue-damaging stimuli are detected by primary afferent neurons termed nociceptors. These cells innervate target tissues and convey excitatory signals to the spinal and medullary dorsal horns, and ultimately to many areas of the brain including the thalamus, somatosensory cortices (S1, S2), insula cortex, anterior cingulate cortex, periaqueductal gray, and other sites. Thus, tissue-damaging stimuli engage a distributed network of brain regions that transform nociceptive inputs into the human experience of pain (see below). The nociceptor has been a main target for new therapeutics because activation of these neurons reliably produces sensations of pain in humans (4, 5), and loss of function in these cells results in an inability to experience pain (6, 7).
As described in the accompanying Review (8), therapeutic targets for the development of new medications might be identified from studies that link gene mutations to altered pain sensation in specific patient populations (1, 9). Examples include calcium channels, where mutations have been identified in familial hemiplegic migraine (10) and migraine without aura (11), and sodium channels, where loss-and gain-of-function mutations in the SCN9A genes, encoding Nav1.7 voltage-gated sodium channels, can result in congenital insensitivity to pain or in abnormal pain such as erythromelalgia (12–14). It is not yet clear, however, that targets identified through familial mutations will be useful targets for pain treatment in the general population; this possibility awaits testing in human trials [see accompanying Review (8)].
Targeting the peripheral nervous system as a valid strategy for treatment of pain is evident by the clinical effectiveness of local anesthetics as well as from examples abundant in nature. Capsaicin, the piquant ingredient in hot chili peppers, activates the TRPV1 cation channel expressed on nociceptors, resulting in sensations of heat and pain; blockers of this channel are under investigation as potential new treatments for pain. The venom of bark scorpions produces pain by activation of the Nav1.7 sodium channel and recruitment of a second sodium channel, Nav1.8, that is required for generating action potentials (15). Some mice have mutations in Nav1.8 that prevent its recruitment after Nav1.7 activation, inhibiting the usual pain from the venom of these scorpions. These evolutionary strategies suggest that both of these sodium channel subtypes could be viable targets for pain therapies in humans (15). Peptides from fish and worm-hunting marine cone snails (for example, ω-conotoxin) selectively target N-type calcium channels that are critical for nociceptive transmission. Ziconotide represents successful translation of one of these peptides to the clinic (16). Other peptides from marine species are also being assessed for their potential as pain therapeutics (17–19).
Molecular targets and mechanisms relevant to human pain states have been identified both from preclinical studies and from drug discovery programs on the basis of human pathophysiology and pharmacology. The NGF-TrkA signaling pathway is important for pain sensation, as shown by the fact that NGF administration produces pain and increased sensitivity to painful stimuli (20–22). Additionally, mutations in NGFB and NTRK1 can result in pain insensitivity. Antibodies to NGF are clinically effective in reducing pain, albeit with potential side effect liabilities (23, 24), and are being evaluated in clinical trials (25, 26). Intriguing data from 2500 volunteers in the Twins UK cohort provide preliminary support for genetic links to experimental, thermal, mechanical, and chemical pain stimuli, suggesting possibilities for drug discovery through phenotyping (27). The angiotensin pathway has recently been implicated in animal models and in human pain conditions (28–30). Therapeutic agents targeting the AT2 receptor have shown clinical efficacy (31).
The triptan drugs, serotonin 1B/1D agonists (32, 33), were the first class of drugs developed specifically for migraine. More recently, the cardinal role of calcitonin gene-related peptide (CGRP) in migraine has been recognized, and efforts are in process to target this mechanism. CGRP receptor antagonists and anti-CGRP antibodies (34) that modulate CGRP actions and trigeminovascular activation during migraine attacks are clinically effective and at various stages of development (34–36). Drugs that affect neurotransmitters such as reuptake blockers (37, 38) and mixed function molecules [(39, 40) and see below] target central pain modulatory pathways and represent significant advances in therapy. For example, serotonergic-noradrenergic reuptake inhibitors can inhibit persistent pain but have little effect on acute pain (41). These compounds likely act in part via endogenous descending pain modulatory circuits (see below) (42).
Despite these successes, choosing targets in the preclinical setting remains difficult, and ultimately, validation only occurs in humans. The de-risking of novel potential targets for drugs is not straightforward and relies on convergent data from multiple systems, ranging from in vitro assays to in vivo function to confirmation of efficacy in pain models. The biology of pain is complex and, because it is a primary survival mechanism, has considerable redundancy and overlap with other sensory functions. Consequently, modulation of single proteins, the strategy most common in drug discovery, may not produce the desired effects in the general patient population. Many of the currently used drugs act on multiple pathways, receptors, and targets. For example, nonsteroidal anti-inflammatory drugs act on multiple inflammatory pathways (43), and even highly selective drugs such as the monoamine reuptake blockers (37, 38) elevate levels of serotonin and norepinephrine, which in turn affect more than 20 serotonergic and adrenergic receptor subtypes. Mixed μ-opioid agonist/monoamine reuptake inhibitor molecules modulate separate central nervous system (CNS) biological pathways, each of which has proven clinical efficacy [tapentadol or tramadol (39, 40)]. The effectiveness of these multitarget drugs that engage several CNS mechanisms suggests that poly-pharmacological strategies for the development of new therapies should continue to be explored (see below).
Plasticity in circuits involved in emotional function has recently been suggested to be an important factor in promoting the transition from acute to chronic pain (44, 45), and it has been hypothesized that some individuals may be genetically predisposed to “chronification” (46). Chronic pain is often associated with comorbidities that are related to multiple CNS circuits. Despite the proven efficacy of analgesic drugs that affect multiple targets or mechanisms, few have achieved this by design. Mindfully addressing a network disease such as chronic pain will require new drug development paradigms and new drug screening approaches (47–49). One strategy is to test mixtures that combine single-activity compounds for safety and efficacy. For instance, the combination of μ-opiate agonists with peripherally acting μ antagonists maintains effects in the CNS but avoids unwanted gastrointestinal adverse effects (50). This combination approach, especially for new chemical entities, may require rethinking of traditional drug registration processes. It may also complicate treatment of patients on multiple nonsynergistic or noncomplementary medications (51, 52).
An alternative method is to synthesize single multitarget drugs that can modulate multiple protein targets simultaneously (53, 54). This approach presents unique medicinal chemistry design challenges to optimize pharmacology at different targets within a single molecule. These combination agents would have an advantage over drug mixtures in getting regulatory approval because only one toxicological, metabolic, pharmacokinetic, and pharmacodynamic and safety profile would be required (55, 56). Whether developed by chance (39) or by design (57), these drugs have the potential to improve clinical outcomes (37–40, 43, 57–59). This strategy also may diminish side effect liability by modulating and stabilizing diverse neural circuits simultaneously. Combination therapies could also harness synergy or additivity: (i) subeffective or minimally effective doses of individual drugs could improve clinical efficacy for pain without enhancing side effects (51) or (ii) one component could inhibit the side effects of another analgesic component, allowing increased efficacy without penalty.
Nociception, pain, and translation
Most analgesics that are effective in humans also show some efficacy in preclinical models (60, 61). The reverse, however, is often not true. Preclinical investigations all too frequently identify pathways that modulate pain in animal assays, but molecules that target these pathways fail in clinical trials (62–65). Examples include the substance P neurokinin-1 (NK-1) receptor antagonists (66–68), the fatty acid amino hydrolase inhibitors that elevate endocannabinoid tone (69), and peripherally restricted cannabinoid CB1 receptor agonists (70). The reasons for such failures are not well understood but may include species differences in pain modulation pathways, that is, the molecular target identified in animals may not be a salient component of pain in patients. More recent strategies have emphasized starting with patients to identify approaches that can improve therapy and then “reverse-translating” to understand mechanism and refine molecules in the preclinical setting in a “bedside-to-bench-to-bedside” strategy.
Failures in clinical trials may also result from other, equally important factors. For example, there may be uncertainty in the underlying pathophysiological mechanisms promoting pain in specific patients, in the dose selected for the clinical trial, in the degree of target engagement required for efficacy, and in the complexity of the design of the clinical trial itself, including unexpected placebo response rates (see below). Strategies to test pain therapies in healthy volunteers as an early validation of molecule and mechanism may be flawed because normal healthy volunteers without disease may be poorly predictive. A straight-to-human strategy would, therefore, optimally test analgesic candidates in chronic pain patients in clinical trials. This approach is complicated by difficulties in selecting appropriate pain populations relevant to the mechanism being tested and would be associated with high early development costs. Thus, preclinical research will likely be needed to triage potential therapeutic targets for pain drug development. Animal- and cell-based assays can inform biochemical and pharmacological questions, provide pharmacodynamic endpoints, and estimate safety and mechanism-based and off-target side effect liability. As preclinical studies are likely to remain a requirement for drug development, approaches to improve their ultimate relevance to clinical outcomes are constantly being explored.
Although recent failures of drugs with new mechanisms in clinical trials have prompted suggestions that preclinical research does not yield good mechanistic insights to human pain (63, 71, 72), this conclusion oversimplifies complex issues. Current understanding of human sensory neurobiology is largely based on information gathered in parallel from both animal and human studies, as well as from astute clinical observations during pharmacotherapy of diverse clinical conditions. For example, the excitability of neurons and the neurobiology of nociception, including the essential role of sodium channels in the action potential (73), are highly conserved across species. The relevance of sodium channels to pain is validated by the preclinical activity and clinical utility of local anesthetics and by evidence from human genetic studies noted above identifying mutations in the Nav1.7, Nav1.8, or Nav1.9 channels that modulate human pain (8). The mechanisms by which these mutations alter the biophysical properties of the channels and resulting neuronal excitability can explain the biology underlying the clinical syndrome (74). Other examples of highly conserved mechanisms also translate across species including the transducers of noxious stimuli (thermal or chemical stimuli) that can elicit sensations of pain [such as, for example, TRP channels (TRPV1 or TRPA1)] (75, 76). The general anatomy of pain systems is also similar in animals and humans, having been demonstrated in humans and characterized mechanistically in animals (77–79): for example, pain and touch pathways are segregated (80, 81), and descending pathways play a critical role in modulating pain. Despite these areas of concurrence, there is still a shortfall in translating preclinical therapeutic findings to patients. Improving preclinical “pain” assessment including reverse translation from the human condition and devising approaches with higher predictive value remains a challenge for the pain community. Such improvements could include modeling clinical conditions in a more effective manner, including consideration of disease duration, effects of previous analgesic use, treatment response or resilience in subpopulations of animals, sex differences, and other considerations. However, the greatest translational barrier lies in the distinction between nociception and pain (82).
Although activation of nociceptors can elicit sensations of pain, the relationship between nociceptor activation and the human experience of pain is not linear. The human experience of pain is multidimensional and complex, and influenced by many factors that include modulation by brainstem and cortical circuits that change over time with the continuation of the pain state. Ultimately, the context (for example, threats to survival, athletic competition, availability of rewards, etc.) in which nociceptors are activated and the ultimate value of responding to pain, or suppressing pain, for the organism determine the human experience of pain (83). Such factors determine, and can override, the biological consequences of a specific pharmacological mechanism, as recently demonstrated in human imaging studies by the lack of analgesic action of hidden administration of potent opiate agonists in humans (82, 84). To date, insufficient emphasis has been placed preclinically on understanding the circuits and mechanisms mediating affective dimensions of pain that are the most troubling to patients (85, 86). Recent advances in understanding evaluation of pain preclinically have studied the brain circuits that are most likely to underlie the various components that together make up the human pain experience. It is, of course, not possible for animal models to capture the human experience that is pain (87, 88), and these models were not developed for this purpose. Nevertheless, deconstructing the pathways involved in pain responses in animals may inform and focus future clinical investigations.
Nociceptive mechanisms for pain resulting from noxious stimuli identified preclinically generally translate well to humans, but these acute conditions are usually not the primary medical need. After injury, patients often develop allodynia (increased sensitivity to normally innocuous stimuli). This symptom can be reliably reproduced in animal assays and human volunteers, making the development of therapies to prevent this escalation in patients seem achievable. Less understood, however, is why some patients show successful resolution of their pain, whereas others go on to develop chronic pain. Injuries to nerves (for example, after surgery) produce a variable incidence of chronic pain; estimates show that some 10 to 50% of patients undergoing surgery ultimately experience chronic pain (89). The reasons for this are unclear, but may include the nature of the injury, the age of the patient, psychological status, and genetic differences in pain susceptibility among patients (90, 91). Possible reasons for resistance to the development of chronic pain in genetically similar animals (92–94) are currently being explored. Combined with strategies aimed at understanding endogenous factors (95, 96) associated with the natural resolution of pain, as observed in many chronic neuropathic pain patients over time (97), such studies could point to genetic and epigenetic clues to identify those patients who are most at risk to develop (98) or limit chronic pain, thereby linking the laboratory to the reality of the clinical world. Indeed, resolution from injury may be an active process that engages signaling molecules such as the resolvins and the protectins (95, 99).
Chronic “spontaneous” or ongoing pain, which is apparently independent of an external stimulus (100), presents a huge challenge for preclinical pain drug discovery research. Our relative inability to measure and study ongoing pain in preclinical models likely has impeded the discovery of new therapeutics (101–103). A number of innovative preclinical approaches, however, are being developed that may be more relevant to ongoing pain (104–108). These include operant protocols that assess complex animal behavior such as, for example, place conditioning and avoidance (105, 107, 109, 110), self-administration of analgesic drugs (111, 112), pain-induced facial expressions of rodents (facial scales) (113), wheel running (114, 115), burrowing (116, 117), and vocalization (118, 119). Determination of the potential translational value of these approaches must await the clinical assessment of drugs aimed at targets identified with these approaches.
Preclinical studies of complex behaviors of animals with presumed chronic pain have pointed to brain circuits that may underlie the affective (aversive) components of human pain. Relief of pain, like relief of other aversive states, can be considered as a reward (120, 121). Clinical studies of experimental (122) and chronic pain (123, 124) have demonstrated that pain cessation has reward value. The mesolimbic dopaminergic reward pathway is activated after peripheral nerve block in injured animals (125), suggesting a neuroanatomical substrate for this effect. The responses of neurons within reward circuits to the onset or offset of nociceptive stimuli (126) are likely to serve as the neural basis of decisions of whether or not to respond to nociceptive stimuli based on context of safety and anticipated reward (83, 127). Such circuit-based analyses may be useful to detect and differentiate mechanisms specifically modulating pain from those that could induce overdrive in motivational processing in the absence of pain, such as the opioids (128).
CLINICAL PAIN DRUG DISCOVERY CHALLENGES
Translational pharmacodynamic and pain biomarkers
Positron emission tomography (PET) is a nuclear imaging technique that uses radioactive tracers (i.e., ligands that bind to specific receptors), allowing measurement of target engagement of drug candidates. This technique can be used in humans and is an increasingly essential component of drug discovery programs (129). All too often, potential CNS drugs have apparently failed in the clinic, without an assessment of whether there was sufficient central target engagement, providing an inadequate test of the clinical hypothesis. PET imaging can demonstrate that clinical drug doses engage the desired target, allowing an adequate proof of concept within the safety and tolerability limits of the molecule. A trial that fails despite appropriate target engagement indicates that the targeted mechanism is not relevant to the tested pain condition. PET imaging is used in animal studies as well, revealing the exposure-occupancy relationships required to produce desired effects and facilitating molecule selection (that is, choosing the drug with the highest on-target occupancy combined with the lowest possible exposure to allow optimal safety margin).
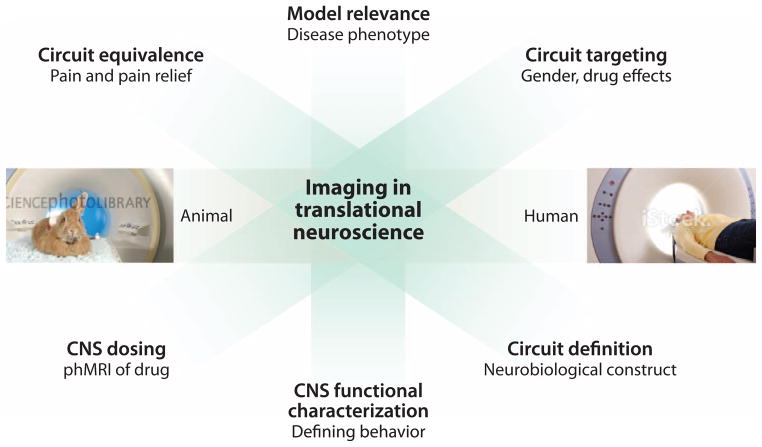
Phase 1 clinical trials can also use pharmacodynamic biomarkers such as functional magnetic resonance imaging (fMRI) (measurement of blood flow as a surrogate for neural activity) (66) and pharmacological MRI (phMRI) imaging (fMRI to evaluate brain activity induced by a pharmacological agent) (130) to accelerate pain drug discovery programs (Fig. 1). Imaging of animals and humans reveals similarities in their responses to evoked pain (131, 132) and resting state activities (132), and phMRI shows correspondence of analgesic effects of drugs across species (133, 134). In addition, anatomical imaging shows parallel changes in morphometric measures of gray matter during neuropathic pain in rats (135) and humans (136, 137).
Fig. 1. Imaging in drug development.
Brain imaging is useful for identifying and investigating various endpoints of translational significance in the preclinical phase of analgesic drug development.
Establishment of disease biomarkers for pain or for analgesia requires validation for disease state and drug responses in well-characterized specific patient populations. In chronic pain conditions, the validation of biomarkers is complicated by the naturally variable and potentially reversible course of the disease. Pain is evaluated by asking patients or subjects to rate intensity on various scales (for example, numerical rating scale where 0 is for no pain and 10 for worst pain imaginable) (138, 139). These values are useful to evaluate the efficacy of therapeutics and can capture the intensity of pain, but they are not fully informative about mechanisms underlying pain. Platforms involving diagnostic assays and methodologies are being developed (140, 141) to discover biomarkers including cytokines and microRNA profiles (142, 143), brain metrics (44, 144, 145), cerebrospinal fluid and tissue changes (146), and skin biopsies (147, 148). These can potentially be used to monitor and track disease progression and therapeutic response in chronic pain syndromes. These markers could also be used in the future to follow disease-modifying effects of therapeutic interventions. For example, reversal of brain gray matter changes has been reported in patients with chronic pain after successful treatment or resolution of pain (149); predicting chronification of chronic back pain may be evaluated using functional imaging (150); genetic measures may predict pain sensitivity and persistence (91); and psychological states such as fear or catastrophizing may predict heightened pain after surgery (151), as well as persistent postsurgical pain (152).
Brain imaging itself is a promising biomarker for chronic pain (144, 145) because it can track functional and morphological changes in multiple brain systems that can be related to the pain condition in animals and humans (135, 153–155). Recent work has begun to define a neural signature of experimental pain in human subjects (156). In this approach, also used for depression, activated brain areas are recognized by a computer algorithm (machine learning) to define a state (such as pain) that can then be used to evaluate other individuals’ responses. This method has proved to be accurate for experimental pain, but its sensitivity in patients with chronic pain is not yet clear. Although imaging can provide insights into CNS responses to noxious stimuli, pain is a subjective phenomenon, and at present, the only reliable biomarker for pain is pain itself.
CHRONIC PAIN AS A BRAIN DISORDER
Peripheral or CNS damage can result in maladaptations to initial nociceptive sensory processing that transform to a state of chronic pain. In the latter, there are alterations in multiple brain processes including motivational, cognitive, physiological (for example, sleep wake), and/or hedonic networks (Fig. 2). To date, therapies for chronic pain that target peripheral (non-CNS) mechanisms have generally not produced complete, sustained pain relief (157). Recent efforts have investigated the effects of chronic pain on the brain itself. Patients suffering from chronic pain show alterations in cognition (158) and decision making (134). There is significant comorbidity between pain and other negative emotional states such as depression and posttraumatic stress disorder (PTSD) (159, 160). Many chronically ill patients suffer from a lack of interest in life activities and diminished reward (anhedonia) (161, 162). Imaging analyses show decreases in cortical volume in patients with chronic pain and other conditions including migraine, irritable bowel syndrome, and back pain (163–166), which appear in some to be reversible when chronic pain is resolved (149).
Fig. 2. Chronic pain affects the brain.
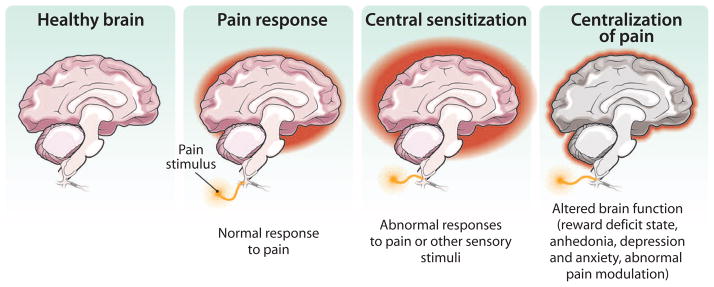
Damage to peripheral nerves (or to the CNS) can produce pain that is associated with ongoing plasticity of the brain. Such plasticity can reflect increased “centralization” of pain within specific (for example, emotional) circuits. Centralization of pain includes changes that are often associated with pain such as altered cognition and affect (depression or anxiety). Understanding the processes promoting such chronification of pain is critical for successful translation of pain therapies.
Preclinical studies have begun to corroborate these clinical observations by demonstrating the consequences of acute and chronic pain on reward (125), attention (167), and dynamic (reversible) changes in brain morphology (149, 168). These approaches can potentially address the disconnect between the complex features of chronic pain in humans and the short-term mechanisms that are commonly evaluated in pre-clinical studies on animals. Understanding the long-term consequences of pain on brain circuits is critical to improving assessment of potential treatments for chronic pain in humans.
Detecting and reporting clinical effects
Patients in clinical trials of pain drugs are heterogeneous with respect to genetics (169), past experience, past medication or drug history, and duration of disease. These factors likely account for the fact that some patients respond to pain treatment, whereas others do not (103). Clinical trials—unlike animal studies, which have homogeneous subject populations—often group patients with heterogeneous pain phenotypes, and probably different underlying pain mechanisms, into broad pain classifications and indications, and then evaluate responses subjectively. Together, these factors can produce large statistical variance, potentially masking clinical activity in subpopulations of patients and thus potential efficacy in these subgroups. Prescreening methods that better define pain genotype and phenotype and enriched trial designs (170) will likely improve clinical signal detection.
Publication of data on failed drug trials is important to prevent redundant and uninformative research, enabling our limited drug discovery and development resources to focus on new targets with improved probability of clinical success (171). The registration of drug trials at clincaltrials.gov provides an opportunity for greater transparency, although detailed descriptions of the clinical protocols may not be available unless results are published in peer-reviewed journals. Timely publication of trial protocols and results, especially if several groups are pursuing the same targets, also supports ethical drug development by preventing the exposure of new volunteers and patient cohorts to drug classes that have not shown efficacy.
CHALLENGES TO THE PAIN RESEARCH COMMUNITY
The National Institutes of Health (NIH) and other federal agencies have supported academic pain research for decades, and this has furthered understanding of fundamental pain neurobiology preclinically and clinically, generating a significant literature base for future investigations. Recently, however, academic pain research has been weakened by reduced NIH funding (declining budget allocations, reduced number of grants being supported, diminished growth of NIH funding, and decreased relative dollar value) (172, 173). This has been compounded by the exodus of many large pharmaceutical companies from analgesic development, decreasing collaborative resources, and access to sophisticated tools and technologies. Reinvigorating industry-academia relations is critical for future pain drug discovery (174).
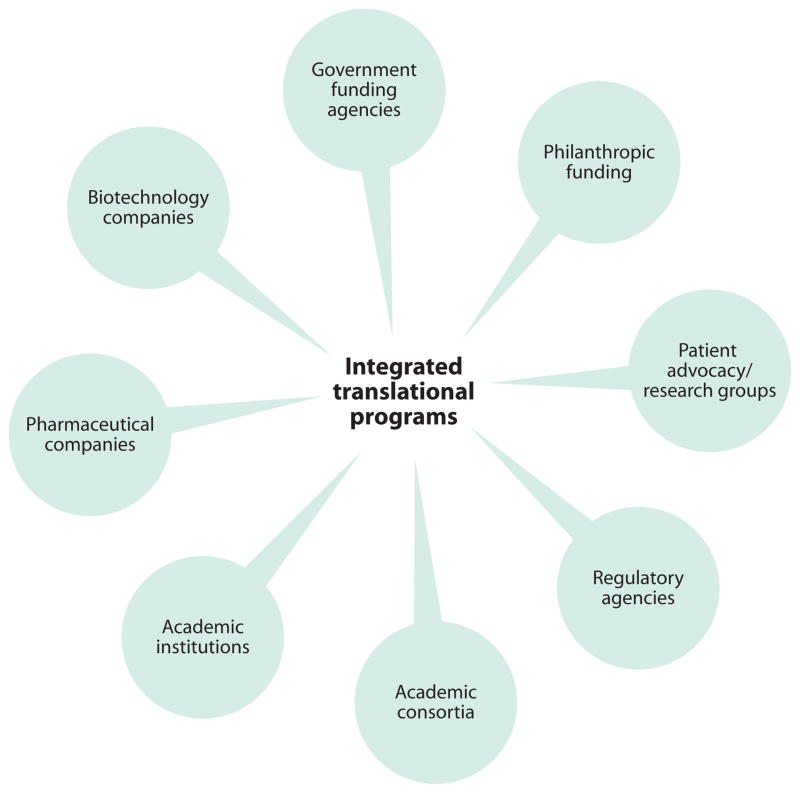
The current productivity crisis in the pharmaceutical industry has spawned the necessity to develop new business models and approaches for open innovation (175) that link external opportunities to internal research and development. New risk- and cost-sharing consortia models that require a “cultural transformation” around intellectual property within both industry and academia (176) are now being explored (Fig. 3). These consortia promote precompetitive sharing of information, early-space interactions with academia, support for investigator-initiated study protocols, imaging consortia, and early assessment of drug candidates through biotechnology companies or newly emerging programs at large universities, private-public partnerships, as well as other national initiatives (Table 1).
Fig. 3. Integrated translational programs.
A concerted effort by different interested stakeholders (patients, academicians, and pharmaceutical companies) can produce more coherent and robust translation of pain therapies. Such efforts would provide a much-needed impetus to define pain phenotypes in animals and humans and to evaluate and develop new and better translational models.
Table 1.
Initiatives for cooperative science and analgesic drug development.
| Program | Reference |
|---|---|
| Academic/university programs | |
| The Clinical and Translational Science Center, Harvard Medical School | (192) |
| Brain Science Institute NeuroTranslational Drug Discovery, Johns Hopkins University | (193) |
| Structural Genomics Consortium, Oxford University | (194) |
| National centers of excellence | |
| London Pain Consortium | (195) |
| German Research Network on Neuropathic Pain | (196) |
| Public-private partnerships | |
| Innovative Medicine Initiative, European Commission and by the European Federation of Pharmaceutical Industries and Associations | (197) |
| NIH Common Fund | (198) |
| NIH Accelerating Medicines Partnership* | (199) |
The initial focus is not on analgesic drug development.
SUMMARY AND NEXT STEPS
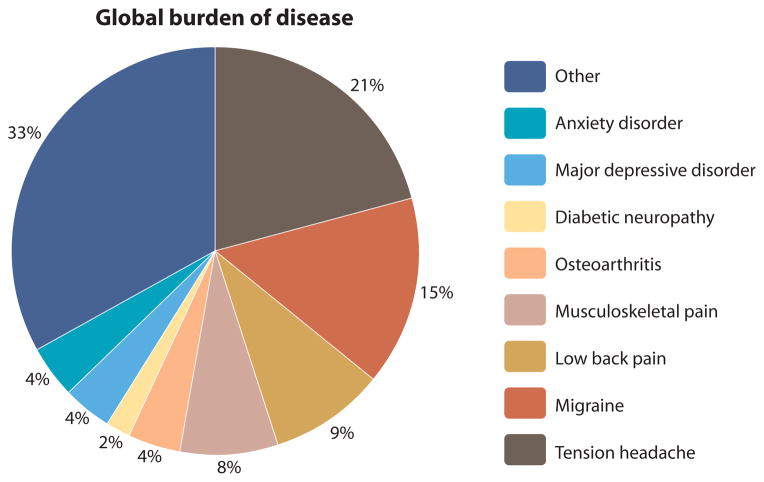
In a recent review on the global burden of disease, chronic pain (including tension headache, migraine, low back pain, musculoskeletal pain, osteoarthritis, and diabetic neuropathy) ranked as one of the most significant health issues—above common psychiatric disorders such as major depressive disorder and anxiety disorder—underscoring the compelling need for more research and investment into treatments for chronic pain (177) (Fig. 4).
Fig. 4. Relative proportion of chronic pain conditions as a medical burden to global society.
Adapted with permission from (177).
Pain is one of the most important global health issues, contributing substantially to humankind’s burden of disease. As a complex brain disease with high unmet medical need, pain is still inadequately treated, despite significant investment in pain research and advances in understanding of its neurobiology. There are many contributors to this situation: (i) difficulties in preclinical target assessment, (ii) poor chemical druggability of targets validated in human studies, (iii) a paucity of clinically qualified biomarkers, (iv) a lack of translational assays (robust, reproducible measures that can predict efficacy across species or from healthy to chronic pain conditions) to guide proof-of-concept testing, (v) poor target engagement of molecules, and (vi) small effect sizes in generalized pain populations that increase the difficulties of informative clinical trials. Drug development is challenging for many CNS disorders (178–182). The problems of analgesic drug development are set in this bleak landscape and the sad context of a retreat from CNS drug discovery by large pharmaceutical companies, despite regulatory encouragement (183). Analgesics often require costly proof-of-concept clinical trials and have a low probability of success (184) compared to other disease areas. Although pain may be considered to be no different than other complex neurological or psychiatric disorders in its translational difficulties, our failure to produce new pain therapeutics is particularly disappointing given our substantial understanding of pain pathways and pain neurobiology.
The de-risking of drug development in academia (185) and industry is essential to encourage further investment in new pain therapies, a need that is fueling the generation of standardized guidelines for analgesic drug development in industry (186, 187). Public-private consortia are forming to enhance clinical pain research, particularly with scientifically validated and clinically qualified human experimental pain models, including patients (188). Such consortia can define new clinical pain paradigms as well as common standards for data capture, processing, and evaluation, so that methodologies and analytical techniques can be easily shared between academia and industry (189, 190). These collaborative efforts (191) will require us to overcome diverse societal, financial, and personal interests to enable rapid progress. Patients are waiting for more effective treatments for pain.
There are, nonetheless, many reasons for optimism. The human genetics of pain are helping to inform decisions on drug discovery targets. Knowledge of the fundamental adaptive changes in the peripheral and CNS that underlie the progression, and possibly the resolution, of pain is growing. New translational efficacy models that bridge the gap between the laboratory and the clinic by the use of common endpoints are being developed and validated through known pharmacological mechanisms. Improvements in imaging of brain pain circuits are progressing rapidly. Biomarkers are being identified that can help to prioritize early decision making and ensure that mechanisms and clinical hypotheses are truly tested. Finally, our understanding of preclinical and clinical chronic pain biology continues to increase, and new approaches promise success in disease modification.
Acknowledgments
Funding: Supported in part by grants from the NIH (F.P. and D.B.).
Footnotes
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Melnikova I. Pain market. Nat Rev Drug Discov. 2010;9:589–590. doi: 10.1038/nrd3226. [DOI] [PubMed] [Google Scholar]
- 2.Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Institute of Medicine; Washington, DC: 2011. [Google Scholar]
- 3.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290:2443–2454. doi: 10.1001/jama.290.18.2443. [DOI] [PubMed] [Google Scholar]
- 4.Serra J, Bostock H, Solà R, Aleu J, García E, Cokic B, Navarro X, Quiles C. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153:42–55. doi: 10.1016/j.pain.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Van Hees J, Gybels J. C nociceptor activity in human nerve during painful and non painful skin stimulation. J Neurol Neurosurg Psychiatry. 1981;44:600–607. doi: 10.1136/jnnp.44.7.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Indo Y. Nerve growth factor and the physiology of pain: Lessons from congenital insensitivity to pain with anhidrosis. Clin Genet. 2012;82:341–350. doi: 10.1111/j.1399-0004.2012.01943.x. [DOI] [PubMed] [Google Scholar]
- 7.Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, Matsuda I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13:485–488. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- 8.Dib-Hajj SD, Waxman SG. Translational pain research: Lessons from genetics and genomics. Sci Transl Med. 2014;6:249sr4. doi: 10.1126/scitranslmed.3007017. [DOI] [PubMed] [Google Scholar]
- 9.Plenge RM, Greenberg JD, Mangravite LM, Derry JM, Stahl EA, Coenen MJ, Barton A, Padyukov L, Klareskog L, Gregersen PK, Mariette X, Moreland LW, Bridges SL, Jr, de Vries N, Huizinga TW, Guchelaar HJ, Friend SH, Stolovitzky G International Rheumatoid Arthritis Consortium (INTERACT) Crowdsourcing genetic prediction of clinical utility in the Rheumatoid Arthritis Responder Challenge. Nat Genet. 2013;45:468–469. doi: 10.1038/ng.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett CF, van den Maagdenberg AM, Frants RR, Ferrari MD. Familial hemiplegic migraine. Adv Genet. 2008;63:57–83. doi: 10.1016/S0065-2660(08)01003-1. [DOI] [PubMed] [Google Scholar]
- 11.Freilinger T, Anttila V, de Vries B, Malik R, Kallela M, Terwindt GM, Pozo-Rosich P, Winsvold B, Nyholt DR, van Oosterhout WP, Artto V, Todt U, Hämäläinen E, Fernández-Morales J, Louter MA, Kaunisto MA, Schoenen J, Raitakari O, Lehtimäki T, Vila-Pueyo M, Göbel H, Wichmann E, Sintas C, Uitterlinden AG, Hofman A, Rivadeneira F, Heinze A, Tronvik E, van Duijn CM, Kaprio J, Cormand B, Wessman M, Frants RR, Meitinger T, Müller-Myhsok B, Zwart JA, Färkkilä M, Macaya A, Ferrari MD, Kubisch C, Palotie A, Dichgans M, van den Maagdenberg AM International Headache Genetics Consortium, Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat Genet. 2012;44:777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The NaV1.7 sodium channel: From molecule to man. Nat Rev Neurosci. 2013;14:49–62. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- 13.Faber CG, Lauria G, Merkies IS, Cheng X, Han C, Ahn HS, Persson AK, Hoeijmakers JG, Gerrits MM, Pierro T, Lombardi R, Kapetis D, Dib-Hajj SD, Waxman SG. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc Natl Acad Sci USA. 2012;109:19444–19449. doi: 10.1073/pnas.1216080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer TZ, Waxman SG. Familial pain syndromes from mutations of the NaV1.7 sodium channel. Ann N Y Acad Sci. 2010;1184:196–207. doi: 10.1111/j.1749-6632.2009.05110.x. [DOI] [PubMed] [Google Scholar]
- 15.Rowe AH, Xiao Y, Rowe MP, Cummins TR, Zakon HH. Voltage-gated sodium channel in grasshopper mice defends against bark scorpion toxin. Science. 2013;342:441–446. doi: 10.1126/science.1236451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pexton T, Moeller-Bertram T, Schilling JM, Wallace MS. Targeting voltage-gated calcium channels for the treatment of neuropathic pain: A review of drug development. Expert Opin Investig Drugs. 2011;20:1277–1284. doi: 10.1517/13543784.2011.600686. [DOI] [PubMed] [Google Scholar]
- 17.Han TS, Teichert RW, Olivera BM, Bulaj G. Conus venoms—A rich source of peptide-based therapeutics. Curr Pharm Des. 2008;14:2462–2479. doi: 10.2174/138161208785777469. [DOI] [PubMed] [Google Scholar]
- 18.Mayer AM, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, McIntosh JM, Newman DJ, Potts BC, Shuster DE. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Teichert RW, Olivera BM. Natural products and ion channel pharmacology. Future Med Chem. 2010;2:731–744. doi: 10.4155/fmc.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O’Brien PC. Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology. 1997;48:501–505. doi: 10.1212/wnl.48.2.501. [DOI] [PubMed] [Google Scholar]
- 21.Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: What went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413. doi: 10.1016/s0074-7742(02)50083-0. [DOI] [PubMed] [Google Scholar]
- 23.Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat Rev Rheumatol. 2013;9:400–410. doi: 10.1038/nrrheum.2013.44. [DOI] [PubMed] [Google Scholar]
- 24.Garber K. Fate of novel painkiller mAbs hangs in balance. Nat Biotechnol. 2011;29:173–174. doi: 10.1038/nbt0311-173. [DOI] [PubMed] [Google Scholar]
- 25.Katz N, Borenstein DG, Birbara C, Bramson C, Nemeth MA, Smith MD, Brown MT. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain. 2011;152:2248–2258. doi: 10.1016/j.pain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic knee pain: Results of a randomized, double-blind, placebo-controlled phase III trial. J Pain. 2012;13:790–798. doi: 10.1016/j.jpain.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Norbury TA, MacGregor AJ, Urwin J, Spector TD, McMahon SB. Heritability of responses to painful stimuli in women: A classical twin study. Brain. 2007;130:3041–3049. doi: 10.1093/brain/awm233. [DOI] [PubMed] [Google Scholar]
- 28.Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, Bountra C, Korchev YE, Anand P. Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur J Pain. 2013;17:1012–1026. doi: 10.1002/j.1532-2149.2012.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MT, Woodruff TM, Wyse BD, Muralidharan A, Walther T. A small molecule angiotensin II type 2 receptor (AT2R) antagonist produces analgesia in a rat model of neuropathic pain by inhibition of p38 mitogen-activated protein kinase (MAPK) and p44/p42 MAPK activation in the dorsal root ganglia. Pain Med. 2013;14:1557–1568. doi: 10.1111/pme.12157. [DOI] [PubMed] [Google Scholar]
- 30.Marion E, Song OR, Christophe T, Babonneau J, Fenistein D, Eyer J, Letournel F, Henrion D, Clere N, Paille V, Guérineau NC, Saint André JP, Gersbach P, Altmann KH, Stinear TP, Comoglio Y, Sandoz G, Preisser L, Delneste Y, Yeramian E, Marsollier L, Brodin P. Myco-bacterial toxin induces analgesia in Buruli ulcer by targeting the angiotensin pathways. Cell. 2014;157:1565–1576. doi: 10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 31.Rice AS, Dworkin RH, McCarthy TD, Anand P, Bountra C, McCloud PI, Hill J, Cutter G, Kitson G, Desem N, Raff M EMA401-003 study group. EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: A randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet. 2014;383:1637–1647. doi: 10.1016/S0140-6736(13)62337-5. [DOI] [PubMed] [Google Scholar]
- 32.Humphrey PP. The discovery and development of the triptans, a major therapeutic breakthrough. Headache. 2008;48:685–687. doi: 10.1111/j.1526-4610.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 33.Tfelt-Hansen P, Hougaard A. Sumatriptan: A review of its pharmacokinetics, pharmacodynamics and efficacy in the acute treatment of migraine. Expert Opin Drug Metab Toxicol. 2013;9:91–103. doi: 10.1517/17425255.2013.744394. [DOI] [PubMed] [Google Scholar]
- 34.Negro A, Lionetto L, Simmaco M, Martelletti P. CGRP receptor antagonists: An expanding drug class for acute migraine? Expert Opin Investig Drugs. 2012;21:807–818. doi: 10.1517/13543784.2012.681044. [DOI] [PubMed] [Google Scholar]
- 35.Diener HC, Barbanti P, Dahlöf C, Reuter U, Habeck J, Podhorna J. BI 44370 TA, an oral CGRP antagonist for the treatment of acute migraine attacks: Results from a phase II study. Cephalalgia. 2011;31:573–584. doi: 10.1177/0333102410388435. [DOI] [PubMed] [Google Scholar]
- 36.Hewitt DJ, Aurora SK, Dodick DW, Goadsby PJ, Ge YJ, Bachman R, Taraborelli D, Fan X, Assaid C, Lines C, Ho TW. Randomized controlled trial of the CGRP receptor antagonist MK-3207 in the acute treatment of migraine. Cephalalgia. 2011;31:712–722. doi: 10.1177/0333102411398399. [DOI] [PubMed] [Google Scholar]
- 37.Lee YC, Chen PP. A review of SSRIs and SNRIs in neuropathic pain. Expert Opin Pharmacother. 2010;11:2813–2825. doi: 10.1517/14656566.2010.507192. [DOI] [PubMed] [Google Scholar]
- 38.Watson CP, Gilron I, Sawynok J, Lynch ME. Nontricyclic antidepressant analgesics and pain: Are serotonin norepinephrine reuptake inhibitors (SNRIs) any better? Pain. 2011;152:2206–2210. doi: 10.1016/j.pain.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 39.Raffa RB, Buschmann H, Christoph T, Eichenbaum G, Englberger W, Flores CM, Hertrampf T, Kögel B, Schiene K, Straßburger W, Terlinden R, Tzschentke TM. Mechanistic and functional differentiation of tapentadol and tramadol. Expert Opin Pharmacother. 2012;13:1437–1449. doi: 10.1517/14656566.2012.696097. [DOI] [PubMed] [Google Scholar]
- 40.Riemsma R, Forbes C, Harker J, Worthy G, Misso K, Schäfer M, Kleijnen J, Stürzebecher S. Systematic review of tapentadol in chronic severe pain. Curr Med Res Opin. 2011;27:1907–1930. doi: 10.1185/03007995.2011.611494. [DOI] [PubMed] [Google Scholar]
- 41.Jones CK, Peters SC, Shannon HE. Efficacy of duloxetine, a potent and balanced serotonergic and noradrenergic reuptake inhibitor, in inflammatory and acute pain models in rodents. J Pharmacol Exp Ther. 2005;312:726–732. doi: 10.1124/jpet.104.075960. [DOI] [PubMed] [Google Scholar]
- 42.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153:1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Botting RM. Inhibitors of cyclooxygenases: Mechanisms, selectivity and uses. J Physiol Pharmacol. 2006;57(Suppl 5):113–124. [PubMed] [Google Scholar]
- 44.Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol. 2013;26:360–367. doi: 10.1097/WCO.0b013e32836336ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borsook D, Becerra L, Carlezon WA, Jr, Shaw M, Renshaw P, Elman I, Levine J. Reward-aversion circuitry in analgesia and pain: Implications for psychiatric disorders. Eur J Pain. 2007;11:7–20. doi: 10.1016/j.ejpain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Denk F, McMahon SB, Tracey I. Pain vulnerability: A neurobiological perspective. Nat Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- 47.Dunn DA, Apanovitch D, Follettie M, He T, Ryan T. Taking a systems approach to the identification of novel therapeutic targets and biomarkers. Curr Pharm Biotechnol. 2010;11:721–734. doi: 10.2174/138920110792927739. [DOI] [PubMed] [Google Scholar]
- 48.Lowe JA, Jones P, Wilson DM. Network biology as a new approach to drug discovery. Curr Opin Drug Discov Devel. 2010;13:524–526. [PubMed] [Google Scholar]
- 49.Schadt EE, Friend SH, Shaywitz DA. A network view of disease and compound screening. Nat Rev Drug Discov. 2009;8:286–295. doi: 10.1038/nrd2826. [DOI] [PubMed] [Google Scholar]
- 50.Mori T, Shibasaki Y, Matsumoto K, Shibasaki M, Hasegawa M, Wang E, Masukawa D, Yoshizawa K, Horie S, Suzuki T. Mechanisms that underlie μ-opioid receptor agonist–induced constipation: Differential involvement of μ-opioid receptor sites and responsible regions. J Pharmacol Exp Ther. 2013;347:91–99. doi: 10.1124/jpet.113.204313. [DOI] [PubMed] [Google Scholar]
- 51.Finnerup NB. Add-on therapy: When two are not better than one. Pain. 2013;154:2579–2580. doi: 10.1016/j.pain.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 52.Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tölle T, Bouhassira D, Cruccu G, Skljarevski V, Freynhagen R. Duloxetine and pregabalin: High-dose monotherapy or their combination? The “COMBO-DN study”—A multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154:2616–2625. doi: 10.1016/j.pain.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 53.Morphy R, Rankovic Z. Fragments, network biology and designing multiple ligands. Drug Discov Today. 2007;12:156–160. doi: 10.1016/j.drudis.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Morphy R, Rankovic Z. Designing multiple ligands—Medicinal chemistry strategies and challenges. Curr Pharm Des. 2009;15:587–600. doi: 10.2174/138161209787315594. [DOI] [PubMed] [Google Scholar]
- 55.Stahl SM. Multifunctional drugs: A novel concept for psychopharmacology. CNS Spectr. 2009;14:71–73. doi: 10.1017/s1092852900000213. [DOI] [PubMed] [Google Scholar]
- 56.Minarini A, Milelli A, Simoni E, Rosini M, Bolognesi ML, Marchetti C, Tumiatti V. Multifunctional tacrine derivatives in Alzheimer’s disease. Curr Top Med Chem. 2013;13:1771–1786. doi: 10.2174/15680266113139990136. [DOI] [PubMed] [Google Scholar]
- 57.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 58.Gilron I, Bailey JM, Tu D, Holden RR, Jackson AC, Houlden RL. Nortriptyline and gabapentin, alone and in combination for neuropathic pain: A double-blind, randomised controlled crossover trial. Lancet. 2009;374:1252–1261. doi: 10.1016/S0140-6736(09)61081-3. [DOI] [PubMed] [Google Scholar]
- 59.Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borsook D, Edwards AD. Antineuropathic effects of the antibiotic derivative spicamycin KRN5500. Pain Med. 2004;5:104–108. doi: 10.1111/j.1526-4637.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 61.Kobierski LA, Abdi S, DiLorenzo L, Feroz N, Borsook D. A single intravenous injection of KRN5500 (antibiotic spicamycin) produces long-term decreases in multiple sensory hypersensitivities in neuropathic pain. Anesth Analg. 2003;97:174–182. doi: 10.1213/01.ane.0000066359.83348.f1. [DOI] [PubMed] [Google Scholar]
- 62.Mogil JS. Animal models of pain: Progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 63.Rice AS, Cimino-Brown D, Eisenach JC, Kontinen VK, Lacroix-Fralish ML, Machin I, Mogil JS, Stöhr T. Animal models and the prediction of efficacy in clinical trials of analgesic drugs: A critical appraisal and call for uniform reporting standards. Pain. 2008;139:243–247. doi: 10.1016/j.pain.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Taneja A, Di Iorio VL, Danhof M, Della Pasqua O. Translation of drug effects from experimental models of neuropathic pain and analgesia to humans. Drug Discov Today. 2012;17:837–849. doi: 10.1016/j.drudis.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Wilkes D, Li G, Angeles CF, Patterson JT, Huang LY. A large animal neuropathic pain model in sheep: A strategy for improving the predictability of preclinical models for therapeutic development. J Pain Res. 2012;5:415–424. doi: 10.2147/JPR.S34977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borsook D, Upadhyay J, Klimas M, Schwarz AJ, Coimbra A, Baumgartner R, George E, Potter WZ, Large T, Bleakman D, Evelhoch J, Iyengar S, Becerra L, Hargreaves RJ. Decision-making using fMRI in clinical drug development: Revisiting NK-1 receptor antagonists for pain. Drug Discov Today. 2012;17:964–973. doi: 10.1016/j.drudis.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 67.Herbert MK, Holzer P. Why are substance P(NK1)-receptor antagonists ineffective in pain treatment? Anaesthesist. 2002;51:308–319. doi: 10.1007/s00101-002-0296-7. [DOI] [PubMed] [Google Scholar]
- 68.Hill R. NK1 (substance P) receptor antagonists—Why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21:244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 69.Bisogno T, Maccarrone M. Latest advances in the discovery of fatty acid amide hydrolase inhibitors. Expert Opin Drug Discov. 2013;8:509–522. doi: 10.1517/17460441.2013.780021. [DOI] [PubMed] [Google Scholar]
- 70.Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease–successes and failures. FEBS J. 2013;280:1918–1943. doi: 10.1111/febs.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mogil JS Preclinical Pain Consortium. What is the reason for lack of translation in the pain field? [accessed 24 June 2014];Pain Research Forum. 2011 Mar 28; http://www.painresearchforum.org/forums/discussion/4561-what-reason-lack-translation-pain-field.
- 72.Whiteside GT, Pomonis JD, Kennedy JD. An industry perspective on the role and utility of animal models of pain in drug discovery. Neurosci Lett. 2013;557:65–72. doi: 10.1016/j.neulet.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 73.Renganathan M, Cummins TR, Waxman SG. Contribution of Nav1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- 74.Waxman SG. Nav1.7, its mutations, and the syndromes that they cause. Neurology. 2007;69:505–507. doi: 10.1212/01.wnl.0000268068.02343.37. [DOI] [PubMed] [Google Scholar]
- 75.Schaffler K, Reeh P, Duan WR, Best AE, Othman AA, Faltynek CR, Locke C, Nothaft W. An oral TRPV1 antagonist attenuates laser radiant-heat-evoked potentials and pain ratings from UVB-inflamed and normal skin. Br J Clin Pharmacol. 2013;75:404–414. doi: 10.1111/j.1365-2125.2012.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maher MP, Bhattacharya A, Ao H, Swanson N, Wu NT, Freedman J, Kansagara M, Scott B, Li DH, Eckert WA, III, Liu Y, Sepassi K, Rizzolio M, Fitzgerald A, Liu J, Branstetter BJ, Rech JC, Lebsack AD, Breitenbucher JG, Wickenden AD, Chaplan SR. Characterization of 2-(2,6-dichloro-benzyl)-thiazolo[5,4-d]pyrimidin-7-yl]-(4-trifluoromethyl-phenyl)-amine (JNJ-39729209) as a novel TRPV1 antagonist. Eur J Pharmacol. 2011;663:40–50. doi: 10.1016/j.ejphar.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 78.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 79.Stein N, Sprenger C, Scholz J, Wiech K, Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain. 2012;153:2210–2217. doi: 10.1016/j.pain.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 80.Craig AD. Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 81.Fields HL, Basbaum AI, Heinricher MM. Textbook of Pain. Vol. 5. Churchill Livingstone; Edinburgh: 2005. pp. 125–142. [Google Scholar]
- 82.Heppelmann B, Messlinger K, Schaible HG, Schmidt RF. Nociception and pain. Curr Opin Neurobiol. 1991;1:192–197. doi: 10.1016/0959-4388(91)90077-k. [DOI] [PubMed] [Google Scholar]
- 83.Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007;32:242–246. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Lee MC, Tracey I. Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr Pain Headache Rep. 2010;14:124–131. doi: 10.1007/s11916-010-0103-0. [DOI] [PubMed] [Google Scholar]
- 85.Danziger N. Neurological basis of the emotional dimension of pain. Rev Neurol. 2006;162:395–399. doi: 10.1016/s0035-3787(06)78570-8. [DOI] [PubMed] [Google Scholar]
- 86.Fields HL. Pain: An unpleasant topic. Pain. 1999;(Suppl 6):S61–S69. doi: 10.1016/S0304-3959(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 87.Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol. 2011;164:1195–1206. doi: 10.1111/j.1476-5381.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: Are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 89.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 90.Costigan M, Belfer I, Griffin RS, Dai F, Barrett LB, Coppola G, Wu T, Kiselycznyk C, Poddar M, Lu Y, Diatchenko L, Smith S, Cobos EJ, Zaykin D, Allchorne A, Gershon E, Livneh J, Shen PH, Nikolajsen L, Karppinen J, Männikkö M, Kelempisioti A, Goldman D, Maixner W, Geschwind DH, Max MB, Seltzer Z, Woolf CJ. Multiple chronic pain states are associated with a common amino acid–changing allele in KCNS1. Brain. 2010;133:2519–2527. doi: 10.1093/brain/awq195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tegeder I, Costigan M, Griffin RS, Abele A, Belfer I, Schmidt H, Ehnert C, Nejim J, Marian C, Scholz J, Wu T, Allchorne A, Diatchenko L, Binshtok AM, Goldman D, Adolph J, Sama S, Atlas SJ, Carlezon WA, Parsegian A, Lötsch J, Fillingim RB, Maixner W, Geisslinger G, Max MB, Woolf CJ. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. doi: 10.1038/nm1490. [DOI] [PubMed] [Google Scholar]
- 92.De Felice M, Sanoja R, Wang R, Vera-Portocarrero L, Oyarzo J, King T, Ossipov MH, Vanderah TW, Lai J, Dussor GO, Fields HL, Price TJ, Porreca F. Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. Pain. 2011;152:2701–2709. doi: 10.1016/j.pain.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dominguez CA, Li L, Lidman O, Olsson T, Wiesenfeld-Hallin Z, Piehl F, Xu XJ. Both MHC and non-MHC genes regulate development of experimental neuropathic pain in rats. Neurosci Lett. 2008;442:284–286. doi: 10.1016/j.neulet.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 94.Dominguez CA, Lidman O, Hao JX, Diez M, Tuncel J, Olsson T, Wiesenfeld-Hallin Z, Piehl F, Xu XJ. Genetic analysis of neuropathic pain-like behavior following peripheral nerve injury suggests a role of the major histocompatibility complex in development of allodynia. Pain. 2008;136:313–319. doi: 10.1016/j.pain.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Ji RR, Xu ZZ, Strichartz G, Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. 2011;34:599–609. doi: 10.1016/j.tins.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ndong C, Landry RP, DeLeo JA, Romero-Sandoval EA. Mitogen activated protein kinase phosphatase-1 prevents the development of tactile sensitivity in a rodent model of neuropathic pain. Mol Pain. 2012;8(34) doi: 10.1186/1744-8069-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thyregod HG, Rowbotham MC, Peters M, Possehn J, Berro M, Petersen KL. Natural history of pain following herpes zoster. Pain. 2007;128:148–156. doi: 10.1016/j.pain.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: Between pro- and antinociception. Pain. 2014;155:663–665. doi: 10.1016/j.pain.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 99.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bennett GJ. What is spontaneous pain and who has it? J Pain. 2012;13:921–929. doi: 10.1016/j.jpain.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 101.Tappe-Theodor A, Kuner R. Studying ongoing and spontaneous pain in rodents—Challenges and opportunities. Eur J Neurosci. 2014;39:1881–1890. doi: 10.1111/ejn.12643. [DOI] [PubMed] [Google Scholar]
- 102.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: Recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 103.Woolf CJ. Overcoming obstacles to developing new analgesics. Nat Med. 2010;16:1241–1247. doi: 10.1038/nm.2230. [DOI] [PubMed] [Google Scholar]
- 104.Boyce-Rustay JM, Zhong C, Kohnken R, Baker SJ, Simler GH, Wensink EJ, Decker MW, Honore P. Comparison of mechanical allodynia and the affective component of inflammatory pain in rats. Neuropharmacology. 2010;58:537–543. doi: 10.1016/j.neuropharm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 105.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Legg ED, Novejarque A, Rice AS. The Three Ages of Rat: The influence of rodent age on affective and cognitive outcome measures in peripheral neuropathic pain. Pain. 2009;144:12–13. doi: 10.1016/j.pain.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 107.Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wallace VC, Segerdahl AR, Blackbeard J, Pheby T, Rice AS. Anxiety-like behaviour is attenuated by gabapentin, morphine and diazepam in a rodent model of HIV anti-retroviral-associated neuropathic pain. Neurosci Lett. 2008;448:153–156. doi: 10.1016/j.neulet.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: Direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci USA. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sufka KJ, Hughes RA, McCormick TM, Borland JL. Opiate effects on isolation stress in domestic fowl. Pharmacol Biochem Behav. 1994;49:1011–1015. doi: 10.1016/0091-3057(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 111.Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain. 2001;91:33–45. doi: 10.1016/s0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- 112.Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: Relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106:312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- 113.Sotocinal SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, Mapplebeck JC, Wei P, Zhan S, Zhang S, McDougall JJ, King OD, Mogil JS. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. Inflammation-induced decrease in voluntary wheel running in mice: A nonreflexive test for evaluating inflammatory pain and analgesia. Pain. 2012;153:876–884. doi: 10.1016/j.pain.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stevenson GW, Mercer H, Cormier J, Dunbar C, Benoit L, Adams C, Jezierski J, Luginbuhl A, Bilsky EJ. Monosodium iodoacetate-induced osteoarthritis produces pain-depressed wheel running in rats: Implications for preclinical behavioral assessment of chronic pain. Pharmacol Biochem Behav. 2011;98:35–42. doi: 10.1016/j.pbb.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andrews N, Harper S, Issop Y, Rice AS. Novel nonreflex tests detect analgesic action in rodents at clinically relevant concentrations. Ann N Y Acad Sci. 2011;1245:11–13. doi: 10.1111/j.1749-6632.2011.06342.x. [DOI] [PubMed] [Google Scholar]
- 117.Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci. 2010;4:165. doi: 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Borszcz GS. Increases in vocalization and motor reflex thresholds are influenced by the site of morphine microinjection: Comparisons following administration into the periaqueductal gray, ventral medulla, and spinal subarachnoid space. Behav Neurosci. 1995;109:502–522. doi: 10.1037//0735-7044.109.3.502. [DOI] [PubMed] [Google Scholar]
- 119.Kurejova M, Nattenmüller U, Hildebrandt U, Selvaraj D, Stösser S, Kuner R. An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer pain and neuropathic pain. Mol Pain. 2010;6:18. doi: 10.1186/1744-8069-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain. 2008;12:866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: A link between personality and the placebo analgesic response. J Neurosci. 2009;29:4882–4887. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: Hedonic and neural responses to safety from pain. PLOS One. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J Neurosci. 2013;33:16383–16393. doi: 10.1523/JNEUROSCI.1731-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Segerdahl AR, Xie J, Paterson K, Ramirez JD, Tracey I, Bennett DL. Imaging the neural correlates of neuropathic pain and pleasurable relief associated with inherited erythromelalgia in a single subject with quantitative arterial spin labelling. Pain. 2012;153:1122–1127. doi: 10.1016/j.pain.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward–valuation circuitry. Proc Natl Acad Sci USA. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 127.Pais-Vieira M, Aguiar P, Lima D, Galhardo V. Inflammatory pain disrupts the orbitofrontal neuronal activity and risk-assessment performance in a rodent decision-making task. Pain. 2012;153:1625–1635. doi: 10.1016/j.pain.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 128.Esch T, Stefano GB. The neurobiology of pleasure, reward processes, addiction and their health implications. Neuro Endocrinol Lett. 2004;25:235–251. [PubMed] [Google Scholar]
- 129.Hargreaves RJ. The role of molecular imaging in drug discovery and development. Clin Pharmacol Ther. 2008;83:349–353. doi: 10.1038/sj.clpt.6100467. [DOI] [PubMed] [Google Scholar]
- 130.Upadhyay J, Anderson J, Schwarz AJ, Coimbra A, Baumgartner R, Pendse G, George E, Nutile L, Wallin D, Bishop J, Neni S, Maier G, Iyengar S, Evelhoch JL, Bleakman D, Hargreaves R, Becerra L, Borsook D. Imaging drugs with and without clinical analgesic efficacy. Neuropsychopharmacology. 2011;36:2659–2673. doi: 10.1038/npp.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Borsook D, Becerra L, Hargreaves R. A role for fMRI in optimizing CNS drug development. Nat Rev Drug Discov. 2006;5:411–424. doi: 10.1038/nrd2027. [DOI] [PubMed] [Google Scholar]
- 132.Becerra L, Pendse G, Chang PC, Bishop J, Borsook D. Robust reproducible resting state networks in the awake rodent brain. PLOS One. 2011;6:e25701. doi: 10.1371/journal.pone.0025701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Borsook D, Pendse G, Aiello-Lammens M, Glicksman M, Gostic J, Sherman S, Korn J, Shaw M, Stewart K, Gostic R, Bazes S, Hargreaves R, Becerra L. CNS response to a thermal stressor in human volunteers and rats may predict the clinical utility of analgesics. Drug Dev Res. 2007;68:23–41. [Google Scholar]
- 134.Becerra L, Upadhyay J, Chang PC, Bishop J, Anderson J, Baumgartner R, Schwarz AJ, Coimbra A, Wallin D, Nutile L, George E, Maier G, Sunkaraneni S, Iyengar S, Evelhoch JL, Bleakman D, Hargreaves R, Borsook D. Parallel buprenorphine phMRI responses in conscious rodents and healthy human subjects. J Pharmacol Exp Ther. 2013;345:41–51. doi: 10.1124/jpet.112.201145. [DOI] [PubMed] [Google Scholar]
- 135.Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: Thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. 2011;31:5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MS, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. doi: 10.1016/j.neuroimage.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 138.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: An overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 139.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res. 2011;63(Suppl 11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 140.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Grace PM, Hurley D, Barratt DT, Tsykin A, Watkins LR, Rolan PE, Hutchinson MR. Harnessing pain heterogeneity and RNA transcriptome to identify blood-based pain biomarkers: A novel correlational study design and bioinformatics approach in a graded chronic constriction injury model. J Neurochem. 2012;122:976–994. doi: 10.1111/j.1471-4159.2012.07833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Slade GD, Conrad MS, Diatchenko L, Rashid NU, Zhong S, Smith S, Rhodes J, Medvedev A, Makarov S, Maixner W, Nackley AG. Cytokine biomarkers and chronic pain: Association of genes, transcription, and circulating proteins with temporomandibular disorders and widespread palpation tenderness. Pain. 2011;152:2802–2812. doi: 10.1016/j.pain.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Orlova IA, Alexander GM, Qureshi RA, Sacan A, Graziano A, Barrett JE, Schwartzman RJ, Ajit SK. MicroRNA modulation in complex regional pain syndrome. J Transl Med. 2011;9:195. doi: 10.1186/1479-5876-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: The need, reality, challenges, and solutions. Discov Med. 2011;11:197–207. [PubMed] [Google Scholar]
- 145.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 2: How, where, and what to look for using functional imaging. Discov Med. 2011;11:209–219. [PubMed] [Google Scholar]
- 146.Backonja MM, Coe CL, Muller DA, Schell K. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol. 2008;195:157–163. doi: 10.1016/j.jneuroim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 147.Lauria G, Devigili G. Skin biopsy as a diagnostic tool in peripheral neuropathy. Nat Clin Pract Neurol. 2007;3:546–557. doi: 10.1038/ncpneuro0630. [DOI] [PubMed] [Google Scholar]
- 148.Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136:1857–1867. doi: 10.1093/brain/awt053. [DOI] [PubMed] [Google Scholar]
- 149.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pavlin DJ, Sullivan MJ, Freund PR, Roesen K. Catastrophizing: A risk factor for post-surgical pain. Clin J Pain. 2005;21:83–90. doi: 10.1097/00002508-200501000-00010. [DOI] [PubMed] [Google Scholar]
- 152.Werner MU, Bischoff JM. Persistent postsurgical pain: Evidence from breast cancer surgery, groin hernia repair, and lung cancer surgery. Curr Top Behav Neurosci. 2014 doi: 10.1007/7854_2014_285. [DOI] [PubMed] [Google Scholar]
- 153.Borsook D, Becerra L. CNS animal fMRI in pain and analgesia. Neurosci Biobehav Rev. 2011;35:1125–1143. doi: 10.1016/j.neubiorev.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Becerra L, Upadhyay J, Chang PC, Bishop J, Anderson J, Baumgartner R, Schwarz AJ, Coimbra A, Wallin D, Nutile L, George E, Maier G, Sunkaraneni S, Iyengar S, Evelhoch JL, Bleakman D, Hargreaves R, Borsook D. Parallel buprenorphine phMRI responses in conscious rodents and healthy human subjects. J Pharmacol Exp Ther. 2013;345:41–51. doi: 10.1124/jpet.112.201145. [DOI] [PubMed] [Google Scholar]
- 155.Borsook D, Hargreaves R, Becerra L. Can functional magnetic resonance imaging improve success rates in CNS drug discovery? Expert Opin Drug Discov. 2011;6:597–617. doi: 10.1517/17460441.2011.584529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dickenson A, Kehlet H. Can we stop pain before it starts? Pain. 2013;155:208–209. doi: 10.1016/j.pain.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 158.Cauda F, D’Agata F, Sacco K, Duca S, Cocito D, Paolasso I, Isoardo G, Geminiani G. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010;81:806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- 159.Carinci AJ, Mehta P, Christo PJ. Chronic pain in torture victims. Curr Pain Headache Rep. 2010;14:73–79. doi: 10.1007/s11916-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 160.Moeller-Bertram T, Keltner J, Strigo IA. Pain and post traumatic stress disorder—Review of clinical and experimental evidence. Neuropharmacology. 2012;62:586–597. doi: 10.1016/j.neuropharm.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 161.Marbach JJ, Richlin DM, Lipton JA. Illness behavior, depression and anhedonia in myofascial face and back pain patients. Psychother Psychosom. 1983;39:47–54. doi: 10.1159/000287720. [DOI] [PubMed] [Google Scholar]
- 162.Elman I, Zubieta JK, Borsook D. The missing p in psychiatric training: Why it is important to teach pain to psychiatrists. Arch Gen Psychiatry. 2011;68:12–20. doi: 10.1001/archgenpsychiatry.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: Potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 164.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 165.Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLOS One. 2011;6:e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: Multiple sex differences in brain function and structure. Brain. 2012;135:2546–2559. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Low LA, Millecamps M, Seminowicz DA, Naso L, Thompson SJ, Stone LS, Bushnell MC. Nerve injury causes long-term attentional deficits in rats. Neurosci Lett. 2012;529:103–107. doi: 10.1016/j.neulet.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 168.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lötsch J. Genetic variability of pain perception and treatment—Clinical pharmacological implications. Eur J Clin Pharmacol. 2011;67:541–551. doi: 10.1007/s00228-011-1012-9. [DOI] [PubMed] [Google Scholar]
- 170.Hewitt DJ, Ho TW, Galer B, Backonja M, Markovitz P, Gammaitoni A, Michelson D, Bolognese J, Alon A, Rosenberg E, Herman G, Wang H. Impact of responder definition on the enriched enrollment randomized withdrawal trial design for establishing proof of concept in neuropathic pain. Pain. 2011;152:514–521. doi: 10.1016/j.pain.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 171.Learning from failure. Nat Rev Drug Discov. 2010;9:499. doi: 10.1038/nrd3222. [DOI] [PubMed] [Google Scholar]
- 172.Hromas R, Abkowitz JL, Keating A. Facing the NIH funding crisis: How professional societies can help. JAMA. 2012;308:2343–2344. doi: 10.1001/jama.2012.45067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boadi K. Erosion of funding for the National Institutes of Health threatens US leadership in biomedical research. Center for American Progress; Mar 25, 2014. [accessed 24 June 2014]. http://americanprogress.org/issues/economy/report/2014/03/25/86369/erosion-of-funding-for-the-national-institutes-of-health-threatens-u-s-leadership-in-biomedical-research/ [Google Scholar]
- 174.Silber BM. Driving drug discovery: The fundamental role of academic labs. Sci Transl Med. 2010;2:30cm16. doi: 10.1126/scitranslmed.3000169. [DOI] [PubMed] [Google Scholar]
- 175.Schuhmacher A, Germann PG, Trill H, Gassmann O. Models for open innovation in the pharmaceutical industry. Drug Discov Today. 2013;18:1133–1137. doi: 10.1016/j.drudis.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 176.New Drug Development: Science, Business, Regulatory, and Intellectual Property Issues Cited as Hampering Drug Development Efforts. Government Printing Office; Washington, DC: 2006. [Google Scholar]
- 177.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gosselin R, Grainger R, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Ma J, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA, III, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: Beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 179.Gomez-Mancilla B, Marrer E, Kehren J, Kinnunen A, Imbert G, Hillebrand R, Bergström M, Schmidt ME. Central nervous system drug development: An integrative biomarker approach toward individualized medicine. NeuroRx. 2005;2:683–695. doi: 10.1602/neurorx.2.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gray JA, Roth BL. The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007;12:904–922. doi: 10.1038/sj.mp.4002062. [DOI] [PubMed] [Google Scholar]
- 181.Rupniak NM. Animal models of depression: Challenges from a drug development perspective. Behav Pharmacol. 2003;14:385–390. doi: 10.1097/01.fbp.0000087738.21047.91. [DOI] [PubMed] [Google Scholar]
- 182.Talevi A, Bellera CL, Di Ianni M, Gantner M, Bruno-Blanch LE, Castro EA. CNS drug development—Lost in translation? Mini Rev Med Chem. 2012;12:959–970. doi: 10.2174/138955712802762356. [DOI] [PubMed] [Google Scholar]
- 183.Woodcock J, Witter J, Dionne RA. Stimulating the development of mechanism-based, individualized pain therapies. Nat Rev Drug Discov. 2007;6:703–710. doi: 10.1038/nrd2335. [DOI] [PubMed] [Google Scholar]
- 184.Kaitin KI, Milne CP. A dearth of new meds. Sci Am. 2011;305:16. doi: 10.1038/scientificamerican0811-16. [DOI] [PubMed] [Google Scholar]
- 185.Collins F. An audience with…Francis Collins. Interviewed by Asher Mullard. Nat Rev Drug Discov. 2011;10:14. doi: 10.1038/nrd3357. [DOI] [PubMed] [Google Scholar]
- 186.Center for Drug Evaluation and Research. Analgesic Indications: Developing Drug and Biological Products. Food and Drug Administration; Silver Spring, MD: 2014. [Google Scholar]
- 187.Potter WZ. New era for novel CNS drug development. Neuropsychopharmacology. 2012;37:278–280. doi: 10.1038/npp.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oertel BG, Lötsch J. Clinical pharmacology of analgesics assessed with human experimental pain models: Bridging basic and clinical research. Br J Pharmacol. 2013;168:534–553. doi: 10.1111/bph.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schwarz AJ, Becerra L, Upadhyay J, Anderson J, Baumgartner R, Coimbra A, Evelhoch J, Hargreaves R, Robertson B, Iyengar S, Tauscher J, Bleakman D, Borsook D. A procedural framework for good imaging practice in pharmacological fMRI studies applied to drug development #1: Processes and requirements. Drug Discov Today. 2011;16:583–593. doi: 10.1016/j.drudis.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 190.Schwarz AJ, Becerra L, Upadhyay J, Anderson J, Baumgartner R, Coimbra A, Evelhoch J, Hargreaves R, Robertson B, Iyengar S, Tauscher J, Bleakman D, Borsook D. A procedural framework for good imaging practice in pharmacological fMRI studies applied to drug development #2: Protocol optimization and best practices. Drug Discov Today. 2011;16:671–682. doi: 10.1016/j.drudis.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 191.Wellenreuther R, Keppler D, Mumberg D, Ziegelbauer K, Lessl M. Promoting drug discovery by collaborative innovation: A novel risk- and reward-sharing partnership between the German Cancer Research Center and Bayer HealthCare. Drug Discov Today. 2012;17:1242–1248. doi: 10.1016/j.drudis.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 192.The Harvard Clinical and Translational Science Center. [accessed 24 June 2014];Harvard Catalyst. http://catalyst.harvard.edu.
- 193.Johns Hopkins School of Medicine, Brain Science Institute. [accessed 24 June 2014]; http://www.brainscienceinstitute.org.
- 194.SGC, Structural Genomics Consortium. [accessed 24 June 2014]; http://www.thesgc.org.
- 195.Kings College London, The London Pain Consortium. [accessed 24 June 2014]; http://www.lpc.ac.uk.
- 196.University of Munich, German Research Network on Neuropathic Pain. [accessed 24 June 2014]; http://www.neuro.med.tumuenchen.de/dfns/e.
- 197.IMI, The Innovative Medicines Initiative. [accessed 24 June 2014]; http://www.imi.europa.eu.
- 198.NIH, NIH Common Fund. [accessed 24 June 2014]; http://commonfund.nih.gov/publicprivate.
- 199.NIH, NIH Accelerating Medicines Partnership. [accessed 24 June 2014]; http://www.nih.gov/amp.