Abstract
Background
Follow-up care is critical for childhood cancer survivors (CCS) who are at high risk for co-morbidities and late effects of cancer treatments. Understanding factors associated with maintaining follow-up care is needed, especially for Hispanic CCS who are underrepresented in previous studies.
Methods
Risk and protective factors for receiving cancer-related follow-up care were examined among 193 Los Angeles County CCS diagnosed between 2000–2007 (54% Hispanic; mean age=19.9, SD=2.8; mean age at diagnosis=12.1, SD=3.0; mean years since diagnosis=7.8, SD=2.0). Self-report surveys assessed follow-up care, insurance status, demographics, clinical factors, and psychosocial risk (e.g., depression) and protective [e.g., self-efficacy (SE)] factors. Multivariable logistic regression was used to determine factors associated with previous (in prior 2 years) and intent for future cancer-related follow-up care.
Results
Seventy-three percent of CCS reported a cancer follow-up visit in the prior 2 years, which was positively associated (p’s<.05) with having health insurance, White ethnicity (vs. Hispanic), younger age and greater treatment intensity. Sixty-nine percent reported intent for follow-up care in the next two years, which was positively associated (p’s<.05) with having health insurance and greater SE.
Conclusions
Hispanics and older CCS are more likely to lack previous follow-up care. Because health insurance was strongly associated with both previous follow-up care and intent to seek care, recent changes in health coverage may improve follow-up among CCS. Interventions targeting improved SE may help increase intent to receive follow-up care for this population.
Keywords: childhood, adolescent, young adult, cancer, survivorship, follow-up care, Hispanic, Insurance
INTRODUCTION
Approximately 80% of children diagnosed with cancer will attain long-term survival or cure, with over 380,000 childhood cancer survivors (CCS) in the United States.1, 2 Improved survival is achieved through complex treatment regimens that can cause specific long-term complications (late effects) for CCS over their lifetime (e.g., heart/kidney failure, liver disease, infertility, second malignancies).3 The National Cancer Institute estimates CCS are five times more likely than siblings to experience adverse health events, with half of CCS reporting >1 late effects, and nearly 25% reporting serious or life-threatening ones. 4, 5 In the Childhood Cancer Survivor Study (CCSS) cohort, after 30 years of follow-up, 73% reported a chronic health condition, with 43% severe or life-threatening,4 and a nearly ten-fold risk of dying earlier than the general population.6, 7
The Children’s Oncology Group (COG) Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers recommend CCS receive lifelong, risk-based monitoring to detect/prevent late effects.8 These evidence-based guidelines assist clinicians in providing appropriate late effects surveillance based on individual treatment exposures and other modifying factors.9
The CCSS indicates cancer-related follow-up drops substantially at older ages.10 Among adult CCS, only 31.5% receive survivor-focused care, with17.8% given specific risk-reduction/screening recommendations.11 Reasons for this decline are largely unknown, but accessing care can be difficult during transition from pediatric to adult healthcare. Further, CCS may lack knowledge about their health risks, desire to move on with their lives, and/or lack health insurance.12, 13
Two limitations to our knowledge of follow-up care among CCS include: First, because the CCSS included only patients diagnosed between 1970–1986, follow-up care among recent CCS is not well described. The past 30 years mark significant improvements in cancer therapy, survivorship care, and possibly, follow-up care. Secondly, Hispanics are underrepresented in survivorship studies, comprising only 5% of the CCSS cohort,14 compared to 16.9% of the US population.15 Hispanics (vs. non-Hispanics) have lower survival from Acute Lymphoblastic Leukemia (ALL), the most common form of leukemia.5, 16, 17 Data from the Surveillance, Epidemiology, and End Results (SEER) cancer registries (1998–2008) indicated Hispanics had a 46% increased risk of dying from ALL compared to non-Hispanic Caucasians. Furthermore, between 1990–2004 mortality rates declined 60% faster for non-Hispanics (vs. Hispanics).18 Among adults, non-adherence to follow-up care/screening recommendations is posited to contribute to higher mortality among minorities.19–21 For CCS, the role of ethnic/racial differences in follow-up care is less clear.22, 23 According to the Institute of Medicine report,4, 6 CCS studies including Hispanics are needed. Los Angeles County (LAC) is a critical region for this research. From 2004–2008, 61.7% of children under 14 diagnosed with cancer in LAC were Hispanic.24
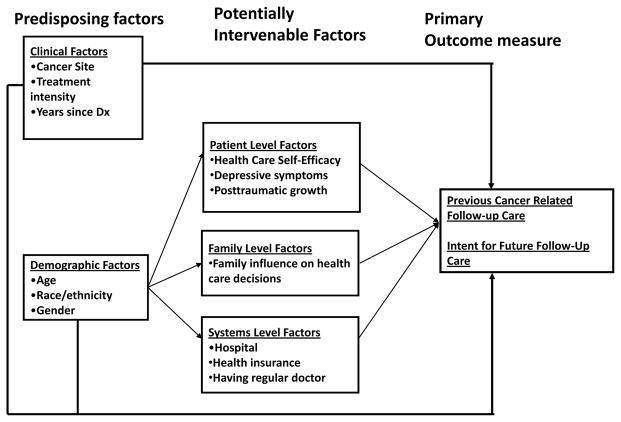
The purpose of this study, entitled “Project Forward”, was to identify risk and protective factors for use of cancer related follow-up care among recently treated Hispanic and non-Hispanic adolescent and young adult CCS. We applied a systems-level approach to the study, assessing factors at patient, family, and healthcare system levels (Figure 1). Selection of these factors was based previous findings concerning CCS survivorship care.11, 25–27 We hypothesized that follow-up care, both previous and intent for care in the future, would be positively associated with younger age, being insured, non-Hispanic (vs. Hispanic), and higher psychosocial functioning.
FIGURE 1.
Conceptual Model
METHODS
Our goal was to examine follow-up care (during adolescence and young adulthood) among childhood cancer survivors (i.e., diagnosed under age 18). A self-administered survey, designed to assess use of follow-up care, was developed in conjunction with focus and advisory group feedback. The language used did not exceed an 8th grade reading level and it took 30–45 minutes to complete. CCS were selected from the Los Angeles Cancer Surveillance Program (CSP), the SEER cancer registry for LAC, diagnosed with cancer (except Hodgkin’s Disease) at age 18 or younger at Children’s Hospital Los Angeles (CHLA) or Miller Children’s Hospital, Long Beach between 2000–2007, and whose age in 2009 was between 14–25 years. Hodgkin’s cases were excluded because they were included in another registry study.
Courtesy letters were mailed to treating physicians of all CCS selected informing them of the study and that we would be contacting their patients unless they asked that their patient not be contacted. (Two requested more information, none requested exclusion.) Two weeks following the physician letter, postcards were sent to CCS notifying them to expect study materials by mail, which enabled early tracing for cases of postal returns for incorrect addresses. Two weeks following the postcard, study packets, including overview letter, study brochure, web address, the survey, and return envelope, were mailed. English and professionally translated Spanish versions of the packets were sent to those with Spanish surnames. Written or verbal consent from CCS over age 18 or parent of CCS ages 15–17 (with child assent) to participate in the study was obtained. In addition the survey could be completed online (in both languages). After 3 weeks, if no response, multiple follow-up phone calls were made and packets resent, as needed. Lost subjects were traced. Bilingual research staff determined ineligibility (i.e. too ill to participate usually based on parent’s assessment, non English or Spanish speaker, denial of cancer, relocation out of country). Upon survey completion, participants received $20 and entry into a lottery for a $300 prize. All study procedures were approved or deemed exempt by the California Committee for the Protection of Human Subjects, California Cancer Registry, University of Southern California, CHLA, and Miller Children’s Hospital.
Measures
Previous use of Cancer Related Follow-up Care
Comparable to a similar CCSS question, participants indicated if they had a healthcare visit related to their cancer in the prior 2 years (cancer follow-up visit) (yes/no).11, 27, 28
Intent to Seek Cancer Related Follow-up Care
Participants were asked “During the next 2 years, what are the chances that you will go for a ‘cancer follow-up’ visit?” Those responding “very likely”/”likely” were classified as high intent, those responding “not likely”/”not sure” as low intent.
Demographics included current age, age at diagnosis, race/ethnicity, education level, and marital status. For analysis purposes, current age was dichotomized as under 21 and 21 years or older. This is a developmental turning point between adolescence and emerging adulthood, a transition period from pediatric to adult healthcare providers, and a time when CCS commonly lose health insurance coverage provided by parental/state/college plans. For example, the California Children Services Program, which provides medical care for children whose families cannot afford all/part of their healthcare needs is provided up to age 21. (Healthcare changes for young adults from the Patient Protection and Affordable Care Act had not been implemented when these data were collected.)
Clinical factors, obtained from the cancer registry, included diagnosis date, cancer site (e.g, leukemia, lymphoma, brain/CNS), and hospital where diagnosed.
Treatment Intensity
The Intensity of Treatment Rating Scale 2.0 (ITR-2)29 was based on cancer registry data and medical chart review, including cancer site, stage at diagnosis, treatment modalities and relapse history. Treatment was categorized by four levels of intensity: 1=least intensive (e.g., surgery only), 2=moderately intensive (e.g., chemotherapy or radiation), 3=very intensive (e.g., two or more treatment modalities), 4=most intensive (e.g., relapse protocols).
Health insurance status and having a regular doctor
Health insurance was categorized by type (public vs. private). Because relationships with follow-up care (and intent) did not differ between public and private insurance, this variable was coded as: any insurance (public or private), no insurance, and don’t know. Participants were asked if they had a regular doctor for regular non-cancer health checkups (yes/no).
Healthcare self-efficacy (SE) was measured using 3 items adapted from the Chronic Disease Self-Efficacy Scales from the Stanford Patient Education Research Center.30 Items assessed confidence in asking your doctor about things that concern you, making doctor’s appointments when you needed care, and getting the cancer follow-up care you need over the next 2 years, with a 3-point Likert scale ranging from “not confident/not sure,” “somewhat confident,” or “very confident.” Scores were summed to provide a composite self-efficacy score (Cronbach’s alpha=.64).
Post-traumatic Growth Inventory (PTGI)
The PTGI short form is a 10-item measure of personal growth experienced by individuals who have experienced a traumatic event, in this case, cancer. 31 Items reflect different areas of growth, including relating to others, new possibilities, personal strength, spiritual change, and appreciation of life. Items are based on a 6-point scale ranging from 0 (“I did not experience this change as a result of my crisis.”) to 5 (“I experienced this change to a very great degree as a result of my crisis.”). A PTG total mean score was calculated, where higher scores indicate more post-traumatic growth (Cronbach’s alpha=.90).
Depressive symptoms, The 20-item Center for Epidemiological Studies Depression Scale (CES-D) was used to assess depressive symptoms.32 Participants indicated how often they experienced symptoms (e.g., depressed mood, loss of appetite, sleep and psychomotor disruption, feelings of guilt and worthlessness and/or helplessness and hopelessness) during the previous week on a four-point ordinal scale ranging from “rarely or none of the time” (less than 1 day) to “most or all of the time” (5–7 days). A total score was calculated with higher scores representing elevated depressive symptoms levels (Cronbach’s alpha=.92).
Parental Involvement with healthcare was assessed with a single item developed from focus groups prior to launching the study, “How often has your family influenced your healthcare decisions?” Participants responded on a 4-item Likert-type scale (often, occasionally, never, not sure). Items were dichotomized to reflect family participation (1 = often/occasionally) vs. non-participation in healthcare decisions (0 = never/not sure).
Statistical Analysis
Descriptive percent distributions of the sample demographic and clinical characteristics were examined, including percent with previous care and intent to seek care (the two primary outcome variables) by categories of these variables. Univariate and multivariable logistic regression analyses were performed to assess factors associated with these two outcome variables. After including demographics (age, sex and race/ethnicity) in each model, other variables that demonstrated a univariate association with each outcome variable (p ≤ 0.10) were selected for inclusion in final multivariable logistic regression models. The final model for previous use of follow-up care included sex, race/ethnicity, health insurance, treatment intensity, family influence on healthcare decisions, having a regular doctor, healthcare self-efficacy, and post traumatic growth. The final model for intent to seek follow-up care included the same variables with the addition of time since diagnosis and the removal of treatment intensity. Data analyses were conducted using SAS statistical software (Version 9.2) (SAS Institute; Cary, NC).
RESULTS
Five hundred fifteen CCS were initially selected from the LA CSP. Forty-five were determined ineligible (25 deceased, 5 out of country, 5 cognitively/developmentally impaired, 10 denied having had cancer). Of the 470 eligible, we recruited 235 (50%). Of the 235 non-respondents, 27 CCS refused, 12 parents of CCS < 18 years of age refused their child’s participation, 84 were never reached (deemed lost-to-follow-up after multiple mailings/phone calls to contact them through tracing, with no known correct contact information), and 112 never responded despite repeat attempts via mail and phone (considered as passive refusals). Participants primarily responded by mail, with only 27 and 4 responding to web/phone based surveys, respectively. Only 6 responded in Spanish. Using cancer registry data, we compared characteristics of respondents to non-respondents and found no difference by age, socio-economic status based on census tract of address at diagnosis, or race/ethnicity. Females were more likely to respond than males (56.4% vs. 44.8%; p<.05).
For these analyses we excluded 42 respondents who had received treatment during the prior two years (based on the self-reported survey results), resulting in an analytical sample of 193. Participants (Table 1) were evenly divided by sex and over half self-reported their ethnicity as Hispanic (54.4%). Age at participation ranged from 15–25 years (mean=19.87, SD=2.82), with 41% 21 or older. The majority was diagnosed at CHLA (85.0%), single (86.5%), and with at least a high school education (70.7%). One third had private health insurance, 30% had public insurance, 19% had no insurance, and 18% had other or unknown health coverage.
Table 1.
Demographic, psychosocial, family, and clinical characteristics of Project Forward participants (n=193)
| Total | ||
|---|---|---|
|
| ||
| No. | % | |
| Demographic Factors | ||
| Gender | ||
| Female | 96 | 49.7 |
| Male | 97 | 50.36 |
| Race/Ethnicity | ||
| Hispanic | 105 | 54.4 |
| White, non-Hispanic | 55 | 28.5 |
| Other | 33 | 17.1 |
| Age at survey completion | ||
| 15–20 yrs | 114 | 59.1 |
| 21+ yrs old | 79 | 40.9 |
| Education | ||
| <HS | 56 | 29.0 |
| HS | 44 | 22.8 |
| Some college | 55 | 28.5 |
| Associate/college degree | 36 | 18.6 |
| Missing | 2 | 1.04 |
| Family Level Factors | ||
| Family influences healthcare decisions | N | % |
| Yes | 172 | 89.1 |
| No | 21 | 10.9 |
| Clinical Factors | ||
| Cancer diagnosis/site | N | % |
| Leukemia | 57 | 29.5 |
| Brain/CNS | 31 | 16.1 |
| Bone | 10 | 5.2 |
| Lymphoma | 38 | 19.7 |
| Other | 57 | 29.5 |
| Treatment intensity | N | % |
| Least intensive 1 | 15 | 7.8 |
| 2 | 65 | 33.7 |
| 3 | 90 | 46.6 |
| Most intensive 4 | 22 | 11.4 |
| Missing | 1 | 0.52 |
| System Level Factors | ||
| Hospital | N | % |
| CHLA | 164 | 85.0 |
| LB | 29 | 15.0 |
| Health Insurance | N | % |
| Public | 61 | 32.1 |
| Private | 69 | 36.3 |
| None | 36 | 19.0 |
| unknown | 27 | 25.7 |
| Has regular doctor for regular (non-cancer) health checkups | N | % |
| Yes | 124 | 64.2 |
| No | 68 | 35.2 |
| Missing | 1 | 0.5 |
Chi-square significance:
p<.10,
p<.05,
p<.01,
p<.001
Clinical characteristics
The most common types of cancer were leukemia (29%), lymphoma (19%) and brain/central nervous system (16%). The majority (81%) received ‘moderate/very’ intensive treatments. Age at diagnosis ranged from 5 to 19 years (mean=12.1, SD=3.0), and years since diagnosis ranged from 4 to 12 (mean=7.8, SD=2.0).
Psychosocial Characteristics
Healthcare Self Efficacy scores ranged from 0 to 6 (M=4.10, SD=1.69). Posttraumatic Growth Inventory scores ranged from 0 to 50 (M=35.71, SD=10.81), while CESD scores ranged from 0 to 46 (M=13.95, SD=11.23).
Previous use of follow-up care/intent to seek follow-up care
Seventy-three percent reported a cancer related follow-up care visit in the prior 2 years. Overall, 69% indicated intent for future follow-up care as either ‘very likely’ or ‘likely’. Differences in the characteristics of the study population by follow-up care and race/ethnicity are provided as supporting information in Tables SI1 and SI2, respectively.
Factors related to previous use of follow-up care
Based on univariate logistic regression analyses, those who had received care in the past two years were more likely to: have higher cancer treatment intensity, be under 21 (vs. ≥21), be insured, report family influenc on healthcare decisions, have a regular non-cancer physician, have higher healthcare self-efficacy (SE), and higher PTG (Table 2). In the multivariable model, treatment intensity, younger age, and insurance remained significantly associated with previous follow-up care and ethnicity became significant, with Hispanics less likely to report previous care than non-Hispanic Whites.
Table 2.
Univariate and multivariable models of previous use of cancer-related follow-up care (within prior 2 years)
| Univariate | Multivariable Model | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Characteristic | Odds Ratio | 95% CI Lower | 95% CI Upper | P Value | Adjusted Odds Ratio | 95% CI Lower | 95% CI Upper | P Value |
|
|
|
|||||||
| Age (age 21 vs. under 21) | 0.23 | 0.12 | 0.45 | <.001 | 0.24 | 0.10 | 0.58 | p<.001 |
| Sex (ref group=male) | 0.93 | 0.49 | 1.74 | .84 | 1.39 | 0.60 | 3.20 | 0.44 |
| Race/Ethnicity | ||||||||
| White, non-Hispanic | 1.00 | 1.00 | ||||||
| Hispanic | 0.55 | 0.25 | 1.21 | .17 | 0.33 | 0.11 | 0.96 | 0.03 |
| Other | 0.78 | 0.29 | 2.20 | .92 | 0.80 | 0.22 | 2.93 | 0.56 |
| Health insurance | ||||||||
| None | 1.00 | 1.00 | ||||||
| Any | 5.52 | 2.47 | 12.34 | <.001 | 3.04 | 1.10 | 8.41 | 0.04 |
| Unknown | 2.88 | 0.96 | 8.69 | .68 | 1.52 | 0.37 | 6.16 | 0.82 |
| Time since Diagnosis | 0.91 | 0.77 | 1.07 | .26 | -- | -- | -- | -- |
| Hospital | 1.55 | 0.67 | 3.61 | .31 | -- | -- | -- | -- |
| Treatment Intensity | 1.58 | 1.04 | 2.39 | 0.03 | 1.83 | 1.09 | 3.06 | 0.02 |
| Family influences healthcare decisions (yes/no) | 2.86 | 1.13 | 7.21 | 0.02 | 2.97 | 0.87 | 10.08 | 0.08 |
| Has regular doctor for regular (non-cancer) health checkups | 2.51 | 1.30 | 4.85 | 0.00 | 1.13 | 0.49 | 2.64 | 0.77 |
| Healthcare self-efficacy | 1.39 | 1.14 | 1.68 | <.001 | 1.20 | 0.94 | 1.53 | 0.15 |
| Post Traumatic Growth | 1.03 | 0.99 | 1.06 | 0.06 | 1.02 | 0.98 | 1.06 | 0.29 |
| Depressive Symptoms | .099 | 0.97 | 1.02 | 0.69 | -- | -- | -- | -- |
Factors related to intent to seek follow-up care
Based on univariate logistic regression analyses, those with high intent to obtain cancer related follow-up care in the next 2 years were more likely to be under 21, male, longer since diagnosis, insured, reported family influence on healthcare decisions, have a regular non-cancer physician, higher healthcare SE, and higher PTG (Table 3). In the multivariable model intent to seek follow-up care intent was significantly associated with having insurance and higher healthcare SE.
Table 3.
Univariate and multivariable models of intent to seek cancer-related follow-up care (within next 2 years)
| Univariate | Multivariable Model | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Characteristic | Odds Ratio | 95% CI Lower | 95% CI Upper | P Value | Adjusted Odds Ratio | 95% CI Lower | 95% CI Upper | P Value |
|
|
|
|||||||
| Age (age 21 vs. under 21) | 0.298 | 0.157 | 0.565 | < .001 | 0.47 | 0.18 | 1.20 | 0.12 |
| Sex (ref group=male) | 0.554 | 0.297 | 1.033 | .063 | 0.54 | 0.22 | 1.31 | 0.18 |
| Race/Ethnicity | ||||||||
| White, non-Hispanic | 1.00 | 1.00 | ||||||
| Hispanic | 0.963 | 0.475 | 1.949 | .744 | 0.74 | 0.26 | 2.12 | 0.40 |
| Other | 1.143 | 0.438 | 2.985 | .724 | 1.23 | 0.31 | 4.86 | 0.57 |
| Health insurance | ||||||||
| None | 1.00 | 1.00 | ||||||
| Any | 18.393 | 7.317 | 46.238 | < .001 | 13.39 | 4.11 | 43.63 | <.001 |
| Unknown | 3.989 | 1.294 | 12.294 | .878 | 2.90 | 0.68 | 12.41 | .69 |
| Time since Diagnosis | 0.870 | 0.743 | 1.020 | .086 | 0.80 | 0.64 | 1.00 | .05 |
| Hospital (ref group=LB) | 1.008 | 0.429 | 2.369 | .985 | -- | -- | -- | -- |
| Treatment Intensity | 0.970 | 0.909 | 1.035 | .355 | -- | -- | -- | -- |
| Family influences healthcare decisions | 2.795 | 1.115 | 7.006 | .028 | 2.70 | 0.73 | 9.95 | 0.14 |
| Has regular doctor for regular (non-cancer) health checkups | 2.532 | 1.342 | 4.776 | .004 | 1.19 | 0.48 | 2.96 | 0.71 |
| Healthcare self-efficacy | 1.674 | 1.363 | 2.055 | < .001 | 1.66 | 1.30 | 2.18 | <.001 |
| Post Traumatic Growth | 1.036 | 1.006 | 1.067 | .017 | 1.04 | 1.00 | 1.09 | .08 |
| Depressive Symptoms | 0.979 | 0.953 | 1.006 | .129 | -- | -- | -- | .-- |
Discussion
We found that, among CCS diagnosed between 2000–2007 at two major hospitals in Los Angeles County, who were now adolescents and young adults, use of previous follow-up care was lower among those over 21 years, compared to younger survivors. Eighty-five percent of CCS under 21 reported previous follow-up care vs. 56% of those over 21. A similar pattern was observed for future intent to seek care, although not significant in the multivariable model. This rapid drop in healthcare utilization during emerging adulthood is likely related to challenges of assuming new adult roles.25 These results are consistent with other findings among late adolescent and emerging adult CCS (ages 16–29), where only 35% recognized their potential for serious health problems related to their cancer treatment and over 50% were not seeking cancer related follow-up care.5 Thus, specialized clinics and broader education efforts focused on CCS transitioning from pediatric to adult care settings may help prevent this decline in follow-up care.
Barriers to survivorship care among young adults are thought to be multifactorial and may involve lack of patient awareness of health risks and need for medical surveillance, geographical inconvenience and limited access to qualified providers, low motivation due to competing life priorities, and lack of health insurance.33,5 Follow-up care (both previous and planned) was significantly higher among the insured, regardless of insurance type (public vs. private). Recent healthcare reform that includes insurance for children through parental coverage up to the age of 26 (among those whose parents have private insurance) and more affordable healthcare options for adults with pre-existing conditions may mitigate this drop off. Future work concerning health insurance change is needed to determine this and, for example, the impact of insurance status on follow-through on referrals for detecting late effects. Supporting young adult survivors in obtaining and maintaining health insurance, as well as addressing other age-related barriers to survivorship care mentioned above, is probably best accomplished through a structured transitional care program that facilitates the planned, systematic transfer of survivorship care from pediatric to adult-focused providers and facilities 11, 12, 34
Previous use of follow-up care, although not future intent to seek it, was lower among Hispanics relative to non-Hispanics. The reasons for this disparity are unknown, but it was not accounted for by insurance or treatment differences. There is a striking lack of research information for Hispanic CSS. For example, in a CCSS report on follow-up care, only 1.6% (n=135) of the participants were Hispanic.11 [Although our study had a larger proportion of Hispanics (54.4%), we note that the absolute number of Hispanics in our study (n=105) is lower than in the much larger CCSS cohort.] Additional research is needed and should include assessments of cultural beliefs and/or differential milestones of emerging adulthood among Hispanic CCS. For example, there may be unique cultural beliefs concerning healthcare management/meaning of cancer that impact follow-up care behavior among Hispanics.35
PTG and healthcare self-efficacy were associated with intent to seek follow-up care. Both of these factors are amenable to intervention,36, 37 and are fruitful targets for improved adherence to follow-up care. For example, interventions can focus on fostering patient-level strengths (e.g., eliciting positive narratives/resources derived from the cancer experience) that can serve as resources/motivators for future healthcare engagement. Similar efficacy/strengths-based approaches have shown effectiveness in improving engagement with HIV care among high risk populations38 and, among CCS, could be especially attractive during the transition to adulthood.
Although the majority of survivorship research among CCS has understandably focused on comorbidities and late effects of treatment, more recent work has documented life adjustment trajectories among CCS that include positive life transformations.39–42 Many CCS report their cancer experience made them a stronger, or better, person. For example, among 150 adolescent CCS, Barakat et al. found the majority to report posttraumatic growth (PTG; positive life changes stemming from their cancer experience).43 PTG is a likely, yet understudied, indicator of successful adaptation to the cancer experience39 and may represent a resource for optimizing health behaviors. 44–47 For example, following treatment, a brief strengths-based case management intervention targeting healthcare self-efficacy/activation and PTG may provide a motivating narrative for CCS to engage in cancer related follow-up care. Developing these and other tailored interventions based on patient resources/characteristics for long term engagement with care may mitigate subsequent declines in follow-up care. 48–50
This study is limited in that it does not include CCS diagnosed under age 5, those with Hodgkins disease (who may have more intense treatment), and only included patients from 2 prominent CCS treating hospitals in the Los Angeles area. Response bias may also affect our results because 50% did not participate. It is possible that more highly educated CCS responded. However our recruitment rate was similar or slightly higher than other recently formed registry cohorts among adolescent and young adults (e.g., 43%; 51) and, differences did not emerge in age, race/ethnicity, an ecological SES variable, or clinical variables compared to registry data. If bias did occur, more motivated CCS would be the more likely to respond, and our results could overestimate follow-up care. In addition, although treatment intensity was determined through chart review, appropriateness and/or venue of the follow-up care could not be assessed. Because CCS are primarily treated at COG institutions (e.g., Children’s hospitals) 52 and more likely to adhere to recommended screenings if followed at an oncology clinic12, future work should assess the site of follow-up care (e.g., pediatric/ transition/adult survivorship clinic vs. community clinic). In addition, longitudinal studies are needed to determine the extent of these relationships over time and the impact of the Affordable Care Act.
Supplementary Material
Acknowledgments
This project was supported by the Whittier foundation. Additional support was provided by P30CA014089 and T32CA009492 from the National Cancer Institute of the National Institutes of Health.
Footnotes
The authors have no financial disclosures.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Prasad PK, Bowles T, Friedman DL. Is there a role for a specialized follow-up clinic for survivors of pediatric cancer? Cancer treatment reviews. 2010 Jun;36(4):372–376. doi: 10.1016/j.ctrv.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014 Mar-Apr;64(2):83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. The New England journal of medicine. 2006 Oct 12;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Jones BL. Promoting healthy development among survivors of adolescent cancer. Family & community health. 2008 Jan-Mar;31(Suppl 1):S61–70. doi: 10.1097/01.FCH.0000304019.98007.ae. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz LA, Mao JJ, Derosa BW, et al. Self-reported health problems of young adults in clinical settings: survivors of childhood cancer and healthy controls. Journal of the American Board of Family Medicine: JABFM. 2010 May-Jun;23(3):306–314. doi: 10.3122/jabfm.2010.03.090215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Council NR. Childhood Cancer Survivorship: Improving Care and Quality of Life. The National Academies Press; 2003. [PubMed] [Google Scholar]
- 7.Mertens AC. Cause of mortality in 5-year survivors of childhood cancer. Pediatric blood & cancer. 2007 Jun 15;48(7):723–726. doi: 10.1002/pbc.21114. [DOI] [PubMed] [Google Scholar]
- 8.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004 Dec 15;22(24):4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Hudson MML, Landier W, Eshelman D, Forte K, Darling J, Hester Allison, Sweeney T. The Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Cure Search. 2006 [Google Scholar]
- 10.Bhatia S, Meadows AT. Long-term follow-up of childhood cancer survivors: future directions for clinical care and research. Pediatric blood & cancer. 2006 Feb;46(2):143–148. doi: 10.1002/pbc.20613. [DOI] [PubMed] [Google Scholar]
- 11.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008 Sep 20;26(27):4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freyer DR, Brugieres L. Adolescent and young adult oncology: transition of care. Pediatric blood & cancer. 2008 May;50(5 Suppl):1116–1119. doi: 10.1002/pbc.21455. [DOI] [PubMed] [Google Scholar]
- 13.Klosky JL, Cash DK, Buscemi J, et al. Factors influencing long-term follow-up clinic attendance among survivors of childhood cancer. Journal of cancer survivorship: research and practice. 2008 Dec;2(4):225–232. doi: 10.1007/s11764-008-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellino SM, Casillas J, Hudson MM, et al. Minority Adult Survivors of Childhood Cancer: A Comparison of Long-Term Outcomes, Healthcare Utilization, and Health-Related Behaviors From the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2005 Sep 20;23(27):6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 15.Census US. [Accessed July 30th 2013];State & County QuickFacts. 2013 http://quickfacts.census.gov/qfd/states/00000.html.
- 16.Kadan-Lottick NS, Ness KK, Bhatia S, Gurney JG. Survival Variability by Race and Ethnicity in Childhood Acute Lymphoblastic Leukemia. JAMA: The Journal of the American Medical Association. 2003 Oct 15;290(15):2008–2014. doi: 10.1001/jama.290.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Hunger SP, Lu X, Devidas M, et al. Improved Survival for Children and Adolescents With Acute Lymphoblastic Leukemia Between 1990 and 2005: A Report From the Children’s Oncology Group. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012 Mar 12; doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. Trends in childhood cancer mortality--United States, 1990–2004. MMWR. Morbidity and mortality weekly report. 2007 Dec 7;56(48):1257–1261. [PubMed] [Google Scholar]
- 19.Weinick RM, Zuvekas SH, Cohen JW. Racial and Ethnic Differences in Access to and Use of Healthcare Services, 1977 to 1996. Medical Care Research and Review. 2000 Nov 1;57(suppl 1):36–54. doi: 10.1177/1077558700057001S03. [DOI] [PubMed] [Google Scholar]
- 20.Coughlin SS, Uhler RJ. Breast and Cervical Cancer Screening Practices among Hispanic Women in the United States and Puerto Rico, 1998–1999. Preventive Medicine. 2002;34(2):242–251. doi: 10.1006/pmed.2001.0984. [DOI] [PubMed] [Google Scholar]
- 21.Ward E, Jemal A, Cokkinides V, et al. Cancer Disparities by Race/Ethnicity and Socioeconomic Status. CA: A Cancer Journal for Clinicians. 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. 2002;100 doi: 10.1182/blood-2002-02-0395. [DOI] [PubMed] [Google Scholar]
- 23.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: a comparison of long-term outcomes, healthcare utilization, and health-related behaviors from the childhood cancer survivor study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005 Sep 20;23(27):6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 24.Lihua LJ, Zhang, Deniis Deapen. Cancer in Los Angeles County, Incidence and Mortality by Race/Ethnicity, 1988–2009. Los Angeles: University of Southern California; 2010. [Google Scholar]
- 25.Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009 May 10;27(14):2363–2373. doi: 10.1200/JCO.2008.21.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudson MMLW, Landier W, Constine LS, Bhatia S, Armstong FD, Darling, Fisher PD, Friedman DL, Green DM. CureSearch. 3.0. 2008. Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolscent, and Young Adult Cancers. [Google Scholar]
- 27.Cox CL, Hudson MM, Mertens A, et al. Medical screening participation in the childhood cancer survivor study. Archives of internal medicine. 2009 Mar 9;169(5):454–462. doi: 10.1001/archinternmed.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hospital SJCsR. Childhood Cancer Survivor Study. 2010 http://ccss.stjude.org/documents/questionnaires.
- 29.Werba BE, Hobbie W, Kazak AE, Ittenbach RF, Reilly AF, Meadows AT. Classifying the intensity of pediatric cancer treatment protocols: the intensity of treatment rating scale 2.0 (ITR-2) Pediatric blood & cancer. 2007 Jun 15;48(7):673–677. doi: 10.1002/pbc.21184. [DOI] [PubMed] [Google Scholar]
- 30.Lorig KSA, Ritter P, González V, Laurent D, Lynch J. Outcome Measures for Health Education and other Healthcare Interventions. Thousand Oaks: SAGE; 1996. [Google Scholar]
- 31.Cann A, Calhoun LG, Tedeschi RG, et al. A short form of the Posttraumatic Growth Inventory. Anxiety, stress, and coping. 2010;23(2):127–137. doi: 10.1080/10615800903094273. [DOI] [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D Scale. Applied Psychological Measurement. 1977 Jun 1;1(3):385–401. [Google Scholar]
- 33.Freyer DR. Transition of care for young adult survivors of childhood and adolescent cancer: rationale and approaches. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 Nov 10;28(32):4810–4818. doi: 10.1200/JCO.2009.23.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson TO, Hlubocky FJ, Wroblewski KE, Diller L, Daugherty CK. Physician preferences and knowledge gaps regarding the care of childhood cancer survivors: a mailed survey of pediatric oncologists. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010 Feb 10;28(5):878–883. doi: 10.1200/JCO.2009.25.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casillas J, Kahn KL, Doose M, et al. Transitioning childhood cancer survivors to adult-centered healthcare: insights from parents, adolescent, and young adult survivors. Psycho-oncology. 2010;19(9):982–990. doi: 10.1002/pon.1650. [DOI] [PubMed] [Google Scholar]
- 36.Wagner B, Knaevelsrud C, Maercker A. Post-Traumatic Growth and Optimism as Outcomes of an Internet-Based Intervention for Complicated Grief. Cognitive Behaviour Therapy 2007. 2007 Nov 01;36(3):156–161. doi: 10.1080/16506070701339713. [DOI] [PubMed] [Google Scholar]
- 37.Knaevelsrud C, Liedl A, Maercker A. Posttraumatic Growth, Optimism and Openness as Outcomes of a Cognitive-behavioural Intervention for Posttraumatic Stress Reactions. Journal of health psychology. 2010 Oct 1;15(7):1030–1038. doi: 10.1177/1359105309360073. [DOI] [PubMed] [Google Scholar]
- 38.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008 Apr 15;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 39.Arpawong TE, Oland A, Milam JE, Ruccione K, Meeske KA. Post-traumatic growth among an ethnically diverse sample of adolescent and young adult cancer survivors. Psycho-oncology. 2013 doi: 10.1002/pon.3286. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eiser C, Hill JJ, Vance YH. Examining the psychological consequences of surviving childhood cancer: systematic review as a research method in pediatric psychology. Journal of pediatric psychology. 2000 Sep;25(6):449–460. doi: 10.1093/jpepsy/25.6.449. [DOI] [PubMed] [Google Scholar]
- 41.Meeske KA, Ruccione K, Globe DR, Stuber ML. Posttraumatic stress, quality of life, and psychological distress in young adult survivors of childhood cancer. Oncology nursing forum. 2001 Apr;28(3):481–489. [PubMed] [Google Scholar]
- 42.Haase JE, Kintner EK, Monahan PO, Robb SL. The Resilience in Illness Model, Part 1: Exploratory Evaluation in Adolescents and Young Adults With Cancer. Cancer nursing. 2013 Mar 20; doi: 10.1097/NCC.0b013e31828941bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barakat LP, Alderfer MA, Kazak AE. Posttraumatic growth in adolescent survivors of cancer and their mothers and fathers. Journal of pediatric psychology. 2006 May;31(4):413–419. doi: 10.1093/jpepsy/jsj058. [DOI] [PubMed] [Google Scholar]
- 44.Milam J, Ritt-Olson A, Tan S, Unger J, Nezami E. The September 11th 2001 Terrorist Attacks and Reports of Posttraumatic Growth among a Multi-Ethnic Sample of Adolescents. Traumatology. 2005 Dec 1;11(4):233–246. [Google Scholar]
- 45.Milam JE, Ritt-Olson A, Unger JB. Posttraumatic Growth among Adolescents. Journal of Adolescent Research. 2004 Mar 1;19(2):192–204. [Google Scholar]
- 46.Love C, Sabiston CM. Exploring the links between physical activity and posttraumatic growth in young adult cancer survivors. Psycho-oncology. 2011 Mar;20(3):278–286. doi: 10.1002/pon.1733. [DOI] [PubMed] [Google Scholar]
- 47.Leung YW, Alter DA, Prior PL, Stewart DE, Irvine J, Grace SL. Posttraumatic growth in coronary artery disease outpatients: Relationship to degree of trauma and health service use. Journal of Psychosomatic Research. 2012;72(4):293–299. doi: 10.1016/j.jpsychores.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiljer D, Urowitz S, Frasca E, et al. The Role of a Clinician-Led Reflective Interview on Improving Self-Efficacy in Breast Cancer Survivors: A Pilot Study. Journal of Cancer Education 2010. 2010 Sep 01;25(3):457–463. doi: 10.1007/s13187-010-0103-0. [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg AR, Macpherson CF, Kroon L, Johnson R. Rethinking Adherence: A Proposal for a New Approach to Risk Assessment. J Adolesc Young Adult Oncol. 2013 Jun;2(2):83–86. doi: 10.1089/jayao.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cox CL, Zhu L, Finnegan L, et al. Survivor profiles predict health behavior intent: the Childhood Cancer Survivor Study. Psycho-oncology. 2012;21(5):469–478. doi: 10.1002/pon.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harlan LC, Lynch CF, Keegan TH, et al. Recruitment and follow-up of adolescent and young adult cancer survivors: the AYA HOPE Study. Journal of cancer survivorship: research and practice. 2011 Sep;5(3):305–314. doi: 10.1007/s11764-011-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Leary M, Krailo M, Anderson JR, Reaman GH. Progress in Childhood Cancer: 50 Years of Research Collaboration, a Report From the Children’s Oncology Group. Seminars in oncology. 35(5):484–493. doi: 10.1053/j.seminoncol.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.